Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos de cardiología de México
versión On-line ISSN 1665-1731versión impresa ISSN 1405-9940
Arch. Cardiol. Méx. vol.80 no.2 Ciudad de México abr./jun. 2010
Investigación Básica: Comentario editorial
Functional and structural regeneration in the axolotl heart (Ambystoma mexicanum) after partial ventricular amputation
Regeneración funcional y estructural del corazón de axolotl (Ambystoma mexicanum) después de amputación parcial del ventrículo
Agustina Cano–Martínez,1 Alvaro Vargas–González,1 Verónica Guarner–Lans,1 Esteban Prado–Zayago,2 Martha León–Olea,3 Betzabé Nieto–Lima.1
1 Departamento de Fisiología, Instituto Nacional de Cardiología Ignacio Chávez.
2 Criadero Tachipa Umbral, Maya 'lk Centro. México.
3 Instituto Nacional de Psiquiatría Ramón de la Fuente.
Corresponding author:
Agustina Cano Martínez Ph.D.
Instituto Nacional de Cardiología Ignacio Chávez,
Departamento de Fisiología, Juan Badiano N°1,
Colonia Sección XVI, Delegación Tlalpan, CP. 14080,
México City, México.
Phone: 011 (52) 5573 2911, ext. 1278.
E–mail: cmamx2002@yahoo.com.mx
Received in November 15, 2007.
Accepted in March 05, 2010.
Abstract
In the present study we evaluated the effect of partial ventricular amputation (PVA) in the heart of the adult urodele amphibian (Ambystoma mexicanum) in vivo on spontaneous heart contractile activity recorded in vitro in association to the structural recovery at one, five, 30 and 90 days after injury. One day after PVA, ventricular–tension (VT) (16 ± 3%), atrium–tension (AT) (46 ± 4%) and heart rate (HR) (58 ± 10%) resulted lower in comparison to control hearts. On days five, 30 and 90 after damage, values achieved a 61 ± 5, 93 ± 3, and 98 ± 5% (VT), 60 ± 4, 96 ± 3 and 99 ± 5% (AT) and 74 ± 5, 84 ± 10 and 95 ± 10% (HR) of the control values, respectively. Associated to contractile activity recovery we corroborated a gradual tissue restoration by cardiomyocyte proliferation. Our results represent the first quantitative evidence about the recovery of heart contractile activity after PVA in an adult urodele amphibian, indicating that the heart of A. mexicanum restores its functional capacity concomitantly to the structural recovery of the myocardium by proliferation of cardiomyocytes after PVA. These properties make the heart of A. mexicanum a potential model to study the mechanisms underlying heart regeneration in adult vertebrates in vivo.
Key words: Partial ventricular amputation; Heart, Amphibian; Contractile activity; Axolotl; Ambystoma; Mexico.
Resumen
En el presente estudio evaluamos el efecto de la amputación parcial del ventrículo (APV) del corazón de un anfibio urodelo adulto (Ambystoma mexicanum) in vivo, sobre la actividad contráctil espontánea del corazón registrada in vitro, a diferentes tiempos después de APV, en asociación a su recuperación estructural. Un día después del daño, los valores de tensión ventricular (TV) (16 ± 3%), tensión auricular (TA) (46 + 4%) y frecuencia cardiaca (FC) (58 + 10%), resultaron ser menores respecto al control. En los días cinco, 30 y 90 después del daño, los valores alcanzaron 61 ± 5, 93 ± 3 y 98 ± 5% (TV), 60 ± 4, 96 ± 3 y 99 ± 5% (AT) y 74 ± 5, 84 ± 10 y 95 ± 10% (FC) de los valores control, respectivamente. Además de la recuperación de la actividad contráctil, corroboramos la recuperación estructural y gradual del tejido miocárdico por proliferación de cardiomiocitos. Nuestros resultados representan la primera evidencia cuantitativa de la recuperación de la actividad contráctil del corazón de un anfibio urodelo adulto después de APV; indicando que el corazón de A. mexicanum recupera su capacidad funcional concomitantemente con la recuperación estructural del miocardio por proliferación de cardiomiocitos. El corazón de A. mexicanum es un modelo potencial para el estudio de los mecanismos de la regeneración miocárdica de vertebrados adultos in vivo.
Palabras clave: Amputación parcial del ventrículo; Corazón; Anfibios; Actividad contráctil; Axolotl; Ambystoma; México.
Introduction
The amphibian heart is characterized by a single ventricular chamber, an atrium partially separated by a perforated septum1 and the absence of coronary circulation.2,3 The heart of amphibians shows contractile activity with a cyclic pattern of alternating steps of contraction and relaxation. It involves a wave of depolarization created by the cells of the sinus venosus that spreads to contractile cells in the two sides of the atrium, which contract simultaneously. Meanwhile the ventricle is in a state of relaxation that allows it to receive the blood from the atrium. After a short delay, the wave of depolarization reaches the ventricle which contracts and pumps the blood to the pulmonary and systemic circuits, where gases and nutrients can be exchanged. As the ventricle contracts, the cells of the atrium repolarize.4 In contrast to the heart of other vertebrates, the heart of amphibians beats spontaneously for long periods of time in vitro at room temperature,5 which allows the evaluation of the contractile activity of cardiac muscle without the necessity of field stimulation.6–8
On the other hand, in contrast to mammals, adult urodele amphibians such as the newt,9,10 axolotl, Ambystoma mexicanum,11,12 and adult zebrafish13,14 restore the structure of myocardial tissue after partial ventricular amputation (PVA). In an ideal situation, structural recovery would result in a fully functional tissue or organ. It is generally assumed that there is a structural restoration, based on the survival time after PVA in newts9,10 and the visual inspection of beating hearts in zebrafish.13 This restoration is related with regeneration and could contribute to the functional reestablishment of the heart function.15 However in none of the cases included, a systematic evaluation of function has been considered. Considering that the functional restoration could be part of the regeneration process, in the present study we evaluated in the adult urodele A. mexicanum the contractile activity of the heart at different time after PVA. Additionally, tissue changes and cardiomyocyte proliferation were evaluated in order to corroborate structural restoration.
Methods
Animals
Neotenic adult specimens of A. mexicanum of either sex, aged 8– to 12–months, were used in this study. All animals received adequate care according to the principles of laboratory animal care (NIH publication No. 86–23, revised 1985) and the specific national and institutional laws on protection of animals. The animals were obtained by reproduction in captivity (Breeding place "Tachipa Umbral", Permission SEMARNAP: INE–CITES–DGVS–CR–IN–0249–D.F./97) and were transported to the laboratory where they were maintained at 20 ± 2ºC, on a 12 h light/ dark cycle, and fed with living fish ad libitum.
Surgical heart injury
Animals were anaesthetized by placing them in a MS–222 water solution, 1:1000 (w:v) (ICN Biomedicals) and transferred to an ice plate to reduce their metabolic rate. Under aseptic conditions, the heart was exposed through thora–cotomy and using microsurgery scissors the ventricular wall was partially resected, reaching the ventricular cavity. The amputated tissue corresponded to the 10 ± 2% of the mean value of total weight of the ventricle. After a blood clot was formed, the wound was cleaned and the incision in the thoracic cavity was closed with a 3–0 silk surgical thread. Sham–operated control organisms were treated in a similar way with the exception of the injury to the ventricular wall.
Functional assay
Six experimental and 4 sham–operated animals were sacrificed at each specific time (1, 5, 30 and 90 days) after surgery. Each whole heart was placed in an isolated organ chamber5 and perfused with amphibian Ringer solution, pH 7.8, with the following composition (mM): NaCl 111, KCl 1.9, NaHCO3 2.4, NaH2PO4 0.07 and CaCl2 1.1, at 16±1°C. This temperature was selected considering our previous results for A. dumerilii, in which the reproducibility of the recordings after pharmacological damage was higher when the bath temperature was between 15 and 17°C.16 The base of the heart was collocated to the bottom of the chamber with a 6–0 surgical thread, whereas the apex was connected to a tension transducer (FTO3 model, Grass, Quincy Mass, USA). A resting tension of 50 to 80 mg was applied to the heart, the cardiac activity was stabilized for 15 to 30 minutes, and spontaneous contractile activity of both the ventricle and atrium was recorded simultaneously in a polygraph (79D model, Grass, Quincy Mass, USA). Isotonic tension and heart rate were evaluated by measuring the amplitude of the spikes and by counting the number of spikes per unit of time in the recording paper, respectively.
Structural evaluation
Macroscopic structural changes were identified in the hearts previously used in the functional assays. Tissue recovery was detected by Masson's trichrome technique (commercial kit, Accustain Trichrome Stain, Sigma Diagnostics) in subsequent serial and longitudinal frozen sections (10 mm thick).
On the other hand, to analyze the cardiomyocyte proliferation, 8 experimental and 4 sham–operated animals for each specific time (5, 10, and 30 days after PVA), received previously their sacrifice 3 administrations of BrdU (250 mg/kg, i.p.) at interval of 24 hr.
Animals were sacrificed by decapitation on days 5, 10 and 30 after surgery, hearts were removed, washed, fixed with Carnoy's solution overnight and equilibrated with 30% sucrose at 4ºC. Subsequently, serial and transverse frozen sections (10 μm thick) of each heart were obtained and a double immunohistochemistry for BrdU and α–sarcomeric actin was carried. Tissue sections were rehydrated with 0.1 M PBS, pH 7.4, and denatured with 2 N HCl at room temperature for 30 minutes, followed by a double immunohistochemistry for mouse anti–BrdU (Sigma Chemical) and α–sarcomeric actin (α–Sr–1, Dako A/S) antibodies. BrdU and α–sarcomeric actin were indirectly detected by using conjugated donkey anti–mouse IgG–fluoresceine isothiocyanate (FITC; green) and mouse IgG–tetramethyl rhodamine isothiocyanate (TRITC; red), respectively. The proportion of cardiomyocyte with BrdU–labelled nuclei in the damaged area with respect to the total was revealed by DAPI staining. The proportion of BrdU–positive nuclei was determined by counting nuclei in 6 random fields under a 10X objective in 4 tissue sections from the regenerating area of each condition. A total of 1000 to 2500 nuclei were counted for each tissue section.
Tissue sections were analyzed by fluorescence (BX51, Olympus) and confocal microscopy (Axiovert 100M, Carl Zeiss). Confocal images were reconstructed from 64 optical sections.
Statistical analysis
Data are presented as mean ± S.D., and the differences between the values of the injured and sham–operated control hearts were established using the Student's t–test. Values of p < 0.05 were considered significant.
Results
Functional evaluation
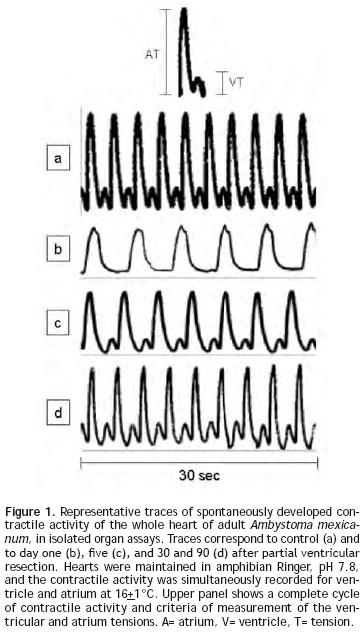
During spontaneous activity of the hearts of sham–operated adult A. mexicanum, a simultaneous record of ventricle and atrium contractions was obtained, showing a cyclic pattern of a peak from ventricular tension (VT) preceded by a peak from atrium tension (AT) (Figure 1a). Traces of contractile activity of injured hearts are shown in Figures 1b–1d. One day (Figure 1b) and 5 days (Figure 1c) after injury, the form, magnitude (mg) and number of peaks (beats/min) were clearly altered; while at 30 and 90 days (Figure 1d) after resection, values were similar to those of control hearts (Figure 1a).
Control VT and AT were 62 + 3 and 284 + 15 mg respectively (Figure 2A), while the mean heart rate (HR) of control was 19 + 2 beats/min (Figure 2B). The effect caused by the PVA was detected 1 day after injury as a decrease in the values of VT (84% ± 3), AT (54% ± 4) (Figure 2A) and HR (42% ± 10) in comparison with control heart (Figure 2B). These values returned gradually to those observed in control hearts; achieving a 61 ± 5, 93 ± 3 and 98 ± 5% of the control values for VT; 60 ± 4, 96 ± 3 and 99 ± 5% of the control values for AT (Figure 3A) and 74 ± 5, 84 ± 10 and 95 ± 10% of control values for HR (Figure 3B); on days five, 30 and 90 after injury, respectively.
Structural evaluation
Concomitant with the heart contractile activity we corroborated a gradual tissue restoration (Figure 4). One day after damage, the ventricle showed an evident gap that was covered with a cloth (Figure 4J); after 30 days the hole was partially occupied by compact tissue (Figures 4C, 4G, 4K), and 90 days after injury the heart showed a ventricular wall surface and myocardic tissue similar to control (Figures 4D, 4H, 4L).
Colocalization of a–sarcomeric actin staining (Figures 5A, 5D) and BrdU–labelled–nuclei (Figures 5B, 5E) revealed by DAPI staining (Figures 5G, 5H, 5I y 5J), by fluorescence microscopy allowed the identification of BrdU–labelled cardiomyocytes. Five days after injury, the proportion of cardiomyocytes with BrdU–labelled nuclei in the damaged area (Figures 5C, 5F) with respect to the total, was 56 ± 5%. Ten days after injury, this value corresponded to the 32 ± 3% and thirty days after lesion it was the 20 ± 2% of the total. In all cases, cell BrdU–labelled nuclei were surrounded by myocardial cytoplasm, as shown in the image reconstructed from 64 optical sections using confocal microscopy (Figure 6).
Discussion
Our results represent the first data concerning about the simultaneous evaluation of the structure and function of the heart of adult A. mexicanum, after surgical injury of the ventricular myocardium. We have obtained evidence showing that the structural recovery of myocardium after ventricular injury in the heart of adult axolotl is accompanied by the restoration of its functional capacity. This finding is in agreement with the proposal that adult organisms of early terrestrial animal species are endowed with a higher capacity of tissue regeneration.17,18
Functional assays of the heart of adult A. mexicanum showed a contractile activity with a cyclic pattern of alternating steps of contraction and relaxation, as occurs in the heart of other vertebrates.4 This pattern was markedly disrupted by the PVA. Besides, the capability to develop tension of both ventricle and atrium was significantly decreased (Figures 2 and 3).
The heart rate of 19 ± 2 beats/minute, found in control hearts of axolotl, lays within the range of other cardiac rates of other amphibians, which vary from 13 to 23 beats/min.19,20 In our study the decrease in HR (42 ± 6%) in comparison to control (Figures 2B and 3B) at one day of injury, was less dramatic than the fall in tension in the ventricle and atrium (84 ± 2 and 54 ± 4%) (Figure 3A).
The force–frequency relationship is an important intrinsic regulatory mechanism of cardiac contractility in which an increase in contractile force is associated with an increase in amplitude,21 however in failing ventricular myocardium this relation is lost. We suggest that the differences in the proportional changes between tension and HR in our study could be a compensatory response in proportion to the tissue and the force–frequency relationship recovery. This might be related to the hypoxic bradycardia, which occurs in lower vertebrates and which could provide a number of direct benefits to the heart when oxygen supply in the spongy myocardium is precarious.22
One day after the insult, the effect of PVA was reflected as a more evident decrease in VT (84%) and AT (54%) (Figures 1b and 2A), reflecting that the injured structure was the ventricle. Considering that the function of this structure in vertebrates is to develop the necessary pressure to pump blood to other organs,1,4 its alterations would induce more evident adverse effects. Additionally even when the injury was made in the ventricle, a smaller decrease in the capability of the atrium to develop tension was also observed (Figure 3A), suggesting that the function of other cardiac structures was also altered on the first day as a consequence of the ventricular damage.
The progressive reestablishment of the heart rate along with the ventricular– and atrium–tension observed between 30 and 90 days after the PVA, shows that the heart of adult A. mexicanum restore its functional capability when it is impaired by a cardiac damage. Furthermore, as proved later on, this functional restoration is also accompanied by the structural recovery of the heart, which is in accordance with the functional restoration in heart of A. dumerilli after pharmacological heart damage.16
Structural evaluation, based on Masson's trichrome results (Figure 4) together with the colocalization of BrdU–labelled nuclei and a–sarcomeric actin, with the surrounding of the BrdU–labelled nuclei by myocardial cytoplasm in the heart of A. mexicanun (Figure 6), shows a notorious recovery of the ventricular tissue and the presence of proliferating cardiomyocyte which were more abundant 5 days after PVA. These results are in accordance with the finding of a subset of cardiomyocytes that progress through mitosis and may enter to successive divisions in the newt, other adult urodele amphibian15 and with previous reports that detect BrdU11 and PCNA12 in cardiomyocyte of A. mexicanum. Our finding suggests that cardiomyocyte proliferation could be responsible of the structural recovery detected. However, other mechanisms proposed for tissue regeneration, such as the reprogramming of stem cell23,24 or differentiated cells,25 cannot be ruled out.
On the other hand, the reason behind the high regenerative capacity of the heart of adult A. mexicanum is unknown; but it is probably a consequence of the biological characteristics of its cardiac tissue. The heart of adult amphibians consists of spongy, loose and trabeculate myocardium,26,27 similar to embryonic myocardium of other vertebrates28,29. These characteristics allow the muscle to acquire oxygen by diffusion from luminal blood within the trabecular spaces,30 which would facilitate the supplement of nutrients and growth factors to cardiac cells, which in turn may result in the production of new cardiomyocyte. However, this remains to be analyzed in future studies.
Finally, it is relevant to mention that in our study we demonstrated that the heart of A. mexicanum beats spontaneously in vitro for long periods of time at room temperature, as has been found for other amphibians,5,16 this allows the evaluation of the contractile activity of cardiac muscle without the necessity of field stimulation.6–8 Furthermore, the procedure that we used to test functional performance allowed us to register simultaneously the spontaneous activity of both ventricle and atrium, and to assess the intrinsic capacity of the cardiac function independently of the compensatory mechanisms from the rest of the body and avoiding the influence of the anesthetics on cardiac function.31
Conclusions
We obtained the first quantitative evidence about the recovery of heart contractile activity after PVA in an adult urodele amphibian which indicates that the heart of A. mexicanum restores its functional capacity after PVA in association to cardiomyocyte proliferation. The evaluation of the post–injury changes in the structure and function in the heart of non–mammalian vertebrates, like urodele amphibians, can be very useful, because the proportion of proliferating myocardial cells after damage is very high when compared to mammalian cardiomyocyte, which show a very low ability to proliferate.32 The possibility of an effective myocardial regeneration in A. mexicanum makes it an interesting model to study the mechanisms underlying heart restoration after an insult.
Acknowledgements
We wish to thank Instituto Nacional de Cardiología Ignacio Chávez for grant 00–303 to ACM and Centro de Instrumentos Hospital de Especialidades C.M.N Siglo XXI (IMSS) for the Carl Zeiss confocal mycroscope facility.
References
1. Kardong KV. Vertebrates. Comparative anatomy, function, evolution. WCB/McGraw–Hill, Boston Massachusetts, pp. 401, 422-470, 1998. [ Links ]
2. Ghiara P, Parente L, Piomelli D. The cyclo–oxygenase pathway in the avascular heart of the frog, Rana esculenta L. Gen Pharmacol 1984;15:300–313. [ Links ]
3. Adler A, Huang H, Wang Z, et al. Endocardial endothelium in the avascular frog heart: role for diffusion of NO in control of cardiac O2 consumption. Am J Physiol Heart Circ Physiol 2004;287:14–21. [ Links ]
4. Opie LH. The heart: physiology, from cell to circulation. Lippincott–Raven, Philadelphia, pp. 343–389, 1998. [ Links ]
5. MacLeod LJ. Pharmacological Experiments on Intact Preparation. Churchill Livingstone, Edinburgh, pp. 113, 1970. [ Links ]
6. Stene–Larsen G, Helle KB. Cardiac beta2–adrenoceptor in the frog. Comp Biochem Physiol 1978; 60C:165–173. [ Links ]
7. Carlsten A. Ericson LE, Poupa O. The healing of frog heart lesions induced by isoproterenol injections. Acta Pathol Microbiol Immunol Scand [A] 1982; 90:57–65. [ Links ]
8. McKean T. Hypoxia and ischemia in buffer–perfused toad hearts. J Exp Biol 1997;200:2575–2581. [ Links ]
9. Becker RO, Chapin S, Sherry R. Regeneration of ventricular myocardium in amphibians. Nature 1974;248:145–147. [ Links ]
10. Oberpriller JO, Oberpriller JC. Response of the adult newt ventricle to injury. J Exp Zool 1974;187:249–260. [ Links ]
11. Flink IL. Cell cycle reentry of ventricular and atrial cardiomyocytes and cells within the epicardium following amputation of the ventricular apex in the axolotl, Amblystoma mexicanum: confocal microscopic immunofluorescent image analysis of bromodeoxyuridine–labeled nuclei. Anat Embryol 2002;205:235–244. [ Links ]
12. Vargas GA, Prado ZE, León OM, et al. Regeneración miocárdica en Ambystoma mexicanum después de lesión quirúrgica. Arch Cardiol Mex 2005;75(Supl 3):21–29. [ Links ]
13. Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science 2002;298: 2188–2190. [ Links ]
14. Poss KD. Getting to the heart of regereration in zebrafish. Semin. Cell Dev Biol 2007;18:36–45. [ Links ]
15. Bettencourt DM, Mittnacht S, Brockes JP. Heterogeneous proliferative potential in regenerative adult newt cardiomyocytes. J Cell Sci 2003;116:4001–4009. [ Links ]
16. Cano MA, Vargas GA, Guarner LV. Temperature effect on contractile activity of the Ambystoma dumerilii heart previously treated with isoproterenol. Comp Biochem Physiol A Mol Integr Physiol 2007;147:743–749. [ Links ]
17. Thouveny Y, Tassava R. Regeneration through phylogenesis: cellular and molecular basis of regeneration. In: Ferretti P. Géraudie JJ (Eds). From invertebrates to humans. Wiley and Sons, New York, pp. 9–43, 1998. [ Links ]
18. Tsonis P. Regeneration in vertebrates. Dev Biol 2000;221 273-284. [ Links ]
19. Wahlqvst I, Campbell G. Autonomic influences on heart rate and blood pressure in the toad, Bufo marinus, at rest and during exercise. J Exp Biol 1988;134:377–396. [ Links ]
20. Dumsday B. Resting heart rate of the toad Bufo marinus: a long term study of individual differences and environmental influences. Physiol Zool 1990;63:420–431. [ Links ]
21. Edoh M. Force–frequency relationship in intact mammalian ventricular myocardium: physiological and pathophysiological relevance. Eur J Pharmacol 2004;500:73–86. [ Links ]
22. Farrell AP. Tribute to P. L. Lutz: a message from the heart––why hypoxic bradycardia in fishes?. J Exp Biol 2007;210(Pt 10):1715–1725. [ Links ]
23. Pomerantz J, Blau HM. Nuclear reprogramming: a key to stem cell function in regenerative medicine. Nat Cell Biol 2004;6:810–816. [ Links ]
24. Laube F, Heister M, Scholtz C, et al. Re–programming of newt cardiomyocytes is induced by tissue regeneration. J Cell Sci 2006;199:4719–4729. [ Links ]
25. Straube WL, Brockes JP, Drechsel DN, et al. Plasticity and reprogramming of differentiated cells in amphibian regeneration: partial purification of a serum factor that triggers cell cycle re–entry in differentiated muscle cells. Cloning Stem Cells 2004;6:333–344. [ Links ]
26. Romenskii O. Blood supply of the compact and spongy myocardium of fish, amphibia and reptiles. Arkh Anat Gistol Embriol 1978;75:91–95. [ Links ]
27. Victor S. Evolution of the ventricles. Tex Heart Inst J 1999;26:168–175. [ Links ]
28. Ben–Shachar G. Ventricular trabeculations in the chick embryo heart and their contribution to ventricular and muscular septal development. Circ Res 1985;57:759–766. [ Links ]
29. Sedmera D, Pexieder T, Vuillemin M, et al. Developmental patterning of the myocardium. Anat Rec 2000;258:319–337. [ Links ]
30. Lillywhite HB, Zippel KC, Farrell AP. Resting and maximal heart rates in ectothermic vertebrates. Comp Biochem Physiol A Mol Integr Physiol 1999;124:369–382. [ Links ]
31. Smith DG. Sympathetic cardiac stimulation in Bufo marinus under MS–222 anesthesia. Am J Physiol 1974;226:367–370. [ Links ]
32. Engel FB. Cardiomyocyte proliferation: a platform for mammalian cardiac repair. Cell Cycle 2005;4:1360–1363. [ Links ]














