Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos de cardiología de México
versión On-line ISSN 1665-1731versión impresa ISSN 1405-9940
Arch. Cardiol. Méx. vol.79 no.3 Ciudad de México jul./sep. 2009
Investigación básica: electrofisiología y arritmias
Antiarrhythmic and arrhythmogenic action of inosine in experimental ventricular tachyarrhythmias
Efectos antiarrítmicos y arritmogénicos de la inosina en taquiarritmias ventriculares experimentales
Alfredo de Micheli,1 Gustavo Pastelín,2 Rafael Chávez Domínguez,3 Pedro Iturralde Torres,4 and Gustavo A Medrano1
1 Instituto Nacional de Cardiología Ignacio Chávez. México.
2 Departamento de Farmacología, Instituto Nacional de Cardiología Ignacio Chávez.
3 Jefe Departamento de Epidemiología, Instituto Nacional de Cardiología Ignacio Chávez.
4 Departamento de Electrofisiología, Instituto Nacional de Cardiología Ignacio Chávez.
Corresponding author:
Dr. Alfredo de Micheli,
Instituto Nacional de Cardiología Ignacio Chávez,
Juan Badiano No. 1, Col. Sección XVI,
México, D.F. CP 14080.
Received: March 31, 2009.
Accepted: July 27, 2009.
Abstract
Objective: To study the possible action of inosine on experimental ventricular tachyarrhythmias.
Material and methods: We used 92 mongrel dogs weighing 13 kg–17 kg, anesthetized with 30 mg/kg sodium pentobarbital applied intravenously. Myocardial lesions were induced by injecting 1ml–1.5 ml of 70% phenol in the free wall of the left ventricle. In 36 dogs, the ventricular arrhythmia (VT) was induced 30 min later with aconitine crystals inserted into the periphery of the damaged area; in 16, VT was due only to myocardial damage and in the other 13 VT was spontaneously originated. Twenty–nine animals constituted the control group; no inosine was administered to them. The possible effects of inosine were studied in 63 animals. Leads II, aVR or aVL, right and left unipolar intraventricular leads and that on the wall of the superior vena cava were recorded under control conditions, once the myocardial damage had been induced, during the ventricular tachycardia, and following the injection of inosine. Of the 63 inosine–treated animals; in 34, VT was due to aconitine; in 16, it was produced only by the myocardial damage and, in 13, VT was presented spontaneously.
Results: Sinus rhythm was not reestablished in the animals of the control group. Inosine reestablished the sinus rhythm in 26 of 34 dogs (76%) that received phenol and aconitine, in 13 of the 16 (81%) presenting only the myocardial damage, and in 6 of the 13 (46%) with spontaneous ventricular tachycardia. In some experiments, inosine induced supraventricular tachycardias, ventricular–atrial blocks, and ventricular pre–excitation phenomena.
Conclusions: In this experimental series, inosine showed antiarrhythmic and arrhythmogenic effects, similar to those of adenosine from which it derives.
Key words*: Inosine; Inosine's antiarrhythmic effects; Inosine's arrhytmogenic effects.
Resumen
Objetivo: Estudiar la posible acción de la inosina en taquiarritmias ventriculares experimentales.
Material y métodos: Se utilizaron 92 perros mestizos de 13–17 kg, anestesiados con 30 mg/kg de pentobarbital sódico por vía intravenosa. En éstos, se produjo daño miocárdico por inyección intramural de 1–1.5 ml de fenol al 70% en la pared libre del ventrículo izquierdo, cerca de la punta. En 34 de ellos, mediante introducción de cristales de aconitina en la periferia del área dañada, se causó una taquiarritmia ventricular (TV). Veinte y nueve animales constituyen el grupo testigo, con evolución libre de dicha arritmia también debida a aconitina. La acción de la inosina se investigó en 63 animales. En todos, se registraron las derivaciones DII, aVR o aVL, las unipolares intraventriculares derecha e izquierda y la de la pared de la vena cava superior. Se hizo esto al comienzo del experimento (control), tras causar el daño miocárdico por fenol, con la TV, al final de la inyección de inosina en bolo por la vena cava superior y durante otra hora u hora y media. La duración total de cada experimento fue de unas 5 horas.
Resultados: El ritmo sinusal (RS) no se restableció en ningún animal del grupo testigo. Por efecto de la inosina, el RS se restableció en 26 de los 34 perros(76%) que habían recibido fenol y aconitina, en 13 de los 16 (81%) con solo daño miocárdico y en 6 de los 13 (46%) con TV espontánea. En algunos experimentos, la inosina provocó también taquicardias supraventriculares, bloqueo ventriculoauricular y fenómenos de preexcitacion ventricular.
Conclusiones: En esta serie experimental, la inosina ha mostrado tener efectos antiarrítmicos y arritmogénicos semejantes a los de la adenosina, de la que deriva.
Palabras clave: Inosina; Efectos antiarrítmicos de la inosina; Efectos arritmogénicos de la inosina.
Introduction
Inosine (IN) is a nudeoside, derived from adenosine by deamination.1 Adenosine converts to inosine by the action of adenosine deaminase. Then, a nudeoside phosphorylase divides IN in hypoxanthine and pentose. In turn, hypoxanthine is oxidized to xanthine by the xanthine oxidase, a flavoprotein containing Fe and Mo. Xanthine is a substrate of xanthine oxidase, which converts it to uric acid, the terminal product of purines' degradation, to be eliminated essentially by urine.
Researches of the group of Bethesda, USA, have extensively studied the electrophysiological,23 pharmacological,4 and therapeutical5 characteristics of adenosine, as well as its action on the specific elements of the atrioventricular conduction system.67 Myocite receptors for adenosine have also been identified.8 Moreover, purines modulate the phenomenon of cellular death produced by free oxygen radicals.9 On its side, adenosine acts as an effective antiarrhythmic on supraventricular tachycardias and on certain ventricular tachyarrhythmias, particularly on those induced by catecholamines, probably due to inhibition of adenylate cyclase.10,11,12
We have already pointed out the antiarrhythmic action of adenosine on certain clinical13 and experimental14 ventricular tachycardias. In our experimental series, the antiarrhythmic effect of adenosine was generally biphasic: one, early and fleeting; the other, delayed.
Herein, we aim at establishing whether IN, derived from adenosine, has effects on experimental ventricular tachyarrhythmias, notwithstanding that some authors15 consider it "mostly inactive".
Methods and materials
This study was realized on 92 mongrel dogs weighing between 13 and 17 Kg. All animals were anesthetized with 30 mg/kg intravenous sodium pentobarbital, connected with an artificial respiration system, and received continuous infusion of Hartmann solution. After opening the thorax to expose the heart on the open pericardium, 1ml–1.5 ml of 70% phenol –depending on the weight of the animal– were injected into the free left ventricular wall close to the apex. This produced a circumscribed, well defined myocardial lesion. Some 30 min–60 min later, a ventricular tachycardia, generally left, was induced by introducing small crystals of aconitine (Sigma) into the myocardium, next to the damaged area. Some 15 min–30 min later, an inosine bolus (12 mg) was injected through the superior vena cava. At the end of injection, a transient fall of the systolic arterial pressure always occurred, as in dogs treated with adenosine.14 The IN's action was investigated in 63 dogs, while another 29 dogs did not receive it. In 34 of those 63 animals, the ventricular tachycardia (VT) was due to the crystals of aconitine inserted into myocardium, in other 16, the VT was produced only by myocardial damage caused by phenol and in 13, the VT was presented spontaneously without myocardial damage nather aconitine.
In all the experiments, leads II, aVR or aVL, the intraventricular right (IVD) and left (IVI) unipolar leads, as well as the unipolar lead on the wall of superior vena cava, were recorded. These were registered by a VR6 polygraph of Electronics for Medicine Co., with a speed of 100 mm/sec. These traces were obtained under control conditions and with VT. After the injection of IN, they were obtained immediately and every 5 min for the first 15 min, then every 30 min for the next hour or more. Nevertheless, traces were continuously monitored on the polygraph screen. The systolic blood pressure was measured at the same time using a U shaped mercury manometer attached to the right femoral artery, because variations in blood pressure were of more interest than its absolute values.
The recovery of sinus rhythm (SR) within 15 min after the IN injection was considered an early effect. If the same occurred between 30 and 60 min, it was considered a late effect. Recovery of SR was considered fleeting if lasting only a few seconds, and transitory when it lasted few minutes or more. This recovery was always followed by the return of VT. This finding is in favor of a cause–effect relation and against some self–limited tachyarrhythmias.
At the end of each experiment, the weight of the heart was established to determine the approximate dose of used IN in milligrams per gram of myocardium (0.07mg/g–0.10 mg/g). Liquid volume, administered to each animal, and released to urine, was measured.
Statistical analysis
To contrast the distribution proportions of the animals treated with inosine that went into SR, and those of the control animals Table 1, the data were submitted to the χ2 statistical test, which revealed 27.22; and 24.88 with Yates correction. The statistical level of significance was p = 0.0000006, and with Fisher's Exact Test, p = 0.000004. Calculations were performed using Epi Info version 6.04b of CDC 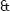 WHO (2007) Geneve, Switzerland. The confidence intervals limits for proportions with a 0.95 significant level (ICL = Inferior Confidence Limit; SCL = Superior Confidence Limit) were consulted from "Confidence Limits for p", in Scientific Tables; Documents Geigy. Konrad Diem, Basel 1965, p 8.
WHO (2007) Geneve, Switzerland. The confidence intervals limits for proportions with a 0.95 significant level (ICL = Inferior Confidence Limit; SCL = Superior Confidence Limit) were consulted from "Confidence Limits for p", in Scientific Tables; Documents Geigy. Konrad Diem, Basel 1965, p 8.
Results
Control animals showed neither fleeting nor transitory recovery of SR after the VT was unchained. This arrhythmia quickly accelerated until originating a ventricular fibrillation in 5 to 20 minutes. Figure 1 shows the electrocardiographic evolution, in a control dog, of ventricular tachycardia with atrio–ventricular dissociation, toward ventricular fibrillation.
Antiarrhythmic effects of inosine
Table 1 shows the different evolution of ventricular tachyarrhythmias in the IN–treated dogs with respect to those developed in control dogs, regarding SR recovery. Inosine reestablished SR in 36 of the 63 treated animals (57.14%, ICL 44.05%; SCL 69.54%), whereas SR was not reestablished in 27 (42.86%). This difference is not statistically significant. However, concerning SR recovery between animals receiving and those not receiving IN (control group), a very significant difference was revealed by the analysis of proportions and confidence limits Table 2 as well as by the distribution of χ2, and p values.

Analyzing the experiments distributed in three subgroups Table 3, it can be noted that IN antiarrhythmic effects were obtained in 26 of 34 animals in which the VT was originated by aconitine in the phenol–damaged myocardium (76.47%), in 13 of 16 animals, in which VT was solely due to phenol (81.25%) and in 6 of 13 dogs with VT spontaneously originated (46.15%). Not statistically significant difference between SR recovery in these subgroups was found.
Examples
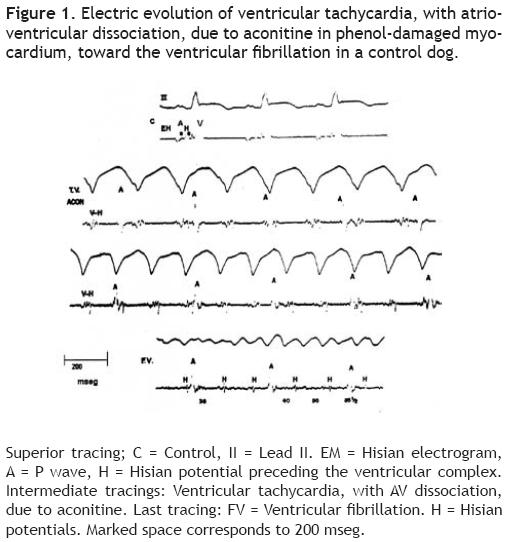
Figure 2 reproduces the electrocardiographic tracings of a 273/min VT, with atrioventricular dissociation, induced by aconitine in the ventricular phenol–damaged myocardium.
The injection of 12 mg of IN through the superior vena cava Figure 3 immediately produced SR recovery (200/min).

Figure 4 exhibits a left ventricular isorhythmic tachycardia, 166/min, and the morphology of ventricular complexes corresponding to an advanced degree RBBB, due only to myocardial damage by phenol. P waves patched to R waves can be seen.

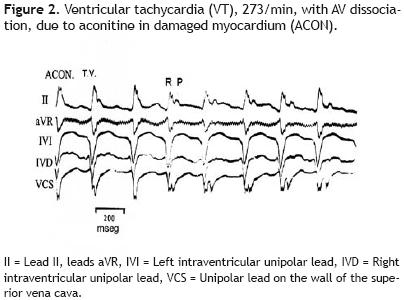
Forty–five minutes after the injection of 12 mg of inosine through the superior vena cava Figure 5 a late and fleeting SR, 162/mln, appeared. Now the RBBB morphologies are absent and P waves precede the ventricular complexes with constant intervals.
Arrhythmogenic effects of inosine
Under the light of its pro–arrhythmic effects, IN, administered to dogs with VT, produced atrial tachycardia in 10 dogs, causing also atrial flutter and fibrillation, as well as ventricular pre–excitation phenomena in two animals.
Concerning the atrioventricular conduction system, the mentioned nucleoside induced ventricular–atrial block of the second degree with progressive periods of Luciani–Wenckebach type in two animals, similar to that observed previously with adenosine.14
Examples
Figure 6 shows, in its superior section, an atrial tachycardia, 273/min, due to inosine. This tachycardia evolved towards an atrial flutter, 545/min, with variable atrioventricular block (intermediate section) and, finally, to atrial fibrillation (inferior section).
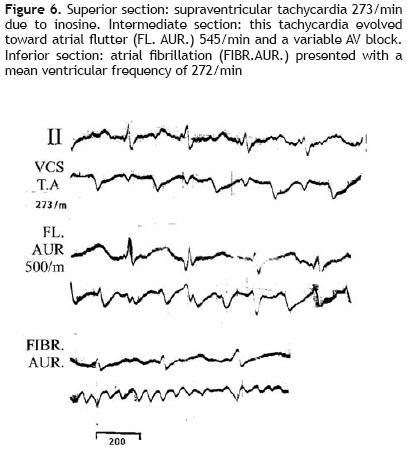
Figure 7 gives an example of both arrhythmogenic and antiarrhythmi'c actions of inosine in the same animal (a dog of 15.8 kg). During a VT, 230/min, due only to myocardial damage by phenol, the first dose of 12 mg of inosine, injected through the superior vena cava, reduced the frequency of this tachycardia to 193/min (not presented in this figure). A second dose of inosine, 5 min after its administration (superior section) originated an atrial flutter, 500/min, with a variable atrioventricular block (early arrhythmogenic effect of inosine). Thirty minutes later (inferior section) a transient SR, 150/min, with prolonged QTc:MV + 0.06 sec appeared, which corresponds to a late antiarrhythmi'c effect.
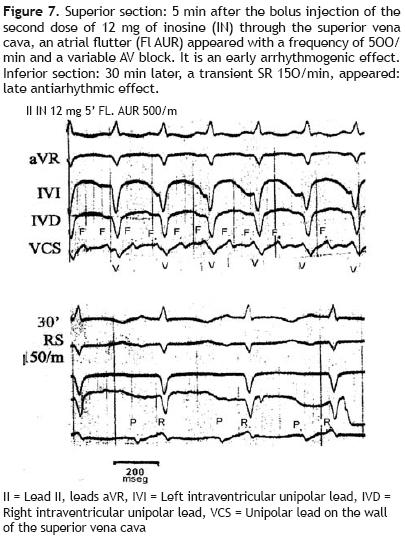
Figure 8 reproduces the manifestation of ventricular pre–excitation: presence of delta wave in the initial portion of the R wave and shortening of the P–R interval, after the administration of 12 mg of IN during an experimental ventricular tachycardia, 300/min.
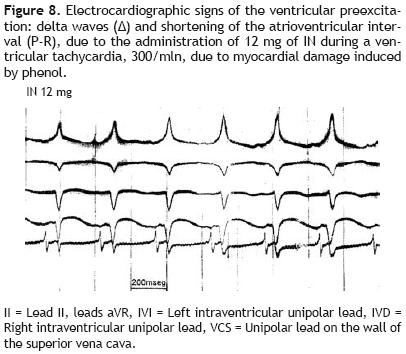
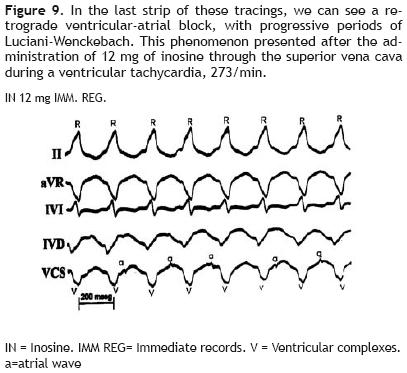
Figure 9 clearly shows, on its last strip, a retrograde ventricular–atrial block of second degree, with progressive periods of Luciani–Wenckebach type: progressive prolongation of ventricular–atrial intervals (V–a) until the interrupted passage of the retrograde activation impulse, and absence of the small atrial wave (a) after the fifth ventricularcomplex(V). This phenomenon was seen on the immediate recordings (IMM REG) after the administration of 12 mg of IN during a ventricular tachycardia, 273/min.
Discussion
In 1987, Lubbe et al.16 pointed out that all the nucleosides derived from adenine have an anticatecholaminic action. When these nudeosides reach a sufficient concentration, they are able to suppress the origin of ventricular arrhythmias due to catecholamines.10,11 These purine compounds, by means of incompletely defined mechanisms, can act as antagonists of the increased myocardial vulnerability mediated by AMPc, which contributes to the genesis of the ventricular fibrillation during the early phase of myocardial ischemia.
The results of our study objectively show that IN has antiarrhythmic and arrhythmogenic effects similar to those of adenosine, from which it is derived. Concerning the relation chemical structure–biological activity, this fact suggests that adenosine deamination does not remove the potential biological activity of this derívate. The IN's antiarrhythmic effects are exerted on atrial and ventricular arrhythmias, probably due to the activation of A, receptors and through other intracellular mechanism.17 Its action could be opposed by blockers of these receptors.
On the contrary, IN's arrhythmogenic effects can be due to sympathetic reflexes, similar to those mentioned by Belardinelli et al.3 concerning adenosine.
Furthermore IN could increase the outward potassium current, like adenosine and acetylcholine, shortening the refractory period of myocytes. In fact, a physiologist of our Institute,18 has observed that, in mouse myocytes, low doses of IN –similar to those used in this study– impel the outward potassium current, while high doses of this nucleoside increase the inward calcium current in myocardial cells. These observations could satisfactorily explain the origin of the atrial flutter and its passage to atrial fibrillation. On the other side, IN, as well as adenosine,19 can allow for the manifestation of a ventricular preexcitation coincident with bradycardia or nodal block, also promoting the conduction through some accessory pathway, usually non–functional: a secondary mechanism to a sympathetic activation.
Inosine apparently acts on the same adenosine receptors of myocytes. These, A, A2A and A38, are stored on the surface of protein G, regulating the effects by stimulating these receptors. Protein G activates the K and Ca–6 channels20 and, probably, phospholypase C. The receptor A1, coupled to a protein G system, reduces the levels of cyclic adenosine monophosphate (cAMP), opposing the increase of adenylate cyclase activity. Inosine could also act on other intracellular and sarcolemmal receptors that are influenced by adenosine, as well as on muscarinic channels of potassium (KACh, KADO), which mediate its chronotropic and dromotropic effects in atria but not in ventricles. When adenosine and IN are accumulated in the extracellular spaces, the outward current of intracellular potassium could increase, thereby inducing a fall in cellular conduction speed.7
We present here the antiarrhythmic and arrhythmogenic effects of IN, an adenosine derívate, which seems to act on the same receptor of adenosine and has a similar action. These effects of IN are shown in this study; although certain authors21 suggest that variations in adenosine's structure implicate the loss of important interactions, and others9 describe its intracellular action independent from other adenosine receptors.
Concerning the arrhythmogenic effects of IN, we have observed that atrial tachycardia, flutter, and fibrillation present in treated animals, as well as in those that received adenosine,2223 are probably due to a shortening of the atrial refractory period. It is worthwhile to mention that, during the treatment of ventricular arrhythmias with intravenously applied IN, electrocardiographic signs of ventricular preexcitation are manifested, as occurs with adenosine.24 This fact already reported by other authors, regarding adenosine,2526 can occur coincidentally with bradycardia or heart block, or could be due to a facilitated conduction through some accessory pathway usually not functional: a secondary phenomenon due to sympathetic activation.
Limitations of the study
The number of experiments included in each subgroup is rather small. A larger number of experiments could confirm the observations already made and support our results. On the other side, it is necessary to state that we did not find specific studies on the electrophysiology and pharmacology of IN, whereas there are numerous studies concerning adenosine. For this reason, we must rely on publications on the characteristics and effects of adenosine. It would be suitable to study also the action of IN infusions.
Conclusions
Inosine, a derívate from adenosine, has antiarrhythmic and arrhythmogenic effects similar to those of adenosine. Its antiarrhythmic action is exerted on atrial and ventricular tachycardias, probably due to the activation of myocyte's receptors, which is opposed by their blockers.
The arrhythmogenic effects of IN are probably due to sympathetic reflexes stimulated by this nucleoside, similar to those stimulated by adenosine.
On the other hand, IN can produce a ventricular preexcitation coincident with bradycardia or heart block, or a facilitated conduction through an accessory, usually not functional pathway. The arrhythmogenic effects of IN can be due to sympathetic reflexes, similar to those stimulated by adenosine.
Concerning the alterations of atrioventricular and ventricular–atrial conduction, IN, as well as adenosine, could reduce the voltage and duration of the action potentials, particularly in the cells of the central region of the atrio–ventricular node.
Bibliography
1. Brandan NC, Aispuru G. Metabolismo de compuestos hidrogenados. http://med.unne.edu.ar/catedras/bioquimica/pdf/nitro.pdf. [ Links ]
2. Belardinelli L, Lerman BB. Adenosine: cardiac electrophysiology. Pacing Clin Electrophysiol 1991;14:1672–80. [ Links ]
3. Belardinelli L, Shreyock KJC , Pelleg A. Cardiac electrophysiologic properties of adenosine. Coron Art Dis 1992;3:1122–6. [ Links ]
4. Pelleg A, Porter RS. Pharmacology of adenosine. Pharmacotherapy 1990; 10:157–74. [ Links ]
5. DiMarco J, Miles W, Akhtar M, Milstein S, Sharma AD, Platia E, et al. Adenosine for paroxysmal supraventricular tachycardia. Dose ranging and comparison with verapamil. Assessment in placebo–controlled, multicenter trials The adenosine for PSVT Study Group..Ann InternMed 1990;113:104–10. [ Links ]
6. Froldi L, Belardinelli L. Species dependent effects of adenosine on heart rate and atrioventricular nodal conduction. Mechanism and physiological complications. Ore Res 1990;67:960–78. [ Links ]
7. Cierno HF, Belardinelli L. Effects of adenosine on atrioventricular conduction. I:Site and characterization of adenosine action in the guinea pig atrioventricular node. Circ Res 1986;59:427–36. [ Links ]
8. Chen Y, Barche RJ. Adenosine. A modulator of the cardiac response to stress. Circ Res 2003;93:691–3. [ Links ]
9. Virág L, Szabó C. Purines inhibit poly (ADP–ribose) polymerase activation and modulate oxidant–induced cell death. FASEB J 2001;15:99–107. [ Links ]
10. Görge B, Kurz T, Katus HA, Richardt G. Endogenous adenosine suppresses norepinephrine–induced ventricular arrhythmias in rat heart. Basic Res Cardiol 1998;93:264–8. [ Links ]
11. Friedrichs GS, Merrill GF. Adenosine deaminase and adenosine attenuate ventricular arrhythmias caused by norepinephrine. Am J Physiol 1991;260(3Pt): H979–84. [ Links ]
12. West G A, Isenberg G, Belardinelli L. Antagonism of forskolin effects by adenosine in guinea pig isolated hearts and ventricular myocytes: evidence that adrenergic effects of adenosine are due to inhibition of adenylate cyclase. Am J Physiol 1986;250:H769–77. [ Links ]
13. Rodríguez–Castellano T J, Iturralde–Torres P, de Micheli A, Ramírez–lnsunza J M, Ramirez J C, Corona Sapien C, et al. Respuesta de la taquicardia ventricular a la administración de adenosina. Arch Inst Cardiol Mex 1994;64:445–54. [ Links ]
14. De Micheli A, Chávez–Domínguez R, Iturralde–Torres P, Pastelín G, Medrano G A. Efectos tempranos y tardíos de.la adenosina en taquicardias ventriculares experimentales. Rev Esp Cardiol 2005;58:159–66. [ Links ]
15. Lerman BB, Belardinelli L. Cardiac electrophysiology of adenosine. Circulation 1991;83:1499–509. [ Links ]
16. Lubbe W F, Gilchrist A I, Holland R K, Pybus J. Adenine nucleotides and ventricular fibrillation. J Mol Cell Cardiol 1987;19 Suppl5:23–33. [ Links ]
17. Szabó G, Stumpf N, Radovits F, Sonnenberg K, Geró D, Hagl S, et al. Effects of inosine on reperfusion injury after heart transplantation. Eur J Cardiothoracic Surg 2006; 30:96–102. [ Links ]
18. Martínez M: Personal communication. [ Links ]
19. Garrat C J, Antoniou M J, Ward DE, Camm AJ. Use of intravenous adenosine in sinus rhythm as a diagnostic test for latent preexcitation. Am J Cardiol 1990;65:868 –73. [ Links ]
20. Bases farmacológicas de la terapéutica. J G Hardman, L E Limbird, editors. México. McGraw–Hill Interamericana. 10th Ed. Vol I. p 958, Cuadro 35–3. [ Links ]
21. Alunni S, Orrü M, Ottavi L. A study on the inhibition of adenosine deaminase enzyme. J Enzyme InhibMed Chem 2008;23:182–9. [ Links ]
22. DiMarco J P. Electrophysiology of adenosine. J Cardiovasc Electrophysiol 1990; 1:340–8. [ Links ]
23. Haines D P, Lerman BB, DiMarco J P. Intravenous adenosine shortens atrial refractoriness in man. Circulation 1988;78 Suppl 11:153. [ Links ]
24. de Micheli A, Iturralde Torres P, Pastelin G. Adenosina en taquicardias ventriculares inducidas. Communication to XX Congreso Mexicano de Cardiología. Mérida, Yucatán, November 1–5,1997. Arch Inst Cardiol Mex 1997;67 Suppl l:No.224. [ Links ]
25. DiMarco J P, Lerman BB, Haines D F. Effects of adenosine on antegrade conduction in patients with manifest and concealed preexcitation. Abstract. Circulation 1987;76:IV–67. [ Links ]
26. Alegret J M, Vínolas X, Palazón O, Vernls J M, Ferrer A, Oter R. Preexcitación intermitente tras administración de adenosina. Rev Esp Cardiol 2000;53:1132–5. [ Links ]
Abbrevations:
LIV: Left intraventricular lead
RIV: Right intraventricular lead
SVC: Superior vena cava
SR: Sinus rhythm
VT: Ventricular tachycardia
LVT: Left ventricular tachycardia
FL: Atrial flutter
FIBR: Atrial fibrillation
AT: Atrial tachycardia
IN: Inosine
RBBB: Right Bundle Branch Block
AAV: Mean Value














