Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos de cardiología de México
versión On-line ISSN 1665-1731versión impresa ISSN 1405-9940
Arch. Cardiol. Méx. vol.81 no.3 Ciudad de México oct./sep. 2011
Investigación clínica
The role of pulmonary pressure/cardiac index to identify pulmonary hemodynamic responders to acute oxygen breathing pulmonary hypertension COPD patients
La utilidad de la relación presión arterial pulmonar – índice cardiaco para identificar respondedores hemodinámicos a la administración aguda de oxígeno en sujetos con enfermedad pulmonar obstructiva crónica e hipertensión pulmonar
Eulo Lupi–Herrera,1 Julio Sandoval,2 Luis Efren Santos,2 Tomás Pulido,2 Martín Rosas–Peralta3
1 Sub–Direction of Clinical Investigation.
2 Cardiopulmonary Department.
3 Clinical Cardiology Department.
Instituto Nacional de Cardiología Ignacio Chávez, México.
Corresponding author:
Eulo Lupi Herrera.
Sur 136 N° 116. Col. Las Américas,
01120, México, D. F. México.
Telephone: 52 308 000, ext. 3762.
E–mail: elupiherrera@hotmail.com.
Received on April 16, 2009;
Accepted on March 30, 2011.
Abstract
Objectives: We sought to analyze exercise–derived mean pulmonary artery pressure (Mpap) – cardiac index (CI) – relationship to expand the concepts regarding its nature and to better identify pulmonary hemodynamic responders to acute oxygen breathing (AOB – 99.5%) in pulmonary hypertension (PH) – COPD patients.
Methods: mPAP/CI and extrapolated pressure (Pext) to zero flow were obtained breathing room air (BRA) and under AOB – 99.5% in 40 stable COPD patients with rest and exercise PH. Hemodynamic characteristics were analyzed for the entire cohort and separate for cases those with resting < or > 30 mmHg mPAP (cohort – A and B, respectively).
Results: mPAP/CI abnormal location, slope (Sp: 5.77; 95% CI: 5.02 – 6.52 mmHg/L min/m2) and Pext values (15.8 mmHg) were associated with hypoxemia/decreased mixed venous – PO2 and lung mechanics abnormalities. Hemodynamic conditions that did not change for Sp (5.47; 95% CI: 3.64 – 7.3 mmHg/L min/m2, p = 0.4) and Pext (15.7 mmHg, p = 0.2) associated with a mPAP/CI significantly decrease in parallel during AOB – 99.5%. For cohort – A, an average–mPAP decline (12.3 mmHg, p <0.004) associated with a slope decrease (from 6.02; 95% CI: 4.04 – 8 to 4.3; 95% CI: 4.11 – 4.49 mmHg/L min/m2, (p <0.008), mPAP/CI – 95% CI down–ward displacement and Pext decrease (from 8.58 ± 3 to 4.7 ± 1.4 mmHg, p <0.01) in relation to BRA were observed. For cohort–B, average–mPAP and mPAP/CI – 95% CI location did not change, Sp show a trend to decrease (p = 0.08) and Pext significantly increase (from 12 ± 2.9 to 20.6 ± 4.9 mmHg, p <0.03) in relation to BRA. Under AOB – 99.5%, significant differences for mPAP/ CI – 95% CI location, average–mPAP (A: 19.5 ± 6 vs. B: 41.2 ± 11.5 mmHg, p <0.001) and Pext (A: 4.7 ± 1.4 vs. B: 20.6 ± 4.9 mmHg, p <0.001), without Sp change between cohorts A and B were documented.
Conclusions: When exercise derived mPAP/CI is analyzed, valuable information for linear–pulmonary vascular resistance – (LPVR) could be obtained for PH – COPD patients. mPAP/CI abnormalities not always reflect "pure arteriolar" increased LPVR for all PH–COPD patients. Hemodynamic benefit on the pulmonary circulation and right ventricular afterload could be expected with long–term oxygen therapy in resting <30 mmHg mPAP–PH–COPD patients.
Keywords: Pulmonary hypertension; Pressure/flow; Acute oxygen breathing; COPD; Mexico.
Resumen
Objetivos: En esta investigación clínica–hemodinámica, analizamos la relación que se establece entre la presión arterial pulmonar media (PAPm) con la del índice cardiaco (IC), obtenida durante el ejercicio, con miras a expandir los conceptos relacionados con su propia naturaleza. Con ello, tratar de identificar mejor a los sujetos portadores de EPOC que se han caracterizado por ser respondedores durante la administración aguda de oxígeno (AAO2 – 99.5%). Métodos: Se obtuvieron la PAPm/IC y la presión extrapolada a cero flujo (Pext = bo)en 40 sujetos con EPOC y portadores de hipertensión pulmonar (HP) clínicamente estables, respirando aire ambiental (RAA) y bajo la influencia de la AAO2 – 99.5% en las condiciones de reposo y durante el ejercicio. Las características hemodinámicas se analizaron para toda la cohorte y para aquellos sujetos con PAPm en resposo < o > de 30 mmHg (Cohorte A y B, respectivamente).
Resultados: La ubicación anormal de la PAPm/IC, de la pendiente (Sp: 5.77; 95% IC: 5.02 – 6.52 mmHg/L min/m2) y la de los valores para Pext (15.8 mmHg) se asociaron con: hipoxemia/ disminución de la presión venosa mezclada del O2, así como con anormalidades de la mecánica pulmonar. Condiciones hemodinámicas que no se modificaron para la Sp (5.47; 95% IC: 3.64 – 7.3 mmHg/L min/m2, p = 0.4) y la Pext (15.7 mmHg, p = 0.2); sin embargo, sí se vieron asociadas a una disminución significativa en paralelo de la PAPm/IC durante la AAO2 99.5%.
Observaciones hemodinámicas que para la cohorte A, se caracterizaron por una reducción de la PAPm promedio (12.3 mmHg, p <0.004), por una disminución de la Sp de 6.02; 95% CI: 4.04 – 8 a 4.3; 95% CI: 4.11 – 4.49 mmHg/L min/m2, (p <0.008) y por el descenso de Pext de 8.58 ± 3 a 4.7 ± 1.4 mmHg, p <0.01, al compararse con las documentadas RAA. En cambio, para la cohorte B, la PAPm promedio y la PAPm/IC no se modificaron, Sp mostró sólo tendencia a disminuir (p = 0.08) y Pext aumento de 12 ± 2.9 a 20.6 ± 4.9 mmHg, (p <0.03) en relación a las registradas RAA. Bajo la AAO2 – 99.5%, se observaron diferencias significativas para la PAPm/ IC – 95% IC en su localización, para la PAPm promedio (A: 19.5 ± 6 vs. B: 41.2 ± 11.5 mmHg, p <0.001) y Pext (A: 4.7 ± 1.4 vs. B: 20.6 ± 4.9 mmHg, p <0.001) y sin cambios en la Sp, entre la cohorte A y la B.
Conclusiones: Cuando se analiza la PAPm/IC, se obtiene información que es valiosa para interpretar la resistencia vascular pulmonar linear en sujetos con EPOC e H P. Sin embargo, las anormalidades de la PAPm/IC, no necesariamente reflejan aumento exclusivo de la resistencia arteriolar pulmonar para sujetos con EPOC e H P. De acuerdo con las observaciones agudas de este estudio, posiblemente solo sea de esperarse beneficio con la oxigenoterapia a largo plazo sobre la circulación pulmonar y la post–carga del ventrículo derecho, para aquellos portadores de EPOC e HP cuando la PAPm en el reposo sea <30 mmHg.
Palabras clave: Hipertensión pulmonar; Relación presión flujo; Oxígeno terapia; EPOC; México.
Introduction
Due to the meaningless significance of calculated pulmonary vascular resistance (PVR),1 a bedside approach to get a deeper insight into the PVR linear component nature is based on the determination of multipoint mean pulmonary artery pressure (mPAP) – cardiac index (CI) – relationship.2–5 In most cardiac/pulmonary diseases mPAP/CI are well described by a linear approximation, but their extrapolation to the pressure axis at zero flow (Pext) may be higher than pulmonary artery occluded pressure or left atrial pressure.1,2,5 An increased Pext could be explained by an increased mean closing pressure in small arterioles or capillaries (or both), or also could result from the forces on the outside of the vessels, such as abnormal alveolar pressure on the pulmonary capillaries.6–11 Approach for PVR determination, that from time to time had been attempted in cohorts composed by few cases with pulmonary hypertension (PH) due to lung diseases in the last 40 years. Although, to our knowledge this hemodynamic approach has not been attempted to evaluate the acute role of oxygen according to mPAP level in PH chronic obstructive pulmonary disease (COPD) patients.3–5 Information that could be important, since it has been suggested that when resting mPAP is moderately to markedly elevated (>30 mmHg) at the onset of long–term oxygen therapy (LTOT), the survival rate is decreased in comparison with patients with no or mild PH (<30 mmHg).12–17 Accordingly, it has been proposed that LTOT should be started earlier at the stage of mild (or even absent) PH to favor the long–term survival in hypoxemic COPD patients.15
Objective
To extend the limited clinical information in relation to multipoint mPAP/CI in PH–COPD; second, if mPAP/CI location, the slope value and Pext analysis could help to better identify pulmonary vasomotion due to acute oxygen breathing and in consequence recognize which cases could be hemodynamically beneficiated by decreasing right ventricular (RV) afterload among PH–COPD patients.
Methods
Patients. The protocol was approved by the ethics review commission. The series of studied patients (n = 40; born, raised and permanent residents of Mexico City (altitude 2240 meters)) were selected from a group of 677 cases, which represented the total number referred to the Cardio–Pulmonary Department for an evaluation of the pulmonary circulation between 1997 and 2007; who's applied diagnostic criteria was the proposed by the American Thoracic Society for COPD.18
All patients should have the following inclusion criteria: 1) Class I – II NYHA/WHO system. 2) None should have a history of atopy or received drugs susceptible of having a vasodilator effect. 3) Absence of a respiratory infection/RV failure syndrome within twelve weeks prior the study. 4) Nonexistence of systemic arterial hypertension, valvular heart, coronary artery or primary myocardial diseases and, 5) Evidence of RV hypertrophy/dilatation, on the basis of either electrocardiographic/echocardiographic methods.
The methodology for lung function test, for the analysis of blood/expired gases and for normal values of blood–gas exchange at our moderate altitude has been previously published.19,20 Pulmonary function results were compared with the normal values reported by Comroe,21 Baldwing22 and Morris23 and expressed as a percentage of the predicted normal value.
For all patients it was its first admission to our institution and were treated before with conventional therapy. This therapy included inhaled bronchodilators, antibiotic agents and mild diuretic as each patient's clinical status indicated. They used supplemental oxygen therapy very irregularly and none were previously on a currently accepted LTOT program.12–16
To compare the results obtained for the pressure/ flow for the studied PH – COPD patients, we analyzed the pressure/ flow in 66 normal subjects (20 to 64 years of age); where 40 cases were collected form the literature; (6 from Harris,2 4 from Lewis,24 3 from Riley,25 8 from Hickam,26 5 from Slonin,27 7 from Fishman,28 7 from Dexter29) and from 26 healthy volunteers studied at our institution. Also, we compare our results obtained for the pressure/ flow from 70 COPD patients where the pertinent pulmonary hemodynamic information could be individually analyzed published by Emirgil30 (n = 6), Evans31 (n = 7), Khaja32 (n = 3), Kitchin33 (n = 2) and by Williams34 (n = 52).
Hemodynamic and exercise measurements. All the procedures were explained in detail and informed consent was obtained for all patients. Our procedure for RHC at rest and during steady – state – exercise has been previously described.19,20 Briefy, all patients were studied in the morning, in the fasting, non–sedated state. Supplemental oxygen therapy was discontinued 4 hours before the RHC study. The hemodynamic evaluation was done with the patient in the supine position and according to the protocol part while breathing room air, on oxygen 99.5% and then after receiving supplemental oxygen by nasal prongs at rest. Pulmonary vascular pressures were measured at end–expiration and sampled at 200 Hz using an analog/digital converter and stored and analyzed on a personal computer. The following measurements were obtained: mPAP, mean pulmonary wedge pressure (mPWP) and mean systemic arterial pressure (mSAP). Cardiac output (CO) was measured by triplicate by the thermodilution method. For 99.5% O2 breathing, the mixture was inspired throughout a non–rebreathing valve from a reservoir bag (surrounded by atmospheric pressure) during 15 minutes previous to the hemodynamic basal measurements and then after while exercising.
Study protocol. Before the hemodynamic study all patients were familiarized with the exercise technique. After steady–state conditions were ensured (stable heart rate (HR), CO, mPAP and mSAP for 10 minutes) a baseline set of hemodynamic measurements, expired gases and blood samples were obtained over – 1 minute period (resting baseline measurements). Systemic and pulmonary pressures were measured by averaging over 3 normal respiratory cycles. After 30 minutes equilibration period between each exercise protocol part, the measurements were then repeated pedaling in the supine position on a bicycle–ergometer for at least 6 minutes [maximum 9 minutes] at a load of 35 W to 40 W; during the following conditions: 1) breathing room air and 2) on 99.5% O2 . The exercise level was so selected that no more than mild to moderate dyspnea or fatigue appeared for all studied patients. An exercise set of hemodynamic measurements, expired gases and blood samples were never collected before 5 minutes of completed exercise (exercise measurements). The collection period for all exercise measurements starts at the moment when the patient refer by a pre–established signal dyspnea/fatigue, and was never <1 minute (maximum 3 minutes). In order that the work done in the two exercise periods should be equal the rate of peddling were kept constant during exercising.
One hour after the last exercise test, while breathing room air, resting baseline measurements was repeated. Supplemental oxygen was initiated and maintained for 4 hours to 6 hours and a second set of measurements were repeated. Each patient received the lowest flow in whole L/min (3 to 4 L/min) that demonstrably increased resting, semi recumbent PaO2 of 65 mmHg to 70 mmHg.
Data analysis. We analyzed the hemodynamic characteristics for the whole cohort and separately for each group PH – COPD patients (with resting < or > 30 mmHg mPAP). The stated results at rest/exercise while breathing room air, oxygen 99.5%, at rest breathing room air and supplemental oxygen represent the average of 3 clustered measurements with less than 10% variation. Harvey and Enson,35 equations were used to assess the relative roles played by active and passive factors for PH genesis.
Plot and interpretation for mPAP/CI and Pext. The mPAP/CI coordinate generated from the resting state to the exercise condition was evaluated by linear regression analysis.36 The slope obtained was considered to be "the true PVR".1 Yield mPAP/CI – 95% confidence interval (CI) was analyzed for all the cohort and for each group.
The following steps were followed for the construction of the pressure/ flow picture: 1) the average–mPAP + 1SD obtained from the resting–exercising maneuver was located in the pressure/CI diagram. 2) The slope was drawn. 3) Pext was traced and 4) CI ± 1SD was over printed on the slope line (solid gross line).
If the tested mPAP/CI – 95% CI were located outside for that derived for the normal population it was consider to be an increased linear PVR. It was considered non similar between or among groups: if, the tested mPAP/CI –95% CI was statistically different located in relation to the control. Slope 95% CI values were considered non similar if they were statistically different from the control.
Pext was considered abnormal for >10 mmHg values,1,9–11 and dissimilar if Pext ± 1SD was significant statistically different in relation to the evaluated. Vascular pulmonary vasomotion was considered to occur if, a significant statistical decrease/increase occurs for mPAP/ CI – 95% CI tested in relation to the control (i.e. if the mPAP/CI 95% CI constructed breathing air do not overlap with the obtained breathing oxygen).
Statistical analysis. Continuous data are expressed as mean 1 ± SD. Independent Student t test, 1 way ANOVA (for multiple comparison Bonferroni's Test (i.e. baseline condition vs. exercise during air or oxygen breathing), X2, or Fisher exact test was used as appropriate. Correlations were calculated using Pearson's correlation test, p values <0.05 were accepted as indicating statistical significance. Analyses were performed by using of SPSS–13 and STATA–9 software.
Results
Clinical and pulmonary function data. All patients were exsmokers with a history of smoking equivalent to at least 20 pack–years. The mean age of the studied population was 59y ± 4y, the estimated symptom duration was 12y and male population predominate over females (2.3/1). Previous respiratory infections or the clinical syndrome of RV failure were observed in 75% and 40%, respectively. According to the NYHA/ WHO system 77.5% of the patients were in class II. Increased residual volume (Rvo), abnormal FEV1/VC ratio and FEF25–75 and decreased MBC were noted for all COPD patients. For COPD patients with a resting >30 mmHg mPAP NYHA/WHO class II and at least one previous respiratory infection treated without respiratory mechanical assistance were more frequently observed; for measurements like VC, Rvo, FEV1 , FEV1/VC and FEF25–75, all were more abnormal when compared to the measured for <30 mmHg mPAP COPD patients (Table 1).

Hemodynamic/blood gas exchange data at rest and during exercise breathing room air. For all patients, hypoxemia at rest (PaO2 <67 ± 2.5 mmHg)19,20 that worsened with exercise associated with a decrease in SvO2 was a constant feature. During exercise a significant increase for mPAP and CI was associated with an increase for VO2 (from 208 ± 38 to 602 ± 44 mL/min, p <0.02). For all patients the increased noted for mPWP was <12 mmHg during exercise (Table 2). A significant negative correlation between FEV1 and mPAP was found (r = –0.63, p <0.05). A significant positive correlation between calculated and measured diastolic–PAP and dPAP–mPWP was found (r = 0.72, r = 0.74, respectively, p <0.001).
Hemodynamic and blood gas exchange data at rest and at exercise during oxygen breathing. After 15 minutes 99.5% O2 breathing at rest, a significant decrease for mPAP, CI, dPAP–mPWP, and HR were documented, associated with a significant increase in PaO2, SaO2 and SvO2 when compared to breathing room in the resting state. During exercise a significant increase for mPAP, CI, mPWP level (that remains <10 mmHg), dPAP–mPWP and HR was observed associated with a decrease in PaCO2 and SvO2 when compared to the resting condition breathing 99.5% O2 (Table 2). When we compare both exercise conditions, mPAP increase less while breathing oxygen (p <0.02), and was not associated with arterial hypoxemia or a signif–cant change for PaCO2 and arterial pH. SvO2 remain increased when compared to the recorded breathing room air at exercise (Table 2).
mPAP/CI and Pext results. mPAP/CI–95%CI location, slope and Pext were abnormal for all PH – COPD patients breathing room air. Breathing oxygen 99.5% a significant decrease for the mPAP/CI – 95% CI in parallel was documented not associated with a slope or a Pext change (Table 3, Figure 1).
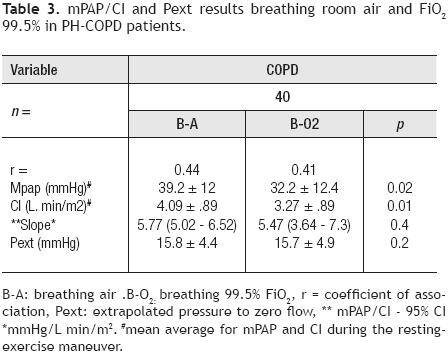

When COPD patients were separated in those with resting or 30 mmHg mPAP, for the first cohort it was noted a significant decline in average–mPAP (12.3 mmHg, p <0.004) associated with a significant slope decrease (p <0.008), a down–ward mPAP/CI – 95% CI displacement and a Pext decrease in relation to breathing room air. For the >30mmHg mPAP group, a non–significant decrease for the average–mPAP (3.3 mmHg, p = 0.23) with no change for the mPAP/CI – 95% CI location, associated with a slope trend to decrease and an increase in Pext in relation to breathing room air was documented.
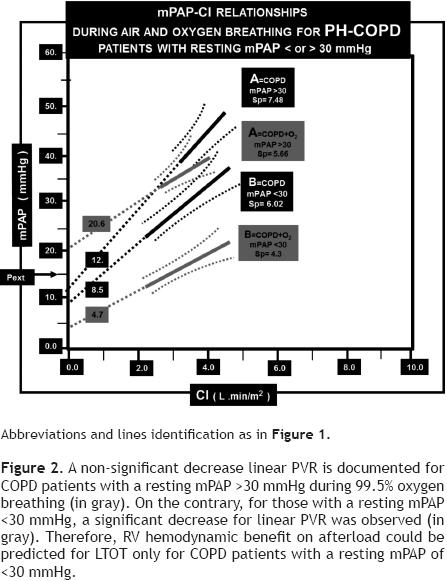
When we compare PH–COPD patients with resting mPAP or 30 mmHg, significant differences for mPAP/CI –95% CI location, for the average–mPAP and Pext values (19.5 ± 6 vs. 41.2 ± 11.5 mmHg; 4.7 ± 1.4 vs. 20.6 ± 4.9 mmHg; respectively, p <0.001 for all comparisons), without Sp differences between the two cohorts were found during 99.5% O2 breathing (Table 4, Figure 2).
Hemodynamic and blood gas exchange data during air and supplemental oxygen breathing at rest. Hemodynamic and blood gas exchange data at rest were not statistically different when compared to the recorded at the first resting baseline measurement during breathing air. After 4h to 6h of receiving supplemental oxygen, PaO2 increased (from 52 ± 3.9 to 67 ± 1.3 mmHg, p <0.001), SvO2, PaCO2 and arterial pH did not change (48 ± 2.3 vs. 47 ± 1.5%, p = 0.3; 35.2 ± 2.1 vs. 37.3 ± 4 mmHg, p = 0.2; 7.43 ± 0.02 vs. 7.41 ± 0.03 Units, p = 0.3; respectively). No differences for blood gas exchange after receiving supplemental oxygen were documented between resting or 30 mmHg mPAP PH – COPD patients.For the <30 mmHg mPAP group, a significant decrease for mPAP (8.9 ± 1.2 mmHg, p = 0.01) was associated with a CI decrease (0.48 ± 0.2 L min/m2, p <0.05) breathing supplemental oxygen when compared to breathing air. For the >30 mmHg mPAP group while breathing supplemental oxygen, mPAP and CI did not change in relation to breathing air (1.9 ± 0.3 mmHg, p = 0.1; 0.32 ± 0.1 L min/m2, p = 0.2; respectively). Hemoglobin (18.7 ± 0.6 g%), hematocrit (65 ± 1.7%) and cumulative fuid balance (1.4 ± 0.4 L) did not change during the different protocol parts.
Comparison of our results in relation to the collected from literature. For the comparison of our results breathing room air in relation to the collected from literature we analyzed the studied normal population; and separate the COPD patients hemodynamically characterized: in those without resting or exercising PH (Group I), those without resting with exercising PH (Group II) and those with resting and exercising PH (Group III). Accordingly, the following hemodynamic information was derived.
When we compare the mPAP/CI – 95% CI location and slope values for the collected normal population our results were not different from that published for normal healthy volunteers by Reeves and collaborators;37 and no differences were observed between the current cases collected from the literature vs. the volunteers studied at our moderate altitude.
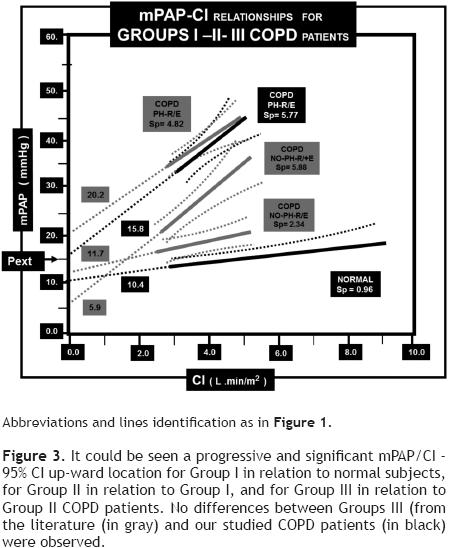
In relation to the previously mentioned COPD groups: 1) mPAP/CI – 95% CI abnormal location and slopes values for all COPD patients. 2) Increased slope values for Groups II – III in relation to Group I (p <0.01). 3) A progressive and significant mPAP/CI – 95% CI up–ward location for Group I in relation to our normal cases, for Group II in relation to Group I and for Group III in relation to Group II. 4) No slope differences between Group II and III (p = 0.1) associated with a Pext significant increase (p <0.001) and 5) no differences for the mPAP/CI – 95% CI location, for the slope or for Pext between Group III collected from the literature when compared to our Group III PH – COPD patients were documented (Table 5, Figure 3).
Discussion
The hemodynamic data collected from the studied COPD patients, who's hemodynamic profile was characterized by an abnormal mPAP at rest that increase with exercise, illustrate that when the pulmonary circulation in this part of the hemodynamic spectrum of the pulmonary circulation is investigated for PH – COPD, the location for mPAP/ CI, for the slope and for Pext value resulted abnormal as has been reported by other investigators.1–5,37 However, differences for linear PVR were observed among the studied PH–COPD patients, characterized by different mPAP/CI, slopes and Pext values when they were separated according to the resting mPAP level < or > 30 mmHg. Therefore, when considered PH–COPD, it is apparent that within the range of a normal mPWP, raised mPAP could result from both an increased in mPAP/CI and Pext for COPD who's mPAP >30 mmHg or could be solely the result from an increased mPAP/CI for those with a resting mPAP <30 mmHg.
mPAP/Q and Pext interpretation. According to our results, mPAP/CI abnormal location, increased slopes and Pext values could not be only attributed to functional (endothelial vasoconstrictor–dilator imbalance, alveolar hypoxia as well as to pulmonary–exercise–vasoconstriction induced by a decrease in SvO2/sympathetic nervous system activation) abnormalities influences in the periphery of the pulmonary vasculature for all PH–COPD. Otherwise, could be also the result of the forces on the outside of the vessels, due to lung remodeling (inflammation–fibrosis) or mechanical abnormalities (increased pleural/alveolar pressure, over infation, bronchial obstruction).1–7,38–42 Therefore, due to the numerous in–volvedness factors for PH genesis, for mPAP/Q and Pext abnormalities in this scenario, is not easy to accept we are just measuring always "the true arteriolar PVR" for all PH – COPD patients. Accordingly, it seems why for PH – COPD patients with a mPAP >30 mmHg, in addition to some non–hypoxemic active factors, or more probably due to factors like more severe bronchial obstruction/over–inflation when compared to those with a resting mPAP <30 mmHg higher slope values were documented. Moreover, according to our findings for slope values, for the mPAP/ CI location and Pext values results seems to depend on the pulmonary hemodynamic characteristic profle of the studied group PH–COPD patients; conditions that in turn seems to be associated with the pathological lung stage of the disease. Our results, support that "true arteriolar" resistance seems to be non–preponderant at advanced stage of this disease, as is supported by lack of vasodilatation (oxygen mediated) in a scenario were other non–active or fxed factors predominate in the genesis for linear PVR in PH–COPD with a mPAP >30 mmHg.1,5,6,38–42 Furthermore, histological studies, had shown that PH – COPD is not mainly a disease of the small muscular pulmonary arteries and arterioles; and the degree of vascular involvement is characterized essentially by medial hypertrophy in arteries and arterioles/cellular intimal proliferation in the smallest muscular arteries, arterioles; vascular pathological panorama that is not, or even close to the degree of vascular damage ascribed for Idiopathic–pulmonary artery hypertension patients which is considered the prototype of a more pure arteriolar pulmonary vascular disease.43,44 With the information collected from the literature and from our own data we demonstrate that COPD patients without resting or exercising PH (Group I) will exhibit lower slopes than those with those without resting with exercising PH (Group II) or with resting and exercising PH (Group III). Hemodynamic aspects that could result important in clinical practice in order to better define, at least in part, some of the current therapeutic strategies (like the impact of LTOT) for COPD patients.
The measured mPAP in the stable state for COPD patients is often <35 mmHg, level for mPAP that is in close agreement with the observed for our studied PH–COPD patients (31.7 ± 8.2mmHg).12–17 LTOT significantly improves the survival of hypoxemic COPD patients, as demonstrated by NOTT and MRC trials.12–14 Remarkable, it has been observed that in COPD patients under LTOT, the 5y survival rate was 66% in those whose initial mPAP was <25 mmHg, but only 36% when initial mPAP was >25 mmHg.14 This improved prognosis could partly be explained by the reduction of episodes of the syndrome of RV failure under LTOT and the duration and the severity of alveolar hypoxia in these patients. However, according to our results and interpretation for the PVR nature, mPAP pointed prognostic information could also be due to the prevail influence of more advanced lung disease component over the "vascular–alveolar–hypoxic potentially reversible component" for stable PH – COPD patients, in a context where mPAP was >25 mmHg. More over for those PH – COPD patients included in NOTT and MRC trials, with a 29 mmHg to 35 mmHg mPAP, little change had been observed over the years for mPAP and only sometimes reverses the progression of PH.12–16 Observation that also give support for the non–preponderant vascular reactivity oxygen mediated PVR nature for PH–COPD patients whose resting mPAP is >30 mmHg.
These observations suggest and support the opinion in relation to supplemental oxygen therapy,15–17 that LTOT should be started earlier in hypoxemic patients (with resting mPAP<30 mmHg) to achieve the reversal or lack for PH related to alveolar hypoxia/induced pulmonary–exercise–vasoconstriction induced by a decrease in SvO2 for PH – COPD patients. Hemodynamic therapeutic gold target that had not been demonstrated to our knowledge for PH – COPD under LTOT; and now is suggested on the mPAP/CI analysis basis for this group PH – COPD patients, where acute oxygen breathing/pulmonary hemodynamic effect benefit was demonstrated for COPD patients with a resting mPAP <30 mmHg. Moreover, our present observation could give support, at least in part, to the benefit in mortality referred by the NOTT group for those COPD patients with a baseline mPAP <27 mmHg (p = 0.03) and not for those with a mPAP >27 mmHg (p = 0.14),13 were the hemodynamic positive benefit on the pulmonary circulation and in consequence on RV afterload could account for a better long–term evolution for the resting mPAP <30 mmHg PH–COPD patients.
Limitations. It is clear, we only studied linear PVR at a clinical mPAP/CI range, and the non–linear segment was not directly investigated at low flows due to the nature of the protocol used.2,9–11,45 Although, we analyzed mPAP/CI behavior for PH – COPD, due to the requisites to perform the exercise–technique, the derived results could only be applied for NYHA/WHO I – II classes PH – COPD stable patients. With the protocol used, we have to accept that this maneuver increase flow but also affect vascular tone due to exercise–induced–pulmonary–vasoconstriction.45 However, if this factor or others (like lung mechanics/ anatomical/blood–gas abnormalities) when present, are taken properly in consideration, will allow us to better precise the resistive information derived from mPAP/CI and in consequence will improve its clinical interpretation at the onset of LTOT. We also are aware, that the obtained "acute hemodynamic information of the pulmonary circulation" in relation to oxygen breathing, information that must be validated in the next coming future at short– and long–term in PH – COPD patients.
Conclusions
Although, CO–exercise changes may led to spurious increased slopes in some PH patients, we demonstrate that when mPAP/CI are constructed according to the logistic provided and the derived information is analyzed in relation to PH pathogenesis, mPAP/CI could give a valuable information regarding the true linear PVR nature. We also found that mPAP/CI abnormalities, due to the influence of active/passive factors related to PH pathogenesis, could not only reflect precisely increased "true PVR for small muscular arteries/arterioles" in all the hemodynamic spectrum for PH – COPD. We should focus on resting mPAP values for PH – COPD patients, because for those with a resting <30 mmHg mPAP could probably benefit more with LTOT by in addition reducing RV afterload, hemodynamic condition which in turn should give a better long–term prognosis for this category PH – COPD patients.
References
1. McGregor M, Sniderman A. On pulmonary vascular resistance: the need for a more precise definition. Am J Cardiol 1985;55:217– 222. [ Links ]
2. Harris P, Segel N, Bishop JM. The relationship between pressure and flow in the pulmonary circulation in normal subjects and in patients with chronic bronchitis and mitral stenosis. Cardiovasc Res 1968;2:73–83. [ Links ]
3. Charms BL, Brofman BL, Eldeer JC, et al. Unilateral pulmonary artery occlusion in man II. Studies in patients with chronic pulmonary disease. J Thorac Surg 1958;35:316–331. [ Links ]
4. Charms BL, Brofman BL, Kohn PM. Pulmonary resistance in acquired heart disease. Circulation 1959;20:850–855. [ Links ]
5. Janicki JS, Weber K T, Likoff M. The pressure– flow response of the pulmonary circulation in patients with heart failure and pulmonary vascular disease. Circulation 1985;72:1270–1278. [ Links ]
6. Permutt S, Bromberger–Barnea B, Bane HN. Alveolar pressure, pulmonary venous pressure and vascular waterfall. Med Thorac 1962;19:239–260. [ Links ]
7. Permutt S, Howell JBL, Proctor D F. Effect of lung infation on static pressure–volume characteristics of pulmonary blood vessels. J Appl Physiol 1961;16:64–70. [ Links ]
8. Permutt S, Riley RL. Hemodynamics of collapsible vessels with tone: the vascular waterfall. J Appl Physiol 1963;18:924–932. [ Links ]
9. Graham R, Skoog C, Oppenheimer L. Critical closure in the canine pulmonary vasculature. Circ Res 1982;50:566–572. [ Links ]
10. López–Muñiz R, Stephens NL, Bromberger–Barnes B, et al. Critical closure of pulmonary vessels analyzed in terms of Starling resistor model. J Appl Physiol 1968;24:625–635. [ Links ]
11. Lupi HE, Furuya MYME, Sandoval J, et al. Effect of hydralazine on vascular mechanics in a canine lobar preparation of pulmonary embolism. Lung 1992;170:291–309. [ Links ]
12. Timms RM, Khaja FU, Williams GW, and the Nocturnal Oxygen Therapy trial group. Hemodynamic response to oxygen therapy in chronic obstructive lung disease. Ann Intern Med 1985;102:29–36. [ Links ]
13. Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease. A clinical trial. Ann Intern Med 1980;93:391–398. [ Links ]
14. Medical Research Council Working Party. Report of long–term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet 1981;1:681–685. [ Links ]
15. Oswald–Mammosser M, Weitzenblun E, Quoix E. Prognostic factors in COPD patients receiving long–term oxygen therapy. Importance of pulmonary artery pressure. Chest 1995;107:1193–1198. [ Links ]
16. Weitzenblum E, Sautegeau A, Ehrhart M. Long–term oxygen therapy can reverse the progression of pulmonary hypertension in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1985;131:493–498. [ Links ]
17. Weitzenblum E. Chronic Cor Pulmonale. Heart 2003;89:225–230. [ Links ]
18. ATS Statement. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease: inpatient management of COPD. Am J Respir Crit Care Med 1995;152:S97–S106,S78–S83. [ Links ]
19. Lupi HE, Sandoval J, Seoane M, et al. Behavior of the pulmonary circulation in chronic obstructive pulmonary disease. Pathogenesis of pulmonary arterial hypertension at an altitude of 2,240 meters. Am Rev Respir Dis 1982;126:509–514. [ Links ]
20. Lupi HE, Seoane M, Verdejo J. Hemodynamic effect of hydralazine in advanced, stable chronic obstructive pulmonary disease with cor pulmonale. Immediate and short–term evaluation at rest and during exercise. Chest 1984;85:156–163. [ Links ]
21. Comroe JH, Forster RE, DuBois AB, Briscoe WA. The lung. 2nd ed. Chicago: Year Book Medical Publishers, Inc. 1962;pp:329,335,339. [ Links ]
22. Baldwin E, Cournand A, Richards DW. Pulmonary insufficiency; physiological classification, clinical methods of analysis, standard values in normal subjects. Medicine 1948;27:243–278. [ Links ]
23. Morris JF, Koski WA, Johnson LC. Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis 1971;103:57–67. [ Links ]
24. Lewis CS, Samuels AJ, Daines MC. Chronic lung disease, Polycythemia and congestive heart failure. Cardiorespiratory, vascular and renal adjustments in cor pulmonale. Circulation 1952;6:874–86. [ Links ]
25. Riley RI, Himmelstein A, Motley HL. Studies of the pulmonary circulation at rest and during exercise in normal individuals and in patients with chronic pulmonary disease. Am J Physiol 1948;152:372–82. [ Links ]
26. Hickam JB, Cargill WH. Effect of exercise on cardiac output and pulmonary arterial pressure in normal persons and in patients with cardiovascular disease and pulmonary emphysema. J Clin Invest 1948;27:10. [ Links ]
27. Slonim NB, Ravin A, Balchum OJ. The effect of mild exercise in the supine position on the pulmonary arterial pressure of five normal human subjects. J Clin Invest 1954;33:1022. [ Links ]
28. Fishman A P, Fritts HW, Cournand A. Effects of acute hypoxia and exercise on the pulmonary circulation. Circulation 1960;22:204. [ Links ]
29. Dexter L, Whittenberger JL, Haynes FW. Effect of exercise on circulatory dynamics of normal individuals. J Appl Physiol 1951;3:439. [ Links ]
30. Emirgil C, Sobol BJ, Herbert WH. Routine pulmonary function studies as a key to the status of the lesser circulation in chronic obstructive pulmonary disease. Am J Med 1971;50:191–199. [ Links ]
31. Evans TO, Van Der Reis L, Selzer A. Circulatory effects of chronic pulmonary emphysema. Am Heart J 1963;66:741–747. [ Links ]
32. Khaja F, Parker JO. Right and left ventricular performance in chronic obstructive lung disease. Am Heart J 1971;82:319–327. [ Links ]
33. Kitchin AH, Lowther CP, Matthews MB. The effects of exercise and breathing oxygen–enriched air on the pulmonary circulation in emphysema. Clin Sci 1961;21:93–106. [ Links ]
34. Williams JF, Behnke RH. The effect of pulmonary emphysema upon cardiopulmonary hemodynamics at rest and during exercise. Ann Intern Med 1964;60:824–842. [ Links ]
35. Harvey RM, Enson Y. Pulmonary vascular resistance. Adv Intern Med 1969;15:73–93. [ Links ]
36. Campbell RC. Associated normal variables. In: Statistics for biologist. Cambridge University Pres, Great Britain, London. 1967;pp:195–214. [ Links ]
37. Reeves JT, Dempsey JA, Grover RF. Pulmonary circulation during exercise. In: Weir EK, Reeves JT, eds. Pulmonary vascular physiology and pathophysiology. Chapter 4. New York: Marcel Dekker. 1989;pp:107–133. [ Links ]
38. Fishman AP. Pulmonary Circulation. In: Handbook of Physiology. The Respiratory System. Circulation and Non–respiratory Functions. Section 3, Vol.1 Bethesda [MD]; American Physiological Society. 1985;pp:93–166. [ Links ]
39. Sandoval J, Long GR, Skoog C. Independent influence of blood flow rate and mixed venous PO2 on shunt fraction. J Appl Physiol 1983;55:1128–1133. [ Links ]
40. Naeije R. Pulmonary hypertension and right heart failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005;2:20–2. [ Links ]
41. Naeije R, Barbera JA. Pulmonary hypertension associated with COPD. Critical Care 2001;5:286–289. [ Links ]
42. Peinado VI, Barbera JA, Ramírez J. Endothelial dysfunction in pulmonary arteries with mild COPD. Am J Physiol [Lung Cell Mol Physiol] 1998;274:L908–L913. [ Links ]
43. Pietra GG. The pathology of pulmonary hypertension. In Robin LJ, Rich S, Eds. primary pulmonary hypertension. New York: Marcel Dekker. 1997;pp:19–61. [ Links ]
44. Barer GR, Wilkinson M, Langhome CA, Heath D. A pathophysiological study of 10 cases of hypoxic cor pulmonale. Quart J Med 1988;249:65–85. [ Links ]
45. Huez S, Naeije R. Exercise stress tests for detection and evaluation of pulmonary hypertension. Eur Heart J 2007;9:H17–H21. [ Links ]














