Services on Demand
Journal
Article
Indicators
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Archivos de cardiología de México
On-line version ISSN 1665-1731Print version ISSN 1405-9940
Arch. Cardiol. Méx. vol.81 n.3 Ciudad de México Oct./Sep. 2011
Investigación clínica
Design and implementation of the TRACIA: intracoronary autologous transplant of bone marrow–derived stem cells for acute ST elevation myocardial infarction
Diseño e implementación del estudio TRACIA: Trasplante intracoronario Autológo de Células madre para Infarto Agudo del miocardio con elevación de segmento ST
Marco A. Peña–Duque,1,2 Marco A. Martínez–Ríos,1,2 Eva Calderón G,3 Ana M. Mejía,1 Enrique Gómez,3 Carlos Martínez–Sánchez,1 Javier Figueroa,1 Jorge Gaspar,1 Héctor González,1 David Bialoztosky,1 Aloha Meave,1 Jhonathan Uribe–González,1 Erick Alexánderson,1 Victor Ochoa,1 Felipe Masso1
1 Instituto Nacional de Cardiología Ignacio Chávez.
2 The contributions by Marco A. Peña–Duque and Marco A. Martínez–Ríos are equal and the order of authorship is arbitrary.
3 Centro Médico ABC
Corresponding author:
Marco Antonio Peña Duque.
Juan Badiano N°1, Col. Sección XVI,
Tlalpan, 14080 México, D. F.
E–mail: penmar@cardiologia.org.mx.
Received on June 29, 2010;
Accepted on February 18, 2011.
Abstract
Objective: To describe the design of a protocol of intracoronary autologous transplant of bone marrow–derived stem cells for acute ST–elevation myocardial infarction (STEMI) and to report the safety of the procedure in the first patients included.
Methods: The TRACIA study was implemented following predetermined inclusion and exclusion criteria. The protocol includes procedures such as randomization, bone marrow retrieval, stem cells processing, intracoronary infusion of stem cells in the infarct–related artery, pre–and–post MRI, pre–and–post SPECT with radioisotope ventriculography, and clinical follow–up at 6 months.
Results: Eight patients with a diagnosis of acute STEMI and duration of symptoms of <24 hours that were perfused successfully through primary percutaneous coronary intervention (PPCI) with a LVEF of <45% were assigned randomly to two groups (n = 4 each). One group treated with stem cells and the other corresponded to the control group. Neither death, re–infarction, no need for revascularization or thrombosis of the stent were observed at follow–up.
Conclusions: The initial experience at the Instituto Nacional de Cardiología Ignacio Chávez in the treatment of acute STEMI by means of autologous transplantation of bone marrow–derived stem cells is encouraging. Implementation was possible in the first eight patients with no complications.
Keywords: Acute infarct with ST elevation; Stem cells; Hematopoietic cells; Cell therapy; Mexico.
Resumen
Objetivo: Describir el diseño y la implementación de un protocolo de transplante autólogo intracoronario de células madre derivadas de médula ósea en infarto agudo al miocardio con elevación del ST y reportar la seguridad del procedimiento en los primeros pacientes incluidos.
Métodos: El estudio TRACIA se implementó con base en criterios de inclusión y exclusión predeterminados. El protocolo incluye la aleatorización, obtención de médula ósea, procesamiento de células madre, infusión intracoronaria de células madre, RM basal y al seguimiento, SPECT con ventriculografía radioisotópica basal y post–procedimiento, y seguimiento clínico a seis meses.
Resultados: Ocho pacientes con diagnóstico de infarto agudo del miocardio con elevación del ST y duración de síntomas <24 horas que fueron reperfundidos exitosamente con angioplastia primaria y con fracción de expulsión <45%, fueron aleatorizados a dos grupos; uno de ellos fue tratado con células madre y el otro grupo permaneció como control. No se observó muerte, re–infarto, necesidad de revascularización o trombosis del Stent durante el seguimiento.
Conclusiones: La experiencia inicial en el Instituto Nacional de Cardiología Ignacio Chávez en el tratamiento del infarto agudo del miocardio con elevación del ST mediante trasplante autólogo de células madre derivadas de médula ósea, es alentadora. La implementación sin complicaciones fue posible en los primeros ocho pacientes.
Palabras clave: Infarto agudo al miocardio con elevación del ST; Células madre; Células hematopoyéticas; Terapia celular; México.
Introduction
Treatment of acute ST elevation myocardial infarction (STEMI) has changed radically in the last two decades, placing as treatment of choice reperfusion therapy with thrombolysis and, preferably, through primary percutaneous transluminal coronary angioplasty (PTCA). Early restoring of the flow in the artery responsible for the infarct has decreased mortality significantly.1 However, the myocardial necrosis process starts rapidly after coronary occlusion and an irreversible loss of muscle is produced in many patients despite successful reperfusion.2 Myocardial necrosis activates a series of repair changes that include formation of scar tissue and ventricular dilation, and this alteration of the ventricular geometry can continue for weeks or months fostering chronic cardiac failure.3–5 One way of reverting or ameliorating this process would be the regeneration of cardiomyocytes and by stimulating neoangiogenesis within the infarcted tissue. Experimental studies have suggested that the use of bone–marrow–derived adult stem cells can contribute to regenerate the infarcted myocardium and foster neoangiogenesis.6,7 The first clinical experience with acute infarctation and injecting intracoronary hematopoietic stem cells derived from the bone marrow of the same patient was reported by Strauer in 2002.8 Several subsequent studies have demonstrated an improvement in the left ventricular ejection fraction (LVEF) of patients receiving stem cells, such as the BOOST,9 REPAIR–AMI10 studies and that of Fernández–Aviléz.11 However, information reported until now is still not conclusive12 and, therefore, it is justified to continue performing prospective and randomized studies.13,14
Objective
To describe the design and implementation of a protocol of intracoronary autologous transplant of bone marrow–derived stem cells for acute STEMI, and to report the safety of the procedure in the first patients included.
Methods
Design of the study and randomization. This study is a controlled, randomized, single–blind, two–arms clinical trial performed at the Hemodynamics Department of the National Institute of Cardiology (Instituto Nacional de Cardiología Ignacio Chávez), in collaboration with the National Center for Blood Transfusion. The study was performed following the principles of good practices of the Helsinki Declaration and the Health Regulations of Mexico, and with the approval of the institutional Research and Ethics Committees. Each patient signed the corresponding informed consent form.
The design of the study is depicted in Figure 1. After the primary PTCA, and between days 3 and 5, patients were assigned randomly at a 1:1 proportion to the stem cells group or the control group. For this, closed envelopes with consecutive numbers were used. In the stem cells group, bone marrow was obtained, performing an intracoronary injection of the tissue, as well as a ventriculography between days 6 and 7. In both groups, a nuclear magnetic resonance imaging (MRI) study, a two–dimensional echocardiogram and simple photon emission computed tomography (SPECT) with isotope ventriculography were performed between days 5 and 7. Clinical follow–up was performed on the first, third, and sixth months in both groups, and cardiac catheterism with ventriculography, SPECT, and MRI were performed.

Inclusion criteria. The study included adult patients of either gender, between 20 and 75 years of age, with a diagnosis of acute STEMI and a duration of symptoms of < 24 hours, who had been perfused successfully with primary PTCA (with or without previous administration of thrombolytics) and with an implanted stent, and who, after the reperfusion, had an LVEF of < 45% measured by post–catheterism ventriculography and/or echocardiogram.
Exclusion criteria. Excluded from the study were patients with previous Q–wave infarction, cardiogenic shock, suspicion or evidence of mechanical complications due to the infarct, a history of sustained ventricular tachycardia or ventricular fibrillation after the first 24 hours of the infarct, treatment during the 4 previous weeks with any drug under research, current use of antineoplastic and/ or immunosuppressor drugs, history of cancer in the last 5 years, women in fertile age unless they had a negative pregnancy test, previous malignant hemopathy, known renal failure with creatinine >2.6 mg/dL, vascular cerebral accident of any type in the last year, and any disease that might affect the survival of the patient during the time of the study.
Technique to obtain the bone marrow. Extraction of stem cells was performed in the hemodynamics study room, with the patient in ventral decubitus, under conscious sedation and analgesia. Along the whole procedure, the patient was kept under surveillance by means of non–invasive arterial pressure monitoring, electrocardiogram, and O2 saturation measurements. Extraction was achieved by repeated punctures in the posterior iliac crest with a puncturing needle connected to a 20 mL syringe, after skin asepsis with an iodine–base solution (Isodine®). In each puncture, an average of 5 to 10 mL was aspirated. After each puncture–aspiration, the needle was washed with saline solution and heparin, repeating the process until 80 to 100 mL of bone marrow was obtained. The sample was placed in a special 100 mL bag for bone–marrow collection with a double fltration system, and containing 10 mL of saline solution and 1 mL of 1% non–fractionated heparin. The bag was transported in fresh to the National Center of Blood Transfusion.
Processing and collecting of stem cells or hematopoietic progenitor cells (HPC). The obtained sample was processed immediately at its arrival to the cell therapy area following the protocol to separate bone marrow–derived mononuclear cells, using a Ficoll® density gradient with the CS–900 kit in the automated Sepax® S–100 system (Biosafe America Inc, USA). The initial documents included request for the processing of hematopoietic progenitor cells (HPC), clinical summary of the patients, demographic data, and informed consent. The total volume of the obtained bone marrow was calculated, and captured in the TESI® Hemodata database, to be assigned a specifc bar code; and samples were obtained for the initial blood count. During collection of mononuclear cells with the Sepax® equipment, 100 mL of pyrogen–free sterile Ficoll® and a washing buffer solution (2.5% albumin) were used. The process lasted approximately 1 hour and 15 minutes. Once the process ended, a bag with a fxed volume of 50 mL of mononuclear cells (MNC) was obtained. From this bag, samples were taken to perform a final blood count, clonogenic cultures (STEMCELL Technologies, USA), to determine CD34+, CD35+, and cell viability through flow cytometry (FACScalibur, Bekton Dickinson, USA). From the residual washing bad, 20 ml were taken to perform aerobic and anaerobic microbiological cultures (BacTALERT, Biomeriux, USA). Cells recovered with the automated process corresponded in average to: MNC, 56%; CD34+, 88%; showing viability, 98%; granulocytes depletion, 88%; erythrocytes depletion, 99.7%; and platelet depletion, 66%. The final dose was adjusted from 1.0 to 2.0 × 106 CD34+ in 20 ml of buffer. HPC were transported in fresh for their immediate infusion.
Intracoronary infusion of stem cells or HPC. This procedure was performed between the 6th and 7th day after the primary PTCA according to conventional an–gioplasty techniques. That is, the femoral artery was punctured with a 6 Fr system and the artery responsible for the infarct was cannulated using the corresponding guiding catheter. Before performing the basal shot with contrast medium, 200 µg of nitroglycerine were administered. Once the responsible artery had been identified, a hydrophilic 0.014" angioplasty guide was advanced from 300 cm until the distal portion of the vessel and at that moment a dose of non–fractionated heparin was administered according to the weight of the patient. Afterwards, an over–the–wire balloon catheter was introduced until the site of the implanted stent (relation balloon–stent, 1:1), insufflating the balloon at 2 or 3 atmospheres (atm). At this moment, the angioplasty guide was removed, infusion of stem cells was started at a rate of 1 mL per min during 2 minutes, and then the balloon was deflated for 1 min. This procedure was repeated until completing the total dose of stem cells (approx. 10 mL to 13 mL).
Magnetic resonance protocol. The used scanner was a 1.5–Tessla Sonata optimized for the heart. The study is initiated with localizing images, followed by orthogonal sections in the three planes in the HASTE (half–acquisition turbo spin echo) sequence as reference for the acquisition of T2–weighted high resolution images, obtaining 16 images of a single pulse of activity by means of the semi–Fourier reconstruction. The functional morphological analysis included 4– and 5–chamber images in gradient plane echo movie, showing the outflow tract of the left and right ventricles. In each projection, at least one cardiac cycle is captured (R–R interval of the electrocardiogram) with a fast Turbo–FLASH sequence. To evaluate myocardial viability, intravenous gadolinium was administered, acquiring immediate images in two cameras and short axis, named "first pass". Fifteen to twenty minutes thereafter, images were acquired in four cameras, short axis, and two cameras in an inversion–recovery sequence in search of delayed reinforcement.
SPECT protocol. A Siemens Orbiter 2000 gamma camera provided with an ICON A/P processor and the quantitative Gated–SPECT software Cedars–Sinai was used. We used the one–day technetium–99m sestamibi (99mTc) protocol. The SPECT images were obtained 45 min to 60 min after the administration of 10 to 15 mCi at rest and 20 to 30 mCi after effort. To perform the radioisotope ventriculography in equilibrium, we used the in vitro labeling of erythrocytes technique with 99mTc (ultra Tag–99Tc Mallinckrodt) at a 30 mCi dose, in a General Electric ELGEMS–Millenium MPS gamma camera.
Statistical analysis. Continuous variables are presented as means and standard deviation (SD), and categorical variables are expressed as frequencies and percentages.
To determine the difference in the LVEF increase in both groups, we used the non–parametric Wilcoxon signed–rank test. Statistical significance was set at p <0.05. The statistics software SPSS, version 13.0, was used for the analyses.
Results
We report the first eight patients included in the protocol, four of them from the stem cells group and four from the control group. The average age of patients was 53.2 years and 87% were men; 50% of patients had antecedents of systemic arterial hypertension and active smoking, 37% had pre–infarct angina, 25% dyslipidemia, and 12.5% diabetes mellitus. Regarding the hemodynamic state, 37.8% were in the Killip–Kimball (KK) 1 classification; 50% in KK 2, and 12.5% in KK 3 (Table 1).
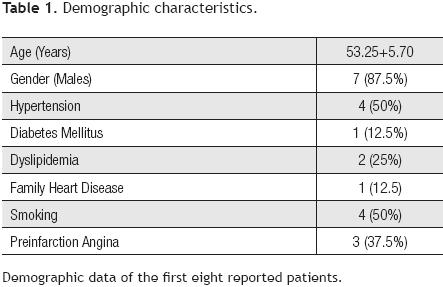
In regard to infarct location, all patients had an anterior wall infarct, with an ischemia time of 430.85 ± 291.55 minutes and a door–to–balloon time of 96 ± 39.5 minutes. All patients were subjected successfully to a primary PTCA and had a bare metal stent implanted (Table 2).

No complications, such as bleeding or hematomas, arouse during bone marrow extraction. Likewise, during intracoronary infusion of stem cells, no complications like diminution of coronary flow, coronary spasm or periprocedural myocardial infarction, as measured by cardiac enzymes, occurred. The dose was adjusted from 1.0 to 2.0 x 106 CD 34+ in 20 mL. The Eclone for all patients was >10%, cell viability was >98%, and microbiological cultures were all negative. The time elapsed between bone marrow collection and intracoronary infusion of hematopoietic cells was of 6 hours at maximum.
Angiographic control at 6 months did not reveal restenosis in any patient. No deaths, fatal re–infarcts, or stent thrombosis occurred, and there was no need for a new revascularization during follow–up.
Discussion
The TRACIA (for its initials in Spanish; autologous transplant of bone marrow–derived stem cells in the acute myocardial infarct with ST segment elevation) study represents the first clinical institutional experience in the setting of acute STEMI. In this randomized, two–institutional study, we report the first eight cases with an angiographic and clinical follow–up by means of SPECT and MRI at 6 months, and illustrate, essentially, the feasibility and safety of performing a stem cells study at our institution.
Regarding the ideal time for the intracoronary injection of stem cells, we chose days 5 and 7, as there is evidence that during the first 5 days of the acute STEMI the infammatory process is more intense and stem cells could easily be destroyed. It has been recommended that the ideal time for the transplant is between days 5 and 14 after presentation of the infarct.15–17 Regarding safety of the procedure, there were no complications during either bone marrow extraction or the intracoronary injection of stem cells, as has been reported previously.
The mechanism of action of stem cells in the treatment of infarcts is still uncertain and can be multi–factorial. Most published data derive from experimental studies, which have suggested that the beneficial effect is exerted through the activation of the stem cells residing in the myocardium. This activation is accomplished through paracrine mechanisms. In fact, it has been demonstrated in mice that bone marrow–derived stem cells transplanted into an infarcted myocardium, after a certain time, loose their hematopoietic characteristics and acquire cardiogenic and endothelial lineages to form functional cardiomyocytes and vascular structures.18
Although the evidence published until now suggests that transplantation of bone marrow–derived stem cells can be clinically relevant, studies with large number of patients are required, as well as new experimental models able to elucidate the biology of these cells and their action mechanism in the infarcted myocardium.
Conclusions
The initial experience at the Instituto Nacional de Cardiología Ignacio Chávez in the treatment of acute STEMI by means of autologous transplantation of bone marrow–derived stem cells is encouraging. Implementation was possible in the first eight patients with no complications.
Acknowledgments
The authors are indebted to: Dr. Antonio Marín López , for his support to this Study. Dr. Marín López was the General Director of the National Center for Blood Transfusion up to 2008.
References
1. Lange RA, Hillis LD. Reperfusion therapy in acute myocardial infarction. N Engl J Med 2002;346:954–955. [ Links ]
2. Keely EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361:13–20. [ Links ]
3. Giugliano R P, Braunwald E. Selecting the best reperfusion strategy in ST–elevation myocardial infarction: it's all a matter of time. Circulation 2003;108:2828–2830. [ Links ]
4. St. John Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction. Pathophysiology and therapy. Circulation 2000;101:2981–2988. [ Links ]
5. Pfeffer MA, McMurray JJV, Velázquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003;349:1893–1906. [ Links ]
6. Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001;410:701–705. [ Links ]
7. Kocher AA, Schuester MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone–marrow–derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001;7:430–436. [ Links ]
8. Strauer BE, Brehm M, Zeus T, et al, Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 2002;106:1913–1918. [ Links ]
9. Wollert KC, Meyer G P, Lotz J, et al. Intracoronary autologous bone–marrow cell transfer after myocardial infarction: the BOOST randomized controlled clinical trial. Lancet 2004;364:141–148. [ Links ]
10. Fernández–Avilés F, San Román JA, García–Frade J, et al. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res 2004;95:742–748. [ Links ]
11. Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow–derived progenitor cells in acute myocardial infarction. N Engl J Med 2006;355:1210–1221. [ Links ]
12. Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med 2006;355:1199–1209. [ Links ]
13. Abdel–Latif A, Bolli R, Tleyjch IM, et al. Adult bone marrow–derived cells for cardiac repair. A systematic review and meta–analysis. Arch Intern Med 2007;167:989–997. [ Links ]
14. Yousef M, Schannwell CM, Köstering M, et al. The BALANCE study. Clinical benefit and long–term outcome after intracoronary auto–logous bone marrow cell transplantation in patients with acute myocardial infarction. J Am Coll Cardiol 2009;53:2262–2269. [ Links ]
15. Sheiban I, Fragasso G, Rosano GM, et al. Time course and determinants of left ventricular function recovery after primary angioplasty in patients with acute myocardial infarction. J Am Coll Cardiol 2001;38:464–471. [ Links ]
16. Frangogiannis NG, Smith C V, Entman ML. The infammatory response in myocardial infarction. Cardiovasc Res 2002;38:464–471. [ Links ]
17. Li RK, Mickle DA, Weisel RD, et al. Optimal time for cardiomyocyte transplantation to maximize myocardial function after left ventricular injury. Ann Thorac Surg 2001;72:1957–1963. [ Links ]
18. Martin–Rendon E, Brunskill SJ, Hyde CJ, et al. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J 2008;29:1807–1818. [ Links ]














