Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos de cardiología de México
versión On-line ISSN 1665-1731versión impresa ISSN 1405-9940
Arch. Cardiol. Méx. vol.81 no.2 Ciudad de México abr./jun. 2011
Artículos de revisión
Function and mechanics of the left ventricle: from tissue Doppler imaging to three dimensional speckle tracking
Función y mecánica del ventrículo izquierdo: de la imagen con Doppler tisular al reconocimiento de patrones acústicos con imágenes tridimensionales
José Antonio Arias–Godínez,1 José Fernando Guadalajara–Boo,2 Ayan R. Patel,3 Natesa G. Pandian4
1 Departamento de Ecocardiografía. Instituto Nacional de Cardiología Ignacio Chávez. México, D.F.
2 Dirección de Enseñanza. Instituto Nacional de Cardiología Ignacio Chávez. México, D.F.
3 Cardiovascular Imaging and Hemodynamic Laboratory. Tufts Medical Center, Boston, MA, EUA.
4 Cardiovascular Imaging and Hemodynamic Laboratory. Tufts Medical Center, Boston, MA, EUA.
Corresponding author:
José Antonio Arias Godínez.
Instituto Nacional de Cardiología Ignacio Chávez.
Juan Badiano Núm. 1, Col. Sección XVI,
Delegación Tlalpan, 14080 México, D.F. México.
E– mail: antonioarias1407@gmail.com
Received on May 13, 2010;
Accepted on February 13, 2011.
Abstract
One of the most common indications in echocardiography is the evaluation of left ventricular function. The traditional measurement of ejection fraction is based upon tracing the left ventricular borders and calculating left ventricular volumes using geometric assumptions. Now, with the introduction of three–dimensional echocardiography, the evaluation of left ventricular function is easier to carry out and with superior accuracy and reproducibility. However, regional myocardial function is more difficult to evaluate because it relies on visual assessment of endocardial motion and wall thickening. Currently, new techniques like tissue Doppler and speckle tracking imaging allow regional and global quantification of myocardial function through new parameters, like deformation/strain, rotation and twist. In this regard, speckletracking echocardiography (STE) has been introduced as a technique for angle–independent quantification of multidirectional myocardial strain and rotation. With the arrival of three–dimensional systems, the entire left ventricle can be evaluated with this technique, lacking the inherent weakness of two–dimensional and tissue Doppler methods. Three dimensional speckle tracking (3DST) has potential to be an ideal tool to assess not only global myocardial function but regional function through deformation, rotation, twist and untwisting parameters.
Keywords: Strain; Rotation; Twist; Speckle tracking; Two dimensional; Three–dimensional; Mexico.
Resumen
La evaluación de la función ventricular es una indicación común en ecocardiografía. La medición de la fracción de expulsión como medida de función ventricular se basa en el trazado del endocardio ventricular y el cálculo de volúmenes ventriculares a través de suposiciones geométricas. Con el advenimiento de la tecnología tridimensional en ecocardiografía, la valoración de la función ventricular se puede realizar de manera más rápida, sencilla, precisa y reproducible. Sin embargo, la estimación de la función regional es una tarea difícil de realizar ya que ésta se basa en la apreciación visual del engrasamiento y movimiento del endocardio ventricular. Actualmente nuevas técnicas como el Doppler tisular y el reconocimiento de patrones acústicos (speckle tracking) han permitido cuantificar la función ventricular regional y global a través del uso de parámetros de deformación, rotación y torsión. El reconocimiento de patrones por ecocardiografía es una técnica que no depende del ángulo de insonación del haz de ultrasonido y que permite la cuantificación multidireccional de la deformación y la rotación del miocardio. Hoy en día, la aplicación de esta técnica a los sistemas de ecocardiografía tridimensional ha permitido una mejor estimación de la función ventricular sin los inconvenientes de la ecocardiografía Doppler y bidimensional. El seguimiento de patrones por ecocardiografía tridimensional tiene el potencial de ser una herramienta ideal para la cuantificación de la función ventricular global y regional a través de parámetros de deformación, rotación y torsión.
Palabras clave: Deformación; Rotación; Torsión; Speckle tracking, Bidimensional; Tridimensional; México.
Introduction
One of the most common indications in echocardiography is the evaluation of left ventricular function. It has traditionally been evaluated as left ventricular ejection fraction and left ventricular end–systolic volume, both remaining as the simplest and most widely used parameters for global assessment of left ventricular function.1
Traditionally, two–dimensional echocardiography has been routinely used in clinical practice to measure left ventricle (LV) dimensions, wall thickness, and function, the latter focused in the measurement of ejection fraction which is based upon tracing the left ventricular borders and calculating left ventricular volumes using geometric assumptions.2 The use of these assumptions may introduce considerable miscalculations, particularly for patients with odd–shaped ventricles and wall–motion abnormalities. Besides, the inadvertent use of foreshortened views can raise its inaccuracy and low reproducibility. Alternatively, LV function can be assessed by Doppler techniques through the measurements of stroke volume using the continuity equation. However, calculations of stroke volume by Doppler are dependent on the accuracy of left ventricle outflow tract measurement (LVOT); errors in the measurement, which are squared in the calculation of the LVOT area, limit the reliability of this parameter.3,4
In search of a method to quantify LV volumes and left ventricle ejection fraction, real–time three–dimensional echocardiography is an important step forward in cardiac ultrasound. Multiple studies have demonstrated the superior accuracy and reproducibility of real–time three–dimensional echocardiography over two–dimensional echocardiography for assessment of cardiac function.5,6
Never the less, segmental wall motion has been more difficult to evaluate. Regional myocardial function by echocardiography is usually evaluated by visual assessment of endocardial motion and wall thickening using two–dimensional echocardiography. This approach, however, suffers from being subjective providing only semi– quantitative data and is limited by huge intra– and inter–observer variability. Visual assessment has limited ability to detect more subtle changes in function and in timing of myocardial motion throughout systole and diastole. Recently, echocardiographic modalities for objective quantification of global and regional function have been developed such as tissue Doppler and speckle tracking imaging.
In this article, we review in a concise manner the methodology behind the development, usefulness, and shortcomings of these echocardiographic techniques.
Deformation – strain
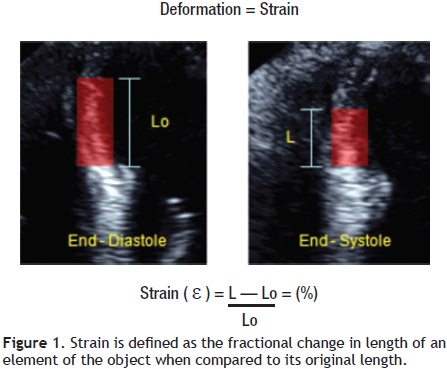
In cardiac muscle physiology, strain is a measurement of deformation representing shortening or stretching of the tissue or myocardial fibers. The Greek letter epsilon (ε) is commonly used as a symbol for strain. The strain value is dimensionless and can be represented as a fractional number or as a percentage change from an object's original dimension (Figure 1). As the ventricle contracts, muscle shortens in the circumferential and longitudinal dimensions (a negative strain) and thickens in the radial and transversal direction (a positive strain). As a result, for the left ventricle, three normal strain values (longitudinal, circumferential, radial/transversal) are used to describe LV deformation in three dimensions (Figure 2).
Theoretically, strain values are not affected by the uniform translational motion of the heart and, as a consequence, they offer a clear advantage over velocity and displacement to assess the local functionality of the myocardium.7,8 The superiority of deformation parameters for assessing cardiac function compared to motion/ velocity/displacement parameters is related to the basic strain–algorithm, which subtracts the motion due to the contraction of neighboring segments (tethering effects and translational motion). Completely passive segments can show motion relative to the transducer due to tethering, but without any deformation, making velocity and displacement information completely unreliable for the characterization of such regions. Strain parameters, on the other hand, are referred to as motion–deformation between two points in the myocardial wall, which is unrelated to the motion towards the transducer, and this fact discriminates the actual passive movement from true contraction in any myocardial region (Figure 3).9
Strain rate (SR) is the rate at which deformation occurs, i.e., change of strain per unit of time. By definition it is the temporal derivative of strain and expresses the rate of shortening or lengthening of a part of the heart. As strain rate describes the velocity of deformation, its unit of measurement is 1/s (Figure 4).

Currently, the principal strain vectors and their velocity derivatives (Strain Rate) can be assessed by tissue Doppler and speckle tracking echocardiography.
Tissue Doppler imaging
Rather than assessing the rapid velocity blood pool as with conventional Doppler, uses filters to remove these signals to concentrate solely on the lower velocity myocardial motion. Velocities in the myocardial wall are much lower than that of the blood pool and are typically less than 15 cm/s. Tissue Doppler imaging allows a quantitative analysis of the motion pattern of the cardiac walls. Some published studies have suggested that tissue velocity, strain, and strain rate by tissue Doppler are more accurate methods for evaluation of global and regional function10–12 when compared with conventional echocardiographic methods (Figure 5). Several groups of investigators have demonstrated that strain analysis of a broader area is probably superior to tissue velocity data at one site and wall motion score for tracking local systolic function.13–17
Clinical applications: Tissue Doppler Imaging (TDI) has been evaluated in several experimental and clinical studies. One of the most important clinical applications of TDI is the quantification of global LV systolic function; this can be done by measuring the myocardial peak systolic velocities of the mitral valve annulus at several locations and to derive an average of them. Gulati et al, reported an excellent correlation between systolic mitral annulus velocity Sm averaged from the apical views and LV ejection fraction.18,19 Others have shown a good correlation between Sm and peak positive dP/dt in patients with dilated cardiomyopathy and hypertensive heart disease.20 In addition, this parameter was found to be the strongest independent echocardiographic predictor of prognosis in patients with chronic heart failure.21 Moreover, TDI permits quantification of individual LV segments by measuring the velocities, strain and strain rate of various LV segments.
In the assessment of LV diastolic function, TDI provides valuable information. In an interesting study, Sohn et al, were able to differentiate subjects with pseudonormal filling from normal by an E' velocity < 8.5 cm/s and an E'/A' ratio < 1, with a sensitivity of 88% and a specificity of 67%.22 Furthermore, this technique can be used in the non–invasive evaluation of LV filling pressure, which is assessed through the ratio of transmitral early peak flow velocity (E) over early diastolic mitral annulus velocity (E') or E/E'. Nagueh et al, demonstrated that the E/E' ratio correlated well with pulmonary capillary wedge pressure measured invasively. An E/E' ratio > 10 detected a mean pulmonary capillary wedge pressure > 15 mmHg with a sensitivity of 97% and a specificity of 78%.23 Ommen et al proposed a higher cut–off value of 15 for E/E', which is now commonly used.24 The different cut–off values can be explained by the fact that Nagueh et al, used the lateral mitral valve annulus whereas Ommen et al used the medial mitral valve annulus.
Other applications of TDI in clinical practice comprise the differentiation between restrictive cardiomyopathy and constrictive pericarditis,25,26 the early identification of cardiomyopathies,27–30 detection of ischemia and evaluation of viability,31–35 and the study of patients with dyssynchrony.36–37
Limitations of tissue doppler imaging: The angle dependency is a serious limitation of all Doppler–based techniques, including Doppler–derived myocardial velocities and strain.12 Tissue Doppler imaging is only able to estimate strain along the ultrasound beam and thus cannot reliably measure strain in the azimuth or perpendicular plane. This limits the use of TDI–derived strain measurements primarily to longitudinal fibers, with the inability to quantify deformation in the radial plane. Therefore, it is essential that the ultrasound beam is aligned parallel to the left ventricle wall in long–axis imaging to obtain longitudinal segmental measurements, and perpendicular to the wall for radial measurements in the short axis. This implies that strain measurements from myocardial segments close to the left ventricle apical curvature cannot be reliably assessed by tissue Doppler imaging.13
Two–dimensional speckle tracking echocardiography
Speckle–tracking echocardiography (STE) has been introduced as a technique for angle–independent quantification of multidirectional myocardial strain. Speckle tracking is an application of pattern–matching technology to ultrasound cine data. Speckles are natural acoustic markers that occur as small and bright elements in conventional gray scale ultrasound images. The speckles are backscattered from structures smaller than a wavelength of ultrasound. These speckles are distributed all through the myocardium on the ultrasound image.1,38
In the speckle tracking methodology, a small area of the myocardium with its unique speckle pattern can be defined (it is called "kernel") and tracked, following a search algorithm based on optical flow method, trying to recognize the most similar speckle pattern from one frame to another. The algorithm searches for an area with the smallest difference in the total sum of pixel values, which is the smallest sum of absolute differences39 (Figure 6).
For accurate speckle tracking, a high frame rate is important. Speckle patterns change over the course of the cardiac cycle because of deformation of the heart and out–of–plane motion. A high frame rate decreases the speckle change between frames, allowing better tracking.40–41 Currently there are different programs available that have the ability to assess myocardial strain, strain rate, velocities and displacement from these speckles. Measurements can be done simultaneously from multiple regions of interest within an image plane from conventional gray–scale B–mode recordings. The distance between selected speckles is measured within a predefined myocardial area as a function of time, and parameters of myocardial deformation can be calculated. This is in contrast to Doppler–based measurements where the sample volume is a fixed area in space and all measurements are done with reference to an external point (the transducer). Strain measurements from the speckle–tracking technique are therefore direct measures of myocardial deformation while tissue Doppler imaging calculates strain by integrating strain rate. Another important advantage by using the new technique is independence from insonation angle and cardiac translation.42–44
In the short–axis view, radial strain and circumferential strain can be calculated. These values are not independent, one is positive (wall thickening) when the other is negative (segment shortening) in a normal heart. In the apical four–, three–, and two–chamber views, transversal strain and longitudinal strain can be calculated45 (Figure 7).
For these reasons, speckle tracking echocardiography provides a direct measure of myocardial deformation and appears to be a more robust method than Doppler–based strain imaging, which estimates strain as the time integral of spatial velocity gradients (Table 1).
Clinical applications: The potential of 2D speckle tracking echocardiography has been investigated in numerous experimental and clinical studies for exploration of systolic and diastolic ventricular function, assessing ischemia, dyssynchrony, and other cardiac conditions,46 some of which will be cited representatively in this review. In initial studies, Becker et al, used 2D speckle tracking imaging to assess regional LV function. They compared a healthy group with a group of patients with previous myocardial infarction. They assessed radial and circumferential strains, and tried to distinguish between normokinesis, hypokinesis, and akinesis based on the 16–segment American Heart Association model. All subjects underwent cardiac magnetic resonance imaging (CMRI). They found excellent correlation between the values obtained for peak radial and circumferential strains and the visual assessment of the MRI images. The sensitivity and specificity of this technique in the detection of dyssynergy were 83.5%. Furthermore, they demonstrated that this technique had good inter– and intra–observer variability when both measurements were performed using the same cardiac cycle.47 Even in acute clinical settings, such as myocardial infarction, this technique seemed to be of value in detection of transmurality and infarct size, systolic recovery after reperfusion, and diastolic dysfunction.48–49 Other groups centered their attention on ventricular torsion mechanics and compared it with tagged magnetic resonance imaging (MRI). The results of these studies demonstrated concordance in the estimation of LV torsion between speckle tracking and tagged MRI and made the assessment of LV rotation and torsion available in clinical cardiology.50,51 Furthermore, some groups have investigated its utility in evaluating diastolic dysfunction in different clinical settings.52,53 Additionally, two–dimensional speckle tracking enables the assessment of LV dyssynchrony and is a valuable tool to identify potential responders to cardiac resynchronization therapy.54
Torsion–twist: Left ventricular torsion is a critical component of cardiac biomechanics because it is important for normal ejection and suction and is a feature of the normal spread of excitation and connections among fibers. The human heart has a specific helical arrangement of the myofibers with a right–hand orientation from the base toward the apex in the endocardial layers and a left–hand orientation in the epicardial layers.55 In systole, the LV apex rotates counterclockwise (as viewed from the apex), whereas the base rotates clockwise, creating a torsional deformation originating in the dynamic interaction of oppositely wound epicardial and endocardial myocardial fiber helices. The difference between apex and base in the rotation angle is called twist and contributes significantly to LV ejection, in addition to myocardial shortening and thickening. It also has an important role in diastole since it contributes to diastolic suction in the early phases of ventricular filling in a process called untwist. Left ventricular torsion can be quantified by speckle tracking echocardiography. By measuring apical and basal rotation from LV short–axis recordings, twist has been explored in both clinical and experimental studies56,57 (Figure 8).
Three–dimensional speckle tracking echocardiography
The interpretation of echocardiographic images requires a complex mental integration of different planes to understand anatomic structures and physiologic functions. Two–dimensional speckle tracking echocardiography and tissue Doppler imaging are limited to two–dimensional analysis (Figure 9).
The recent development of ultrasound systems with the capability to acquire real–time full volume data of the whole left ventricle makes it possible to perform speckle tracking in three dimensions, and thereby track the real motion of the myocardium. Instead of using two–dimensional templates to view two–dimension movement, cubic templates allow motion analysis of the entire ventricle in three–dimension.58,59 As a consequence, three–dimensional speckle tracking is emerging as a new instrument that can be used for regional wall motion analysis of the entire left ventricle, allowing acquisition and evaluation of real three–dimensional indices and wall motion accurately.60
Three–Dimensional speckle tracking takes into account the motion of the cardiac wall not only in a concrete plane (radial, longitudinal, circumferential, and transversal) but also in three dimensions. The rationale for this is that from only one apical position, the entire ventricle may be analyzed; a 90 x 90° triggered full volume is obtained and the echocardiographer does not need to change to different positions to obtain different planes61 (Figure 10). That is why the three–dimensional format more closely represents reality and better accuracy than the two–dimensional format.62
Clinical application: Three dimensional speckle tracking is a recent modality and its clinical application is currently being evaluated. In an early work with 3D speckle tracking echocardiography, Pérez de Isla et al, studied 30 patients with different pathology (namely, coronary artery disease, diabetes mellitus, hypertension, and hyperlipidemia), and they found that this technique was able to quickly and accurately evaluate cardiac deformation in its three main vectors when it was compared with 2D speckle tracking imaging.61 Saito and coworkers performed further research measuring radial, longitudinal, and circumferential strain in a normal population and comparing it with 2D speckle tracking. They concluded that 3D speckle tracking "is a simple, feasible, and reproducible method to measure strain". They also reported differences in strain measurements and time to perform a complete 3DSTE analysis compared with 2D speckle tracking results.60 Maffessanti et al recently published work exploring the utility of this technique in normal populations and patients with cardiac disease (dilated myocardiopathy, coronary artery disease, myocardiopathy secondary to myocarditis and valvular disease), finding superiority of this technique with respect to 2D STE in regards to quantification of global and regional function and detection of regional abnormalities.63 Three–dimensional speckle tracking echocardiography is a promising technique that has the advantage of measuring real myocardial function compared with conventional 2D speckle tracking echocardiography. Currently 3D STE is being applied in the clinical field by several groups around the world.64,65 Examples of the current clinical applications of 3D speckle tracking are demonstrated in Figure 11.
Current limitations of speckle tracking echocardiography
The dependence of speckle tracking echocardiography on frame–by–frame tracking of the myocardial pattern makes it dependent on image factors including reverberation artifacts and attenuation; technical proficiency remains important in image processing: (1) misplacing points at the time of tracing endocardial borders might result in apparent wall motion abnormalities and misleading outcomes or result in poor tracking, (2) excessive region of interest width (e.g., including the pericardium) might have an adverse influence on tracking quality, and (3) insufficient region of interest width might increase the variability of strain by compromising the reproducibility of these measurements. In three–dimensional speckle tracking, the number of beams needed in full–volume acquisition of the left ventricle limits its frame rate (commercial systems are currently able to assess full volume images of the left ventricle at a rate of about 20 to 30 frames per second)46,66 resulting in low temporal and spatial resolution (Table 1 Advantages and Disadvantages of Speckle Tracking). Further improvement of spatial and temporal resolution of 3D STE is likely to overcome these drawbacks. As speckle tracking echocardiography evolves and becomes familiar, it will be mandatory to ensure standardization of terminology, steps in data acquisition, and optimal training to increase data accuracy and reproducibility.
Conclusion
A developing body of evidence suggests that assessment of LV mechanics by tissue Doppler imaging and speckle tracking echocardiography offers valuable information in several clinical scenarios. Understanding such events could provide novel insight into the mechanisms of LV dysfunction, and may have the potential to identify subtle changes in LV mechanics in patients with subclinical myocardial dysfunction. With the advent of 3D echocardiography, three dimensional speckle tracking is emerging as a new tool that combines the usefulness of myocardial motion tracking with a better integration of the anatomic structures and physiologic function of the heart. The evidence for the utility of this tool is growing and offers valuable advantages over TDI and two dimensional speckle tracking echocardiography, holding the promise for a better understanding of the mechanisms of LV dysfunction and tracking the impact of current and future therapies.
References
1. Edvardsen T, Helle–Valle T, Smiseth O. Systolic Dysfunction in Heart Failure with Normal Ejection Fraction: Speckle–Tracking Echocardiography. Prog Cardiov Diseases 2006;49 207–214. [ Links ]
2. Lang R, Bierig M, Devereux R, et al. Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–1463. [ Links ]
3. Quinones M, Otto C, Stoddard M, et al. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr 2002;15:167–184. [ Links ]
4. Pemberton J, Li X, Kenny A, et al. Real–time 3–dimensional Doppler echocardiography for the assessment of stroke volume: an in vivo human study compared with standard 2–dimensional echocardiography. J Am Soc Echocardiogr 2005;18:1030–1036. [ Links ]
5. Jacobs L, Salgo I, Goonewardena S, Weinert, et al. Rapid online quantification of left ventricular volume from real–time three–dimensional echocardiographic data. Eur Heart J 2006;27:460–468. [ Links ]
6. Jenkins C, Bricknell K, Hanekom L, et al. Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real–time three–dimensional echocardiography. J Am Coll Cardiol 2004;44:878–886. [ Links ]
7. Kawagishi T. Speckle Tracking for Assessment of Cardiac Motion and Dyssynchrony. Echocardiography 2008;10:1167–1171. [ Links ]
8. Marwick Th. Measurement of Strain and Strain Rate by Echocardiography Ready for Prime Time? J Am Coll Cardiol 2006;47:1313–1327. [ Links ]
9. Pavlopoulos H, Nihoyannopoulos P. Strain and strain rate deformation parameters: from tissue Doppler to 2D speckle tracking. Int J Cardiovasc Imaging 2008; 24:479–491. [ Links ]
10. Edvardsen T, Gerber B, Garot J, et al. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans: Validation against three–dimensional tagged magnetic resonance imaging. Circulation 2002; 106:50–56. [ Links ]
11. Heimdal A, Stoylen A, Torp H, et al. Real–time strain rate imaging of the left ventricle by ultrasound. J Am Soc Echocardiogr 1998;11:1013–1019. [ Links ]
12. Urheim S, Edvardsen T, Torp H, et al. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation 2000;102:1158–1164. [ Links ]
13. Edvardsen T, Skulstad H, Aakhus S, et al. Regional myocardial systolic function during acute myocardial ischemia assessed by strain Doppler echocardiography. J Am Coll Cardiol 2001 37:726730. [ Links ]
14. Stoylen A, Heimdal A, Bjornstad K, et al. Strain rate imaging by ultrasonography in the diagnosis of coronary artery disease. J Am Soc Echocardiogr 2000; 13:1053–1064. [ Links ]
15. Voigt JU, Exner B, Schmiedehausen K, et al. Strain rate imaging during dobutamine stress echocardiography provides objective evidence of inducible ischemia. Circulation 2003;107:2120–2126. [ Links ]
16. Armstrong G, Pasquet A, Fukamachi K, et al. Use of peak systolic strain as an index of regional left ventricular function: comparison with tissue Doppler velocity during dobutamine stress and myocardial ischemia. J Am Soc Echocardiogr 2000;13:731–737. [ Links ]
17. Greenberg N, Firstenberg M, Castro P, et al. Doppler–derived myocardial systolic strain rate is a strong index of left ventricular contractility. Circulation 2002;105:99–105. [ Links ]
18. Galiuto L, Ignone G, DeMaria A. Contraction and relaxation velocities of the normal left ventricle using pulsed–wave tissue Doppler echocardiography. Am J Cardiol 1998;81:609–614. [ Links ]
19. Gulati VK, Katz WE, Follansbee WP, et al. Mitral annular descent velocity by tissue Doppler echocardiography as an index of global left ventricular function. J Am Soc Echocardiogr 1998;11:105– 111. [ Links ]
20. Yamada H, Oki T, Tabata T, et al. Assessment of left ventricular systolic wall motion velocity with pulsed tissue Doppler imaging: comparison with peak dP/dt of the left ventricular pressure curve. J Am Soc Echocardiogr 1998;11:442–449. [ Links ]
21. Nikitin N, Loh P, Silva R, et al. Prognostic value of systolic mitral annular velocity measured with Doppler tissue imaging in patients with chronic heart failure caused by left ventricular systolic dysfunction. Heart 2006;92:775–779. [ Links ]
22. Sohn D, Chai I, Lee D, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 1997;30:474–480. [ Links ]
23. Nagueh S, Middleton K, Kopelen H, et al. Doppler tissue imaging: a non–invasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 1997;30:1527–1533. [ Links ]
24. Ommen S, Nishimura R, Appleton C, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler–catheterization study. Circulation 2000;102:1788–1794. [ Links ]
25. Garcia M, Rodriguez L, Ares M, et al. Differentiation of constrictive pericarditis from restrictive cardiomyopathy: assessment of left ventricular diastolic velocities in longitudinal axis by Doppler tissue imaging. J Am Coll Cardiol 1996;27:108–114. [ Links ]
26. Ha JW, Ommen S, Tajik A, et al. Differentiation of constrictive pericarditis from restrictive cardiomyopathy using mitral annular velocities by tissue Doppler echocardiography. Am J Cardiol 2004;94:316–319. [ Links ]
27. De Backer J, Matthys D, Gillebert T, et al. The use of TDI for the assessment of changes in myocardial structure and function in inherited cardiomyopathies. Eur J Echocardiogr 2005;6:243–250. [ Links ]
28. Nagueh S, Bachinski L, Meyer D, et al. Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy. Circulation 2001;104:128–130. [ Links ]
29. Ho C, Sweitzer N, McDonough B, et al. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation 2002;105:2992–2997. [ Links ]
30. Dutka D, Donelly J, Palka P, et al. Echocardiographic characterization of cardiomyopathy in Friedreich's ataxia with tissue Doppler echocardiographically derived myocardial velocity gradients. Circulation 2000;102:1276–1282. [ Links ]
31. Derumeaux G, Ovize M, Loufoua J, et al. Doppler tissue imaging quantitates regional wall motion during myocardial ischemia and reperfusion. Circulation 1998;97:1970–1977. [ Links ]
32. Bolognesi R, Tsialtas D, Barilli A, et al. Detection of early abnormalities of left ventricular function by hemodynamic, echotissue Doppler imaging, and mitral Doppler flow techniques in patients with coronary artery disease and normal ejection fraction. J Am Soc Echocardiogr 2001;14:764–772. [ Links ]
33. Katz W, Gulati V, Mahler C, et al. Quantitaive evaluation of the segmental left ventricular response to dobutamine stress by tissue Doppler echocardiography. Am J Cardiol 1997;79:1036–1042. [ Links ]
34. Von Bibra H, Tuchnitz A, Klein A, et al. Regonal diastolic function by pulsed Doppler myocardial mapping for the detection of left ventricular ischemia during pharmacologic stress testing. J Am Coll Cardiol 2000;36:444–452. [ Links ]
35. Bountioukos M, Schinkel A, Bax J, et al. Pulsed–wave tissue Doppler quantification of systolic and diastolic function of wable and nonviable myocardium in patients with ischemic cardiomyopathy. Am Heart J 2004;148:1079–84. [ Links ]
36. Yu C, Zhang Q, Fung J, et al. A novel tool to assess systolic asynchrony and identify responders of cardiac resynchronization therapy by tissue synchronization imaging. J Am Coll Cardiol 2005;45:677–684. [ Links ]
37. Van de Veire N, Bleeker G, De Sutter J, et al. Tissue synchronization imaging accurately measures left ventricular dyssynchrony and predicts response to cardiac resynchronization therapy. Heart 2007;93:1034–1039. [ Links ]
38. Leitman M, Lysyansky P, Sidenko S, et al. Two dimensional strain—a novel software for real–time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr 2004;17: 1021–1029. [ Links ]
39. Bohs L, Trahey G. A novel method for angle independent ultrasonic imaging of blood flow and tissue motion. IEEE Trans Biomed Eng 1991;38:280–286. [ Links ]
40. Ogawa K, Hozumi T, Sugoka K, et al. Usefulness of automated quantification of regonal left ventricular wall motion by a novel method of two–dimensional echocardiographic tracking. Am J Cardiol 2006;98:1531–1537. [ Links ]
41. Tanabe M, Suffoletto M, Pinsky M, et al. Validation of novel echocardiographic speckle tracking radial strain to assess ventricular dyssynchrony: Comparison with angle corrected tissue Doppler strain imaging. J Am Coll Cardiol 2007;49:806–808, 100A. [ Links ]
42. Amundsen B, Helle–Valle T, Edvardsen T, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: Validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol 2006;47:789–793. [ Links ]
43. Helle–Valle T, Crosby J, Edvardsen T, et al. New noninvasive method for assessment of left ventricular rotation: Speckle tracking echocardiography. Circulation 2005;112:314–315. [ Links ]
44. Langeland S, D'hooge J, Wouters P, et al. Experimental validation of a new ultrasound method for the simultaneous assessment of radial and longitudinal myocardial deformation independent of insonation angle. Circulation 2005;112:2157–2162 [ Links ]
45. Hurlburt H, Aurigemma G, Hill J, et al. Direct Ultrasound Measurement of Longitudinal, Circumferential, and Radial Strain Using 2–Dimensional Strain Imaging in Normal Adults. Echocardiography 2007;24: 723–731. [ Links ]
46. Artis N, Oxborough D, Williams G, Pepper CB, Tan LB. Two–dimensional strain imaging: A new echocardiographic advance with research and clinical applications. Inter J Cardiology 2008;123:240–248. [ Links ]
47. Becker M. Analysis of myocardial deformation based on pixel tracking in 2D echocardiographic images allows quantitative assessment of regional left ventricular function. Heart 2005;92:1102–1108. [ Links ]
48. Sjøli B, Ørn S, Grenne B, et al. Diagnostic capability and reproducibility of strain by doppler and by speckle tracking in patients with acute myocardial infarction. J Am Coll Cardiol Img 2009;2:24 –33. [ Links ]
49. Ishii K, Suyama T, Imai M, et al. Abnormal regional left ventricular systolic and diastolic function in patients with coronary artery disease undergoing percutaneous coronary intervention clinical significance of post–ischemic diastolic stunning. J Am Coll Cardiol 2009;54;1589–1597. [ Links ]
50. Takeuchi M, Otsuji Y, Lang R. Evaluation of left ventricular function using left ventricular twist and torsion parameters. Current Cardiology Reports 2009;11:225–230. [ Links ]
51. Kim W, Lee B, Kim Y, et al. Apical Rotation Assessed by speckle–tracking echocardiography as an index of global left ventricular contractility. Circ Cardiovasc Imaging 2009;2:123–131. [ Links ]
52. Wang J, Khoury D, Yue Y, et al. Left ventricular untwisting rate by speckle tracking echocardiography. Circulation 2007;116:2580–2586. [ Links ]
53. Burns A, La Gerche A, Prior D, MacIsaac A. Left ventricular untwisting is an important determinant of early diastolic function. J Am Coll Cardiol Img 2009;2:709–716. [ Links ]
54. Delgado V, Ypenburg C, Van Bommel RJ, et al. Assessment of left ventricular dyssynchrony by speckle tracking strain imaging comparison between longitudinal, circumferential, and radial strain in cardiac resynchronization therapy. J Am Coll Cardiol 2008; 51:1944–1952. [ Links ]
55. Torrent–Guasp F, Kocica M, Corno AF, et al. Towards new understanding of the heart structure and function. Eur J Cardiothorac Surg 2005;27:191–201. [ Links ]
56. Notomi Y, Lysyansky P, Setser R, et al. Measurement of Ventricular Torsion by Two–Dimensional Ultrasound Speckle Tracking Imaging. J Am Coll Cardiol 2005;45:2034–2041. [ Links ]
57. Bertini M, Marsan N, Delgado V, et al. Effects of Cardiac Resynchronization Therapy on Left Ventricular Twist. J Am Coll Cardiol 2009; 54:1317–1325. [ Links ]
58. Abe Y, Kawagishi T, Ohuchi H, et al. Accurate detection of regional contraction using novel 3–dimensional speckle tracking technique. J Am Coll Cardiol 2008; 51(Suppl A): A11. [ Links ]
59. Crosby J, Amundsen B, Hergum T, et al. 3–d speckle tracking for assessment of regional left ventricular function. J Ultras Med Bio 2009;35 458–471. [ Links ]
60. Saito K, Okura H, Watanabe N, et al. Comprehensive Evaluation of Left Ventricular Strain Using Speckle Tracking Echocardiography in Normal Adults: Comparison of Three–Dimensional and Two–Dimensional Approaches. J Am Soc Echocardiogr 2009;22:1025–1030. [ Links ]
61. Pérez de Isla L, Vivas D, Fernández–Golfín C, et al. Three–dimensional–wall motion tracking: a new and faster tool for myocardial strain assessment: comparison with two–dimensional–wall motion tracking. J Am Soc Echocardiogr 2009;22:325–330. [ Links ]
62. Hung J, Lang R, Flachskampf F, et al. 3D echocardiography: a review of the current status and future directions. J Am SocEchocardiogr 2007;20:213–233. [ Links ]
63. Maffessanti F, Nesser H, Winert L, et al. Quantitative evaluation of regional left ventricular function using three–dimensional speckle tracking echocardiography in patients with and without heart disease. Am J Cardiol 2009;104:1755–1762. [ Links ]
64. Evangelista A, Nesser J, De Castro S, Faletra F, Pandian NG, et al. Three–dimensional speckle tracking study of myocardial mechanics in normal humans: demonstration of regional and segmental heterogeneity in radial, circumferential and longitudinal strain. J Am Coll Cardiol 2009;53:A231–A304. [ Links ]
65. Arias A, Urbano Moral JA, Kuvin J, Patel A, Pandian NG. Twist mechanics of the left ventricle: quantitative assessment of systolic and diastolic rotational parameters in normal humans employing three–dimensional speckle tracking echocardiography. J Am Soc Echocardiogr 2010;23:B62–B88. [ Links ]
66. Marwick T, Leano R, Brown J, et al. Myocardial strain measurement with 2–dimensional speckle–tracking echocardiography: Definition of normal range. J Am Coll Cardiol Img 2009;2:80–84. [ Links ]














