Serviços Personalizados
Journal
Artigo
Indicadores
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Archivos de cardiología de México
versão On-line ISSN 1665-1731versão impressa ISSN 1405-9940
Arch. Cardiol. Méx. vol.82 no.2 Ciudad de México Abr./Jun. 2012
Comunicación breve
Anterograde flow compromise of a patent left internal mammary artery graft from a proximal subclavian artery stenosis. Myocardial ischemia not driven by the coronary–subclavian steal syndrome mechanism
Compromiso anterógrado del flujo de un puente de arteria mamaria izquierda debido a estenosis de la arteria subclavia. Isquemia miocárdica por un mecanismo distinto al síndrome de robo coronario–subclavio
Alejandro Alcocer, Genaro Castillo, Juan M. Rivera–Capello, Vidal González, Eduardo Meaney.
Cardiovascular and Hemodynamic Unit. Hospital Regional 1° de Octubre, ISSSTE. Mexico City, Mexico.
Corresponding author:
Alejandro Alcocer, MD.
Av. Politécnico Nacional 1669, Gustavo A. Madero.
C.P. 07300. Mexico City, Mexico.
Telephone: 5586 2557.
E–mail address: alcocerchauvet@gmail.com
Received on January 27, 2011.
Accepted on November 16, 2011.
Abstract
A 54–year male with previous triple vessel coronary artery and aorto–bifemoral bypass graft surgeries complained of crescent angina. Stress induced myocardial ischemia on echocardiography was demonstrated. We performed direct stenting of a saphenous vein graft to the right coronary artery, via right radial approach. Subsequently stenting of a severe left subclavian artery proximal stenosis was performed via right brachial approach in order to relieve an overt myocardial ischemia in the territory supplied by a patent left internal mammary artery graft originated distally to the left subclavian stenosis. The finding of a total left axillary artery occlusion complement the pathogenesis of myocardial ischemia produced by limited anterograde flow and not driven by the common flow reversal mechanism of a typical coronary–subclavian steal syndrome.
Keywords: Coronary–subclavian steal syndrome; Saphenous vein bypass; Brachial artery approach; Direct stenting; Angioplasty; Multivascular intervention; Mexico.
Resumen
Se trata de un paciente masculino de 54 años de edad, con antecedentes de cirugía de revasuclarización miocárdica por enfermedad coronaria trivascular, así como puentes aortobifemorales. Se presentó en nuestro centro aquejando de angina de pecho, de patrón creciente. Se demostró la existencia de isquemia miocárdica, mediante ecocardiografía con estrés farmacológico. Realizamos una angioplastia con stent a un puente de safena inversa a la coronaria derecha por vía radial derecha. Posteriormente debido a una estenosis proximal severa de la arteria subclavia izquerida, implantamos un stent mediante vía braquial derecha, con la idea de mejorar la isquemia existente en el territorio previamente revascularizado por un puente de arteria mamaria izquierda, que se originaba distalmente a la estenosis de la arteria subclavia. El hallazgo de una oclusión total crónica a nivel de la arteria axilar izquierda completó la patogénesis de la isquemia miocárdica no condicionada por robo al flujo coronario, como tradicionalmente sucede en el síndrome de robo subclavio coronario. En este caso particular, el mecanismo isquémico fue debido al compromiso anterógrado de flujo, provocado por la estenosis severa de la arteria subclavia.
Palabras clave: Síndrome de robo sub–clavio–coronario; Puente de safena inversa; Abordaje braquial derecho; Implantación de stent; Angioplastía; Intervencionismo multivascular; México.
Introduction
Coronary artery graft bypass surgery (CAGB) late complications are not infrequent. When dealing with this sort of patients the Interventional Cardiologist must always think about different possibilities that could explain the presence of myocardial ischemia other than just the existence of coronary artery or bypass graft significant stenosis, specially in patients with known multi–vessel artery disease, in whom is always wise to take a thoroughly angiographic exploration of the related arterial tree. So when facing with anterior myocardial ischemia in a patient with a patent left internal mammary artery graft it is particularly important to look after the presence of a left subclavian artery proximal disease. Therefore the publication of cases with different ischemia physiopathologies in post CABG surgery patients means a great added value to comprehend these certainly difficult ischemia origins.
Case presentation
A 54–year man was referred from his local clinic to our regional hospital, due to crescent angina. He had history of long standing diabetes mellitus requiring insulin treatment. He also had hypertension and dyslipidemia. He was a current smoker at the time of our first assessement. Triple vessel CABG surgery was performed on year 2000, due to severe left main and right coronary artery disease. Aorto–bifemoral bypass graft surgery was also performed on year 2001, due to severe bilateral iliac artery disease.
Recently stress echocardiography with dobutamine was performed in order to evaluate a chronic angina pattern. The study showed anterior and lateral myocardial ischemia of the left ventricle, therefore a new coronary catheterization was decided. The procedure was performed on May 2008. Right radial artery approach was employed, due to previous aorto–bifemoral bypass graft surgery and the lack of left arm artery pulses. The angiographic findings were severe left main disease and total anterior descending, circumflex and right coronary artery occlusions. Also a total saphenous vein graft occlusion to the circumflex coronary artery and severe saphenous vein graft stenosis to the right coronary artery were found. During attempts, via right radial approach to engage the left subclavian artery with a Cordis Corp. XB® 3,5 6F left coronary guiding catheter, in order to perform direct visualization of the left internal mammary artery graft (LIMA), a 74% proximal left subclavian artery stenosis was demonstrated (Automatic edge–detection algorithm, MEDIS, CMS, Leiden, The Netherlands) which precluded the possibility to perform LIMA graft selective engagement.
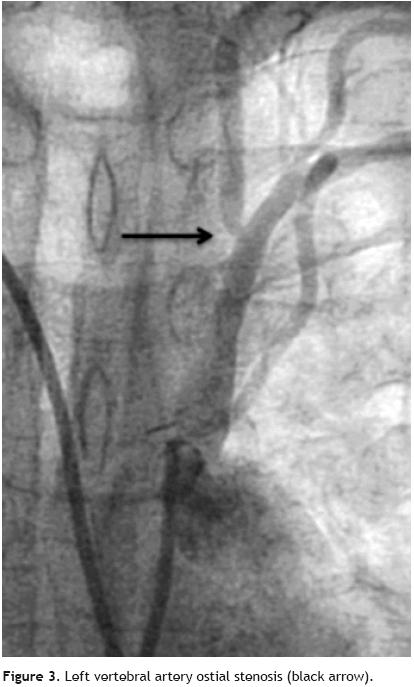
Nevertheless it was possible to see mild anterograde LIMA graft angiographic flow reaching the mid–distal anterior descending coronary artery during dye injection performed from the left subclavian artery ostium (Figure 1). We also demonstrated a total axillary artery occlusion and an 82% ostial left vertebral artery stenosis (Figure 2 and 3). With these findings, we performed direct stenting with an Abbot Vascular Multi–link Vision® 3,5x12mm stent to the stenosis of the saphenous vein graft to the right coronary artery with satisfactory result. Then, in a second procedure we performed angioplasty to the proximal left subclavian artery stenosis in order to relieve the echocardiographically proved anterior myocardial ischemia of the left ventricle, taking on knowledge that there was no structural commitment of the LIMA graft as the cause of myocardial ischemia. Also, we decided not to treat the total axillary artery occlusion and the ostial vertebral artery stenosis due to the patient had not left arm or neurologic ischemic clinical manifestations.

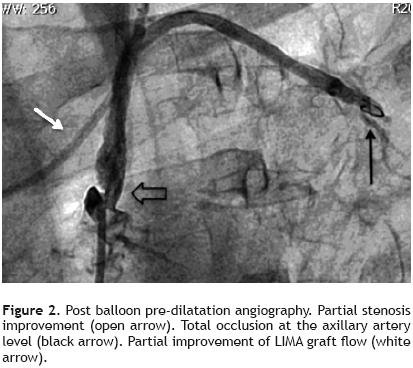
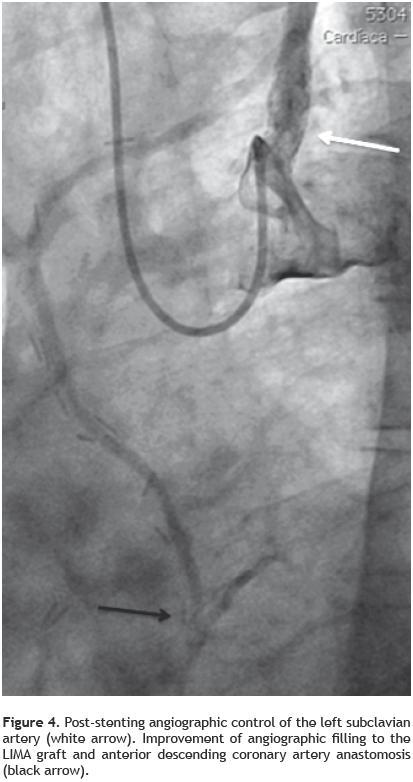
The second procedure was conducted via right brachial approach, performing the engagement the left subclavian artery ostium with a Cordis Corp. XB® 4,0 7F left coronary guiding catheter, balloon pre–dilatation was made and further Boston Scientific Express® Biliary LD balloon expandable Stent System 7.0 x 20mm implantation was done, with good angiographic result and without residual translesion gradient (Figure 4).
Three months later, dobutamine stress echocardiography was performed, resulting negative for myocardial ischemia whilst the patient remained asymptomatic.
Discussion
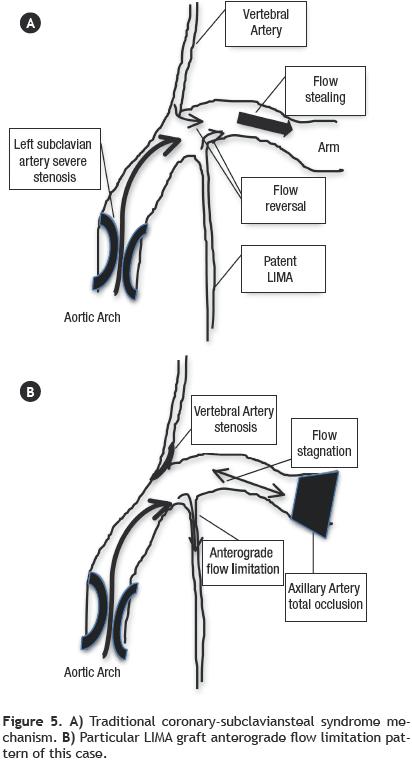
Multiple cases of coronary–subclavian steal syndrome have been published until now,1,2 and also cases with unusual or difficult technical approaches to relief this relatively common myocardial ischemia pathophysiology.3 Nevertheless, as far as we know this is the first published case with a distinct myocardial ischemia mechanism, that could initially appeared as a typical coronary–subclavian steal syndrome. The typical syndrome, was described in patients after a CABG surgery in whom a proximal subclavian artery stenosis resulted in reversal of flow through the LIMA graft due to flow stealing from the upper extremity (Figure 5). This process can lead to myocardial ischemia, which may manifest as angina pectoris or even ischemic cardiomyophaty. The clear pathophysiology distinction between our case and one of a coronary–subclavian artery steal syndrome relies on our patient total left axillary artery occlusion. The association with proximal left subclavian artery stenosis and the total occlusion at the axillary artery level, precludes direct flow to the left arm, which sets this case apart from the typical flow stealing mechanism exerted by the left arm, which was previously described as pathognomonic of the coronary–subclavian steal syndrome. We are also certain that the vertebral artery, in this particular case does not produced flow stealing, because it had a significant ostial stenosis.
We did not perform vascular doppler ultrasonography previous to the treatment, because we did not have enough experience in the interpretation of this sort of useful studies. Despite this fact, our case etiology and mechanism of myocardial ischemia appeared to be due to direct flow limitation to the LIMA graft, produced by the severe left subclavian artery stenosis in a clearly distinct mechanism of the traditional described syndrome.
This case also represents one of a very diffuse vascular disease with subsequently difficult vascular access; cases like this, are not frequent and poses a big challenge in dealing with non–favorable vascular approaches and allows to the utilization of devices initially designed for another purposes; like the use in this particular case of a coronary guiding catheter to perform a subclavian artery angioplasty. Generally speaking, is a wise decision to treat this sort of CABG long–term complications by the use of percutaneous techniques, because repeated surgery is burdened by a higher risk of death and provides less symptomatic improvement.4
The decision to perform left subclavian angioplasty was driven by the presence of proved anterior myocardial ischemia of the left ventricle despite a patent LIMA graft to the anterior descending coronary artery. The vascular right radial and right brachial artery approaches certainly appeared to be a non–favorable way to reach the left subclavian artery. Nevertheless there was no access via left arm or femoral arteries, as the usual ways to deal with left subclavian artery stenosis. The decision to use a XB® 4,0 7F left coronary guiding catheter, finally suited well and gave correct catheter support to perform the entire procedure.
The subsequently proved absence of inducible anterior myocardial ischemia of the left ventricle after subclavian artery stenting, confirmed the resolution of the ischemia, just by the improvement of LIMA graft anterograde flow, achieved by relieving of the proximal subclavian artery stenosis.5
References
1. Ishii K, Hirota Y, Kita Y, et al. Coronary–subclaviansteal corrected with percutaneous transluminal angioplasty. J Cardiovasc Surg 1991;32:275–277. [ Links ]
2. Marques KM, Ernst SM, Mast EG, et al. Percutaneous transluminal angioplasty of the left subclavian artery to prevent or treat the coronary–subclavian steal syndrome. Am J Cardiol 1996;78:687–90. [ Links ]
3. Kang WC, Han SH, Ahn TH, et al. Percutaneous transluminal angioplasty and stent implantation for coronary subclavian steal syndrome: unusual approach. Int J Cardiol 2005;101:335–7. [ Links ]
4. Foster ED, Fisher LD, Kaiser GC, Myers WO. Comparison of operative mortality for initial and repeat coronary artery bypass grafting: The Coronary Artery Surgical Study (CASS) Registry Experience. Ann Thorac Surg1984;38:563–570. [ Links ]
5. Coats AJ. Ethical authorship and publishing. Int J Cardiol 2009;131:149–50. [ Links ]














