Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos de cardiología de México
versión On-line ISSN 1665-1731versión impresa ISSN 1405-9940
Arch. Cardiol. Méx. vol.83 no.3 Ciudad de México jul./sep. 2013
https://doi.org/10.1016/j.acmx.2013.04.003
Investigaciones clínicas
Cardiopulmonary exercise testing in healthy children and adolescents at moderately high altitude
Prueba de esfuerzo cardiopulmonar a niños y adolescentes sanos en altitud moderadamente elevada
Hermes Ilarraza-Lomelía,*, Javier Castañeda-Lópeza, Jonathan Myersb, Irma Mirandac, Paula Quirogad, María-Dolores Riusa, César Lopez-de-la-Vegaa, Enrique Vallejoe, Juan Calderónc, Javier Figueroac, Alfonso Buendíac
a Servicio de Rehabilitación Cardiaca, Instituto Nacional de Cardiología Ignacio Chávez, México, DF, México
b Division of Cardiology, Veterans Affairs Palo Alto Health Care System, Stanford University, Palo Alto, California, United States
c Departamento de Cardiología Pediátrica, Instituto Nacional de Cardiología Ignacio Chávez, México, DF, México
d Servicio de Rehabilitación Cardiaca, Instituto Modelo de Cardiología, Córdova, Argentina
é Departamento de Cardiología Nuclear, Instituto Nacional de Cardiología Ignacio Chávez, México, DF, México
*Corresponding author at:
Juan Badiano 01, Colonia Sección XVI,
Tlalpan, CP 14080, México, DF, México.
Tel.: +55 732911; fax: +55 731994.
E-mail address: hermes_ilarraza@yahoo.com (H. Ilarraza-Lomelí).
Received 29 January 2013.
Accepted 12 April 2013.
Abstract
Objective: Cardiopulmonary exercise testing is a tool that helps clinicians to establish diagnosis and calculate risk stratification in adults. However, the utility of this test among children with congenital heart disease has not been fully explored. The goal of this study was to describe reference values for cardiopulmonary performance of healthy children. Methods: This study included 103 apparently healthy children (aged from 4 to 18 years; 61 boys), who underwent cardiopulmonary test using a treadmill protocol. All tests took place at 2240m above sea level (Mexico City).
Results: Exercise time was 11 ± 4 min. There were no complications. Peak oxygen uptake correlated closely with height in both genders (girls r = 0.84; boys r = 0.84, p < 0.001). A multivariable linear regression model showed that body surface area, exercise time, gender and heart rate reserve were significant predictors of peak oxygen uptake (R2 =0.815, p<0.001). Peak oxygen uptake was strongly associated with age even among children younger than thirteen years (r = 0.74, p <0.001).
Conclusion: This study provides physiological values for the major cardiopulmonary variables obtained from exercise testing using a treadmill among healthy children. Cardiopulmonary exercise test can be safely and effectively performed in young children even as young as 4 years old. Variables including age, gender and height are strongly associated with exercise time, peak heart rate and peak oxygen uptake. Regression equations for predicting peak heart rate and peak oxygen uptake are presented as reference values that allow researchers to compare children with heart disease versus those who are healthy.
Key words: Cardiopulmonary exercise testing, Congenital heart disease, Children, Oxygen uptake, México.
Resumen
Objetivo: La prueba de esfuerzo cardiopulmonar es una herramienta que ayuda a los médicos a establecer el diagnóstico y estratificar el riesgo en adultos. Sin embargo, su utilidad en los niños no se ha explorado a fondo. El objetivo fue describir los valores de esta prueba en niños sanos en altitud moderadamente alta.
Métodos: Se realizaron pruebas de esfuerzo cardiopulmonar a 103 niños sanos (4 a 18 años, 61 varones) mediante tapiz rodante y a 2240m sobre el nivel del mar (Ciudad de México).
Resultados: El tiempo de ejercicio fue de 11 ± 4 min, sin complicaciones. El consumo de oxígeno pico se correlacionó estrechamente con la talla en ambos géneros (niñas r = 0.84; niños r = 0.84, p < 0.001). El modelo multivariado que incluyó superficie corporal, tiempo de ejercicio, género y la frecuencia cardíaca de reserva fue un fuerte predictor del consumo de oxígeno pico (R2=0.815, p<0.001).
Conclusión: Las pruebas de esfuerzo cardiopulmonar mediante tapiz rodante se pueden realizar con seguridad y eficacia en niños, incluso de 4 años de edad. Variables como la edad, el género y la talla están fuertemente asociados con el tiempo de ejercicio, la frecuencia cardiaca máxima y el de oxígeno pico. Las ecuaciones de regresión obtenidas para calcular la frecuencia cardíaca máxima y el consumo de oxígeno pico pueden ayudar, tanto a clínicos como a investigadores, a comparar el comportamiento de niños con cardiopatías frente a los que no las tienen.
Palabras clave: Prueba de ejercicio cardiopulmonar, Cardiopatía congénita, Niños, Consumo de oxígeno, México.
Introduction
Exercise testing is a well recognized tool for assessing cardiovascular function in a wide range of individuals, from patients with heart disease to high performance athletes.1,2 In recent years the test has been used for the evaluation of children and adolescents with cardiovascular disease,3 especially those with congenital heart disease who survive until adulthood. In the past three decades, normal cardiopulmonary values during exercise among adults have been widely documented in the medical literature. However, these values have not been sufficiently described among children, among whom the physiological response to exercise differs considerably from that of adults. The value of cardiopulmonary exercise testing (CPET) lies in its power to make a global assessment of the integrated response to exercise, allowing a comprehensive evaluation of the pulmonary, cardiovascular, hematopoietic, neuropsychological, and skeletal muscle systems.4 CPET includes the measurement and analysis of work rate, electrocardiographic signals, blood pressure and respiratory gas exchange variables including oxygen uptake (VO2), carbon dioxide output (VCO2) and minute ventilation (VE), among others.
These responses are considered useful markers for risk stratification in adult patients with cardiovascular disease,5 particularly those with heart failure. Appropriate risk stratification requires comparison between the data obtained from the patient versus normal values from healthy individuals, adjusted for gender and age. However, few groups have published data obtained from CPET in children.6-9 There is also a lack of studies using a treadmill, which is the most widely used device on the American Continent. Exercise performance on a treadmill is known to be different from that observed on a cycle ergometer.2,10,11 Moreover, the CPET response to exercise at moderately high altitude has not been adequately studied in children. Therefore, the purpose of this study was to describe the cardiopulmonary response to exercise and assess associations among exercise and demographic variables, in a group of apparentlyhealthy children and adolescents.
Methods
Children and adolescents, from 4 to 18 years of age, were evaluated at the pediatric cardiology consultation room, most of them suspected of having a cardiac murmur. Demographic variables recorded included birth date, weight and height, body surface area (BSA)12 and body mass index (BMI).13 Once they were evaluated by a cardio-pediatrician and the presence of cardiovascular disease was ruled out, they were asked to perform a cardiopulmonary stress test. Written consents were obtained from the parents or legal guardian of the children. The children's parents were present during the test, and they were requested to encourage their child to provide a maximal effort. A Schiller CS-200 © device with a Trackmaster treadmill was used to perform all exercise tests. An electrocardiographic signal (ECG) was recorded throughout the test.10 Blood pressure (BP) was measured every minute during exercise, and at the 1st, 3rd, 5th and 8th min of recovery using a calibrated aneroid sphygmomanometer. Cuff size was selected according to the child's age and arm circumference.10 An automated medical gas analysis device (PowerCube ©) was used to measure the volume, airflow and the fractional concentrations of oxygen and carbon dioxide in the exhaled air. Thirty second samples were printed every 10s.2 A face mask was used to collect expired air, taking care that it fitted properly and that there were no air leaks during exercise.
A cardiac defibrillator and a fully stocked resuscitation cart were present at all times.
Exercise protocol
All exercise tests were performed at an altitude of 2240 m above sea level. The gas analyzer was calibrated according to the manufacturer specifications before each test was performed. ECG leads were placed according to the Mason-Likar2 method. Initially, skin was cleaned with an alcohol saturated cotton swab, and gentle abrasion of the skin's superficial layer was made with a fine abrasive paper.14 A resting 12-lead ECG and spirometry were performed prior to the test. CPET began with a 3 min resting period, and all subjects performed the same treadmill ramp-protocol,2,15 where they were subjected to an increase of 1 MET per minute (ACSM16) (Table 1). Once maximal exercise was achieved, subjects continued to walk for 3 min at 2 km/h at 0% elevation. Following the 3 min walking period, subjects rested in the supine position for an additional 5 min.
Standard exercise testing
All patients were instructed to express symptoms during exercise including chest pain, dyspnea or palpitations. Heart rate (HR) was obtained from the continuous ECG signal. HR at rest (HRrest) was measured when the child was seated for at least 3 min immediately prior to beginning exercise. Peak HR (HRpeak) was defined as the highest HR value achieved during exercise. HR reserve (RHR) and HR increase index17 (HRII) were used to evaluate the chronotropic response to exercise. RHR was the difference between HRrest and HRpeak, and HRII expresses this difference as a percentage of the HRrest. Heart rate recovery was obtained by subtracting HR at the first minute of recovery from the HRpeak.18 The predicted maximal HR was calculated as follows: HRmax pred =220 - age in years.2 Systolic blood pressure (SBP) was assessed using the systolic blood pressure exercise index (SBPEI) and the systolic blood pressure recovery index (SBPRI). SBPEI was calculated using SBP at peak exercise divided by SBP at rest. Similarly, SBPRI was determined by SBP at the third minute of recovery divided by the SBP value at the first minute of recovery.19 Myocardial oxygen uptake (MVO2) was estimated using the product of heart rate and systolic blood pressure (double product, DP). The presence of arrhythmias, ST-segment anomalies, or other ECG disturbances was recorded.
Gas-exchange analysis
The cardiopulmonary response to exercise was evaluated using expired gases, and the following variables were obtained: minute ventilation (VE), respiratory exchange ratio (RER),2,20 oxygen uptake (VO2) at the anaerobic threshold (AT) and maximal effort (VO2peak), predicted VO2 peak (VO2peak_pred),21 ventilatory equivalent for carbon dioxide slope (VE/VCO2),4 oxygen pulse (PO2),1 half time of VO2 recovery (VO2T1/2),4 and cardiac power during exercise (CPE).22
Statistics
Statistical analysis was performed using SPSS 15.0 software. Nominal and categorical variables were presented as frequencies and percentages, and compared using the chi square testor the Fisher exacttest. Continuous variables are presented as mean and reference intervals (CI 95%).23 A two sample t-test for independent variables and one-way ANOVA were used to compare means of those variables with normal Gaussian distribution (Kolmogorov-Smirnov test), and the Mann-Whitney U test was used for numerical variables without normal distribution. Variables were plotted, bivariate analyses were performed, and all r and p values were derived using the Pearson test. Variables that were demonstrated to be statistically significant were included in a multiple regression model (foreword stepwise).24 All p values less than 0.05 were considered significant.
Results
Demographic variables
Data from 103 children (42 girls and 61 boys), ranging in age from 4 to 18 years (mean 11.0 ±4), were studied. Height, weight, body surface area and body mass index are presented (Table 2), according to age quartile and gender. Five boys (4.9%) and 3 girls (2.9%) were obese25 (BMI >95th percentile). All CPETs were symptom limited, and fatigue was the cause for cessation in 98% of tests (n = 101, p = ns between girls and boys). One girl expressed chest pain without any ischemic changes on the ECG, and a 5-year-old boy asked to stop the test prematurely. Ninety-one children (88%) reached an objective maximal effort (RER ≥1.1 and/or HRpeak ≥85% of predicted HRpeak).
Complications and other findings
All participants performed CPET without anycomplications. Twelve children exhibited arrhythmias: supraventricular premature beats (n = 8), ventricular ectopy (n = 6), and frequent ventricular ectopy (n= 2), with no statistical differences between genders or age. No significant changes on the ST-T segments were observed.
Standard cardiovascular response to exercise
Cardiovascular variables obtained from the tests are shown in (Table 3). Exercise time was higher in boys. Linear regression showed a significant association between age and exercise time in both girls and boys (r = 0.64, p < 0.001). Among the youngest (children <13 years old), exercise time increased with age (r = 0.60, p < 0.001), but this association was inverse among adolescents (≥13 years old) and was not significant (r = -0.234, p = ns). Heart rate at rest decreased with age (r = -0.54), p < 0.001) after adjusting for potential confounders (height, weight, SBPrest and DBPrest), and was higher among girls. HRpeak was lower in boys, and showed a significant correlation with age (r = 0.33, p = 0.008). Among girls, no significant correlation was observed (p = ns). RHR correlated significantly with age (r = 0.53, p<0.001), especially among boys (r = 0.60, p < 0.001), and also with exercise time (r = 0.65, p = 0.001) for all children. HRR decreased slightly with age (r = 0.27, p <0.01), and was poorly related to RHR (r = 0.20, p = 0.04). Blood pressure (both SBP and DBP) at rest and during exercise increased with age and was higher among boys (p < 0.001). Double product at peak exercise was closely related to age (r= 0.70, p=0.001), and no differences were found at rest. Blood pressure indexes (SBPEI or SBPRI) were not associated with age or gender. On the other hand, CPE rose with age, without differences according to gender.
Analysis of respiratory gas exchange
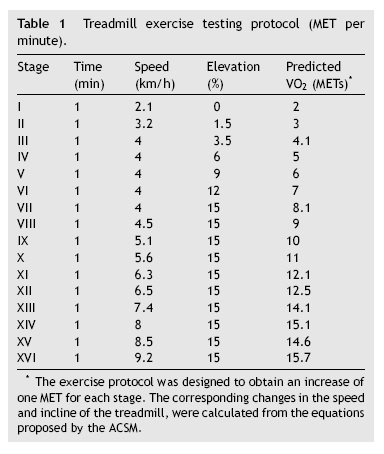
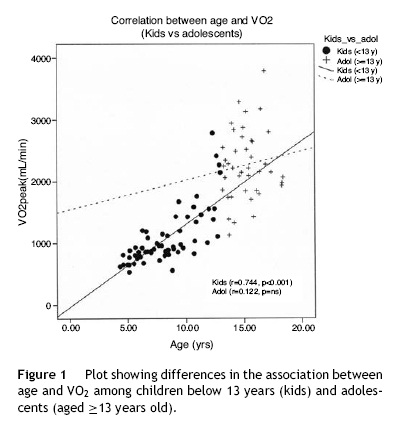
Data obtained from cardiopulmonary variables are shown in Table 4. RERpeak increased with age and correlated positively with HRpeak and exercise time. Peak oxygen uptake was higher in boys at every age and had strong positive associations with age, weight, height and BSA. However, the correlation between age and VO2peak was reduced when children were older than 13 years (Fig. 1). We performed a multiple regression model to predict VO2peak achieved, including age, weight, and height. Among these variables, height was the strongest predictor of VO2peak, among both boys and girls (Fig. 2) (Table 5).
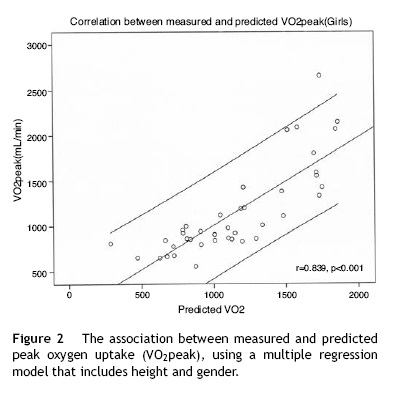
VO2 was estimated in the absence of expired gas analysis using exercise test performance variables. The multiple linear regression model included BSA, exercise time, gender and RHR (R2= 0.813, p < 0.001) (Fig. 3). VO2 at the anaerobic threshold was higher in boys and was modestly correlated with VO2peak among adolescents (r = 0.42, p < 0.001), but not in younger subjects. The VE/VCO2 slope decreased with age, VO2peak and oxygen pulse. The VE/VCO2 slope values were higher in girls. The oxygen pulse was higher in boys and was closely related to age (r = 0.81, p<0.001). Oxygen kinetics during recovery (VO2T1/2) were higher among girls and did not correlate with heart rate recovery (r= 0.15, p= ns). Finally, minute ventilation increased with age and was higher among boys.
Discussion
The main goal of this analysis was to provide CPETreference values for children and adolescents. CPET behavior has been reported by other research groups7,9 in children above 8 years using a cycle ergometer. This current study adds information in terms of younger children (beginning at 4 years), and among individuals using a treadmill. However, further studies are needed to achieve a better understanding of cardiopulmonary performance at younger ages. Although energy transformation is closely correlated with tissue oxygen uptake, this association can varywidely, since workload is only one of several factors that require higher energy expenditure. Traditionally, in a treadmill exercise protocol, speed and elevation increase with time, so ET can be used as a surrogate variable for workload. In our study, children reached maximal effort at 11 ± 2 min, close to what is recommended by various guidelines on exercise testing.1,2,4,5 Contrary to what is observed among adults, ET increased with age in our sample of children. The maximum heart rate is traditionally described as a major determinant of VO2 at peak exercise. Among adults, HRpeak decreases with age (r = -0.40),2 and when HRpeak is impaired it can reflect chronotropic incompetence, probably as part of the aging process. Interestingly, we found that among children, HRpeak and age were positively associated. HRpeak was slightly lower in our population than those reported by Ten Harkel et al.,9 and this could be attributed in part to differences in altitude between The Netherlands (at sea level) vs. Mexico City (2240m above sea level).
Performing exercise at high altitude can result in a reduced sympathetic nervous system effect on heart rate. Other factors affecting maximal heart rate in response to dynamic exercise include age, gender, level of fitness, cardiovascular disease, bed rest, type of exercise, and the extent to which maximal exertion was achieved.2 We observed that RERpeak values rose significantly with age, exercise time and HRpeak.
Children in the current study reached higher values for RERpeak (1.15) than those reported by Ten Harkel et al.9 (1.01), or among patients with congenital heart disease26 (RERpeak=1.10). VO2peak increased closely with height, and the regression equation that we obtained to estimate VO2peak paralleled that proposed by Cooper et al. for children using a cycle ergometer (r = 0.99, p <0.001).21 The current equations to predict VO2peak were superior to those more commonly used based on age only9 (r = 0.27, p = ns). We also observed that children reached higher RERpeak values and lower VO2peak values than those reported by other groups. This could be due to environmental conditions such as air pollution, altitude or even individual variables such as genetics, height or weight.
Among adults, it is common in clinical practice to estimate VO2peak indirectly using one of several regression equations; most of these equations use only the work rate achieved. However, these regression equations provide only modest accuracy (r values ranging from 0.60 to 0.90), due to variability introduced that is attributable to such factors as habituation to exercise, fitness level, handrail holding and the exercise protocol used.2 We found that the estimation of VO2 in children was improved by adding other variables including body surface area, gender and heart rate reserve (R2=0.82, p<0.001). As other authors have described, we found that VO2 values correlate better with height than with age or weight in children.7 Therefore, it appears that VO2 is better expressed in terms of BSA (ml O2/m2/min), than in terms of weight (ml O2/kg/min), based on a higher regression coefficient (r = 0.84 vs r = 0.80, respectively). Ten Harkel et al. proposed that HR can be an inexpensive surrogate for exercise capacity,9 although we did not find a strong correlation between HRpeak and VO2peak. The VE/VCO2 slope correlated positively with age and VO2peak, and was lower than has been reported previously in children.9
These results suggest some potentially useful avenues for future investigation, including a comparison of cardiopulmonary variables between children with congenital heart disease and healthy populations, assessing physiological phenomena related to the cardiorespiratory response to exercise in children and to establish new prognostic variables in children with heart disease. Among adults, CPET variables are frequently used to help make decisions regarding listing for heart transplantation. More information is needed to determine the predictive power of CPET responses among children with heart disease. Another possible application of these data is the comparison of normal standards from CPET to those obtained from young high-performance athletes.27
Limitations
We studied a group of children who were initially referred to a pediatric cardiologist to assess cardiovascular disease. Although illness was ruled out, the sample was no doubt biased. There are some variables that could provide more information about exercise performance, such as air pollution levels, socioeconomic, dietary, or other factors that were not considered.
Conclusions
This study provides physiological response to cardiopulmonary exercise testing using a treadmill among children without heart disease. CPET can be safely and effectively performed in children, even as young as 4 years of age. Age, gender and height are strongly associated with exercise time, heart rate (peak and reserve) and oxygen uptake. Regression equations to predict peak heart rate, VO2peak, and VE/VCO2 slope are presented as reference values to permit comparisons between children with heart disease and those who are healthy.
Financial support
None.
Conflict of interest
Authors declare no conflict of interest.
References
1. Wasserman K, Hansen J, Sue D, Stringer W, Whipp B. Principles of exercise testing and interpretation. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. [ Links ]
2. Froelicher V, Myers J. Exercise and the heart. 5th ed. Philadelphia: Ed. Saunders; 2006. [ Links ]
3. Connuck D. The role of exercise stress testing in pediatric patients with heart disease. Prog Pediatr Cardiol. 2005;20:45-52. [ Links ]
4. Piepoli MF, Corrà U, Agostoni PG, Belardinelli R, Cohen-Solal A, Hambrecht R. Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: recommendations for performance and interpretation. Part I. Definition of cardiopulmonary exercise testing parameters for appropriate use in chronic heart failure. Task Force of the Italian Working Group on Cardiac Rehabilitation Prevention (Gruppo Italiano di Cardiologia Riabilitativa e Prevenzione GICR) endorsed by the Working Group on Cardiac Rehabilitation and Exercise Physiology of the European Society of Cardiology. Eur J Cardiovasc Prev Rehabil. 2006;13:150-64. [ Links ]
5. Weisman I, Marciniuk D, Martinez F, Sciurba F, Sue D, Myers J. Indications for cardiopulmonary exercise testing. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003:211-77. [ Links ]
6. Washington RL, van Gundy JC, Cohen C, Sondheimer HM, Wolfe RR. Normal aerobic and anaerobic exercise data for North American school-age children. J Pediatr. 1988;112:223-33. [ Links ]
7. Cooper DM, Weiler-Ravell D, Whipp BJ, Wasserman K. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol Respir Environ Exerc Physiol. 1984;56:628-34. [ Links ]
8. Takken T, Blank AC, Hulzebos EH, van Brussel M, Groen WG, Helders PJ. Cardiopulmonary exercise testing in congenital heart disease: equipment and test protocols. Neth Heart J. 2009;17:339-44. [ Links ]
9. Ten Harkel AD, Takken T, Van Osch-Gevers M, Helbing WA. Normal values for cardiopulmonary exercise testing in children. Eur J Cardiov Prev Rehabil. 2010;18:48-54. [ Links ]
10. Paridon SM, Alpert BS, Boas SR, et al. Clinical stress testing in the pediatric age group: a statement from the American Heart Association Council on Cardiovascular Disease in the Young, Committee on Atherosclerosis, Hypertension, and Obesity in Youth. Circulation. 2006;113:1905-20. [ Links ]
11. Quiroga P, Ilarraza-Lomelí H, Rius-Suárez MD, Miranda I, Zamora C, Buendía A. Cardiac rehabilitation program in pediatric patients. Circulation. 2008;117:99. [ Links ]
12. Mosteller RD. Simplified calculation of body surface area. N Engl J Med. 1987;317:1098. [ Links ]
13. Behrman R, Jenson H, Kliegman R. Essential of pediatrics. 5th ed. Philadelphia: Elsevier Saunder; 2006. [ Links ]
14. Ilarraza H, Torres-Domínguez C, Mendoza-Pena B, et al. Efecto de la preparación de la piel en su impedancia eléctrica. Rev Arg Cardiol. 2005;73:171. [ Links ]
15. Barbosa O, Ribeiro L, Sobral D. Treadmill stress test in children and adolescents: higher tolerancia on exertion with ramp protocol. Arq Bras Cardiol. 2007;89:354-9. [ Links ]
16. American College of Sports Medicine. Guidelines for exercise testing and exercise prescription. 5th ed. Philadelphia: Lea and Febiger; 1995. p. 269-87. [ Links ]
17. Pacheco N, Ceron N, Alonso J, Ilarraza H. Conduct relation comparison between cardiac frequency and work load during stress test on patients with cardiopathy. Circulation. 2008;117:115. [ Links ]
18. Cole Ch Blakstone E, Pashkow F, Snader C, Lauer M. Heart rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351-7. [ Links ]
19. McHam S, Marwick T, Pashkow F, Lauer M. Delayed systolic blood pressure recovery after graded exercise an independent correlate of angiographic coronary disease. J Am Coll Cardiol. 1999;34:754-9. [ Links ]
20. Corrà U, Mezzani A, Bosimini E, Giannuzzi P. Cardiopulmonary exercise testing and prognosis in chronic heart failure. A prognosticating algorithm for the individual patient. Chest. 2004;126:942-50. [ Links ]
21. Cooper CM, Wieler-Ravell D. Gas exchange response to exercise in children. Am Rev Respir Dis. 1984;129:547-8. [ Links ]
22. Scharf C, Merz T, Kiowski W, Oechslin E, Schalcher C, Brunner-La Rocca HP. Noninvasive assessment of cardiac pumping capacity during exercise predicts prognosis in patients with congestive heart failure. Chest. 2002;122:1333-9. [ Links ]
23. Altman Douglas. Practical statistics for medical research. London: Chapman & Hall/CRC; 1999. [ Links ]
24. McCulloch C, Searle S. Generalized, linear, and mixed models. New York: John Wiley & Sons, Inc.; 2001. [ Links ]
25. Kaufer-Horwitz M, Toussaint G. Indicadores antropométricos para evaluar sobrepeso y obesidad en pediatría. Bol Med Hosp Infant Mex. 2008;65:502-18. [ Links ]
26. Karila C, de Blic J, Waernessyckle S, Benoist MR, Scheinmann P. Cardiopulmonary exercise testing in children. Chest. 2001;120:81-7. [ Links ]
27. Padilla J, Eguía Lis MC, Licea J, Taylor AW. Capacidad aeróbica máxima y actividad deportiva en mexicanos de 13 a 56 años de edad. Arch Inst Cardiol Mex. 1998;68:224-31. [ Links ]














