Liposarcomas represent the second most common type of soft tissue sarcomas after malignant fibrous histiocytoma. There are four histological subtypes according to the World Health Organization: well-differentiated (atypical lipomatous) liposarcoma, dedifferentiated, myxoid (round-cell) liposarcoma (ML), and pleomorphic liposarcoma1. Their primary anatomical sites of origin from liposarcomas are the retroperitoneum in 25-50% and the lower limbs in 25-35%. The most frequent distant sites for metastasis arising from ML include lungs, retroperitoneum, abdominal cavity, and chest wall1. ML accounts for 30-50% of all liposarcomas, with a peak incidence between the third and the fifth decade of life, being more frequent in males than females, exhibiting an overall metastatic rate of 30%. Interestingly, it has an expansive growth pattern, with less aggressive infiltrative behavior, thus, causing frequently obstructive clinical syndromes at the main site of metastasis, however, metastatic intracardiac soft tissue sarcomas represent an extremely rare clinical entity1. Some authors believe that in patients with a known history of liposarcoma, the time interval between diagnosis and the occurrence of distant metastatic disease ranges from 3 to 25 years1.
Fulminant right ventricular (RV) failure from RV metastasis causing outflow obstruction, cardioembolic complications, such as ischemic stroke or significant massive pulmonary tumor embolism (PTE) could occur, carrying catastrophic consequences2-6. PTE complicating RV metastasis from ML represents an extremely difficult premortem diagnostic challenge for clinicians. Herein, we describe, to be best of our knowledge, the first case, of a rapidly progressive hematogenous metastatic ML to the RV complicated with extensive PTE with catastrophic outcomes.
Case description
A 63-year-old Hispanic woman with medical history of hypertension and hyperlipidemia was transferred to our hospital, with a significant history of 5-week progressively worse dyspnea; in the previous hospital, she was diagnosed with pneumonia; however, despite antibiotics given, her condition started to deteriorate over the following weeks. The patient also reported unknown quantity of weight loss in the past 4 months, as well as progressively worse right hip pain and discomfort with subjective swelling for the past 2 months, provoking her a couple of falls.
On physical examination, her blood pressure was 105/70 mmHg, heart rate at 105 beats per minute, afebrile with an oxygen saturation of 94% at 4L/NC; cardiac auscultation revealed significant tachycardia; lung auscultation with bilateral lungs clear; right hip with pain on deep palpation, and exacerbation of pain on extension and flexion of it; and no skin discolorations noted.
Laboratory findings were notable for severe thrombocytopenia of 25,000 cells/mm3 (normal > 140,000 cells/mm3) and lactate dehydrogenase of 1042 U/L (normal menor 250 U/L). 12-lead electrocardiogram showed sinus tachycardia. Chest radiography showed bilateral basal multifocal airspace opacities, suggesting possible infarcts or atelectasis. Bilateral lower extremity venous Doppler ultrasonography did not reveal any deep vein thrombosis.
Transthoracic echocardiography showed a "D"-shaped morphology of the left ventricle during systole, left ventricular ejection fraction between 60% and 65%; moderate right atrial enlargement, severe RV dilation, and hypokinesis with positive McConnell's sign, with multiple intraventricular echodensities, suggestive of RV thrombi, some of them pending in the posterior leaflets of the tricuspid valve, and other attached to the free wall of the RV, biggest thrombi dimensions are 1.5 cm x 1.7 cm; lack of microbubble (transpulmonary) contrast agent uptake, suggesting possible avascular structures, estimated pulmonary arterial pressure at 61 mmHg (Fig. 1A).
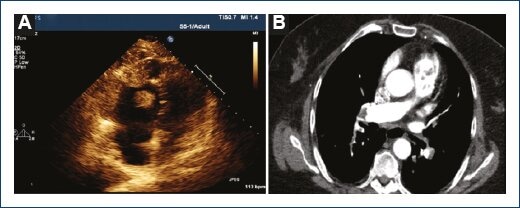
Figura 1 Transthoracic echocardiogram at apical four-chamber view showing severe right ventricular (RV) dilation, presence of the McConnell sign (significant distal RV lateral free wall hypokinesis) suggestive of multiple RV thrombi; biggest thrombi dimensions are 1.5 cm x 1.7 cm (A). Computed tomographic angiogram of the chest showing intracavitary RV thrombi with right main and left lobar bilateral pulmonary emboli extension, and absence of contrast enhancement within the filling defects (B). Please, also, see supplementary videoclip of the transthoracic echocardiography in the electronic version of this article.
Computed tomographic (CT) angiography of the chest for pulmonary embolism protocol showed central right and left main bilateral pulmonary emboli, extending into the lobar arteries; multiple thrombi filling the RV, with bilateral diffuse lower lobes ground glass opacities (Fig. 1B). CT with contrast of the abdomen and pelvis showed extensive peripherally enhancing fluid collections within the right hip and right thigh musculature, measuring 14.3 cm x 14.8 cm. Pathologic macroscopic and microscopic descriptive findings are shown (Fig. 2A-2F).

Figura 2 Gross fresh appearance, short axis section. Right ventricle occupied by tumor (A). Low-power field, the tumor invades endocardium and cardiomyocytes HyE x10 (B). Conspicuous myxoid stroma and malignant cells with "floating" aspect HyE x25 (C). Pulmonary artery filled by tumoral thrombus (d). Histological view tumoral thrombus and lung parenchyma, HyE x10 (E). Acute pulmonary thromboembolism concomitant HyE x10 (F).
During her hospital course, she was managed with intravenous argatroban drip, given her severe consumptive thrombocytopenia. Orthopedic surgery performed a fine-needle aspiration biopsy on her right hip, given her extensive lesions concerning for malignancy, and given her very poor surgical candidacy and unacceptable high risk for major bleeding events. The patient's clinical condition rapidly deteriorated in the next 24-48 h, to the point that she became severely hypoxemic, hypotensive, requiring mechanical ventilatory support, and vasopressor therapies. Unfortunately, the patient passed away, after her family members decided to withdraw care through her 4th day of hospital stay.
Discussion
Although primary cardiac tumors are rare (between 0.01% and 0.1% in postmortem studies), the frequency of cardiac metastasis is more common, ranging between 0.5% and 3.7% in the general population;2-4 however, its frequency in patients with known malignancy varies from 9.1% to 14.5% in patients with advanced metastatic malignancies4,5. The symptomatology from cardiac metastasis is non-specific, and the clinical features are highly variant and dependent on the location and disseminated tumor burden2-5.
Cardiac metastasis can spread to the heart mainly by four mechanisms: direct extension or contiguity, hematogenous spread, lymphatic spread or by transvenous and/or intracavitary invasion4,5. Melanomas, lymphomas and sarcomas are amongst the most common tumors that cause hematogenous invasion. Certain malignancies such as renal cell carcinomas and hepatocarcinomas can extend into the inferior vena cava and right atrium, through transvenous diffusion6.
Pericardial metastasis is the most common site of invasion, with an estimated frequency of 60-69% of all necropsy studies; epicardial and myocardial involvement represent the second and third most common sites, in approximately 25-34% of cases; endocardial and intracavitary are rare, representing 3-5% on necropsy studies; however, they can present with exaggerated and life-threatening clinical features with immediate negative outcomes2-6. Cardiogenic shock due to fulminant RV failure from RV metastasis causing outflow obstruction, significant cardioembolic complications from tumor embolism, causing ischemic stroke or pulmonary tumor emboli, like in our patient, can carry catastrophic outcomes.
Intracardiac metastatic solid sarcomas represent extremely rare clinical entities. From menor que 40 cases of metastatic cardiac ML that has been reported in medical literature in English language since 1968, only 13 cases have been reported in the medical literature involving the RV since the first case reported by Godwin et al., in 19817,8.
In an imaging-based review series, Chiles et al.9 reported an incidence of 0.6% of intracardiac metastases from sarcomas, with an increased incidence in males, the primary ML usually located in the lower extremities, with a long interval between the occurrence of the primary ML and metastasis (usually months to years), indicating slow tumor growth, although, in our patient, she had a subacute clinical presentation, with relatively rapid progression and clinical deterioration.
Among many diagnostic imaging techniques currently available for the evaluation of cardiac metastasis, contrast-enhanced transthoracic echocardiography typically represents the best initial diagnostic imaging modality of choice9,10.
Macroscopic PTE has been reported in soft tissue sarcomas, hepatocellular, breast, and renal cell carcinomas, whereas microscopic PTE has been found in gastric, hepatocellular, pancreatic, and choriocarcinomas11. Tumor embolism possesses a significant level of resistance to recanalization, leading to progressive and irreversible vascular obstruction. Even in patients with a history of known malignancy, accurate diagnosis is only made in as few as 6% antemortem11. PTE has a frequency of 0.3-26% of solid malignancies in necropsy studies, with a mortality rate of approximately 8%.11 Latchana et al.12 performed a focused literature review with a particular focus of soft tissue sarcomas and PTE, finding 45 cases, in which 14 cases arise from soft tissue sarcomas and 31 cases from bone sarcomas; the most common symptom was dyspnea (87%), followed by cough (29%), chest pain (7%), and hemoptysis (4-5%); most of the patients presented acutely (0-2 weeks), seven patients presented subacutely (2-8 weeks), and only one patient had a chronic presentation (>8 weeks).
Conclusions
Metastatic hematogenous ML involving the RV with extensive/massive PTE represents an extremely rare clinical entity, with significant challenges while trying to establish the diagnosis premortem. Even in the early stages of the disease, despite offering prompt and adequate therapies for ML, prognosis has been generally poor, even in the short term. In this particular case, PTE turned out to be subacute in onset with rapid progression, with extremely difficult premortem diagnosis. This case is extremely rare, authentic, and unique because, to the best our knowledge, this is the first case in which its clinical presentation was compatible with extensive/massive PTE, complicating RV metastasis from ML, and ending up with devastating consequences.











 nueva página del texto (beta)
nueva página del texto (beta)


