Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Archivos de cardiología de México
On-line version ISSN 1665-1731Print version ISSN 1405-9940
Arch. Cardiol. Méx. vol.78 n.2 Ciudad de México Apr./Jun. 2008
Investigación clínica
Respiratory and non respiratory oscillations of the skin blood flow: A window to the junction of the sympathetic fibers to the skin blood vessels
Oscilaciones respiratorias y no respiratorias del flujo sanguíneo de la piel: una ventana para estudiar la función de las fibras simpáticas a los vasos de la piel
Bruno Estañol*, Horacio Sentíes–Madrid*, Yolanda Elías***, Plácido Coyac*, Raúl Martínez–Memije**, Óscar Infante**, José Francisco Tellez–Zenteno, Guillermo García–Ramos*
* Laboratory of Clinical Neurophysiology, Department of Neurology and Psychiatry, Instituto Nacional de Ciencias Médicas y Nutrición, Salvador Zubirán, Mexico City (EB, CP, SMH, GRG)
** Department of Medical Instrumentation Instituto Nacional de Cardiología Ignacio Chávez, Mexico City (MMR, IO, LC).
*** Laboratory of Clinical Neurophysiology Instituto Nacional de la Comunicación Humana, Mexico City (EY).
Correspondence:
Bruno Estañol. M.D.
Cerro Chinaco Núm. 139,
Colonia Campestre Churubusco,
Mexico City, 04200.
Tel 55683450, Fax 55688460,
E mail: brunoest@hotmail.com
Recibido: 8 de enero de 2007
Aceptado: 28 de enero de 2008
Abstract
Objective of the study: The skin blood flow (SBF) has been known to oscillate in frequency and amplitude. The nature and type of these oscillations have remained obscure. We studied the oscillations of the SBF in frequency and amplitude with non invasive techniques during normal breathing at rest and compared it to the oscillations during rhythmic paced breathing at 6 cycles per minute.
Subjects and methods: Thirty healthy subjects were studied under normothermic conditions. The following variables were recorded: 1) EKG signal; 2) SBF signal given by an infrared photoplethysmograph; 3) respiratory movements (RM). A correlation of the frequency of the respiration, the SBF and the EKG was made. The variability of the amplitudes of the SBF, RR intervals and pulse intervals was analyzed in the time domain and with spectral analysis using Fourier analysis.
Results: We found no clear respiratory modulation of the amplitude of the SBF during natural breathing at rest. With default breathing there was a low frequency oscillations (LF 0.04 to 0.15 Hz) modulation of the amplitude of the SBF that was non respiratory in nature. During rhythmic breathing at 0.1 Hz there was a strong modulation at LF of the SBF with a typical waxing and waning appearance, decreasing in amplitude during the tachycardia period and increasing in amplitude during the bradycardia period.
Conclusions: Under normothermic conditions there is a consistent variability of the frequency and amplitude of the SBF with normal and rhythmic breathing. While breathing at rest the modulation of SBF amplitude was clearly seen at LF and non respiratory related. With rhythmic breathing there is a strong modulation of amplitude and frequency at the respiratory frequency.
Key words: Skin blood flow. Low frequency oscillations. High frequency oscillations. Respiratory variability. Non respiratory variability.
Resumen
Objetivo: Se conoce que el flujo sanguíneo de la piel (FSP) oscila en frecuencia y amplitud. La naturaleza y tipo de estas oscilaciones han permanecido oscuras. Estudiamos las oscilaciones del FSP en frecuencia y amplitud con técnicas no invasivas durante la respiración en reposo y la comparamos con la oscilación inducida por la respiración rítmica a 6 ciclos por minuto.
Sujetos y métodos: Se estudiaron treinta sujetos sanos bajo condiciones normotérmicas y registramos las siguientes variables. 1) la señal del EKG; 2) la señal del FSP dada por un fotopletismógrafo infrarrojo; 3) los movimientos respiratorios. La variabilidad de las amplitudes del FSP y el intervalo RR fueron analizados en el dominio del tiempo y con análisis espectral.
Resultados: No se observó una modulación clara de la amplitud del FSP durante la respiración en reposo. En esta condición observamos una modulación no respiratoria de la amplitud del FSP en oscilaciones de baja frecuencia (LF). Durante la respiración rítmica se observó una modulación respiratoria muy robusta en 0.1 Hz. La amplitud disminuyó durante los períodos de taquicardia y aumentó durante los períodos de bradicardia.
Conclusiones: Bajo condiciones normotérmicas se observó una variabilidad consistente de la frecuencia y amplitud del FSP durante la respiración en reposo se observó una variabilidad en baja frecuencia y no relacionada con la respiración y con la respiración rítmica se observó una modulación robusta en la amplitud a la frecuencia respiratoria estudiada (0.1Hz).
Palabras clave: Flujo sanguíneo de la piel. Oscilaciones de baja frecuencia. Oscilaciones de alta frecuencia. Variabilidad respiratoria. Variabilidad no respiratoria.
Introduction
The sympathetic nervous system innervates the blood vessels of the skin. The cutaneous blood flow regulates the convective transfer of heat and thereby regulates the body temperature.1 Sympathetic nerves to the extremities travel in the somatic nerves although they originate from the spinal autonomic neurons of the intermediolateral column. Preganglionic fibers to the upper extremities exit in the thoracic ventral roots from T2 to T9 but they may extend from the spinal roots of C8 to T10.2,3 Postganglionic neurons of the sympathetic system are found in the prevertebral and paravertebral ganglia.3 Because sympathetic fibers accompany the somatic nerves the distribution of sympathetic functions corresponds to the somatic motor innervations.2 The efferent pathway of vasomotor skin reflexes travel from the hypothalamus, medulla oblongata and spinal cord via the intermediolateral cell column and sympathetic nerves, to neurovascular synapses and adrenergic receptors. Human digital vasculature contains ∞1 and ∞2 adrenergic receptors. It seems that the latter are more important for vasoconstriction. Vasodilation may result from the withdrawal of sympathetic activity but peptidergic and cholinergic mechanisms and local axon reflexes have also been postulated.4
In 1948 Gilliat5,6 found that a sudden brisk inspiration decreased the skin blood flow of the finger pad for a few seconds. He reasoned that the transient decreased blood flow was due to vasoconstriction of the skin arterioles and probably also of the venules and thought that the response was reflex mediated through a sympathetic discharge to the skin blood vessels. The sympathetic discharge probably traveled through the intermediolateral columns of the spinal cord.3 Delius found that the skin blood flow of the fingers also decreased with the immersion of the other hand in cold water and, therefore, postulated this response as a spinal reflex. Hagbarth7 thought that the decreased skin blood flow was apparently not modulated by baroreceptor reflex action in contrast to the muscle blood flow. The afferent side of the reflex is unknown and it may involve the low pressure cardiopulmonary baroreceptors or the high pressure arterial baroreceptors.7–9 Recent studies using laser Doppler measurements of SBF have shown that during the time of the forced expiration against a closed glottis of the Valsalva maneuver (phases II and III) the skin blood flow decreases.7 This also occurs during the act of standing.7 Microneurographic investigations, using a tungsten electrodes inserted in a peripheral nerve (e.g. the peroneal nerve) have shown that sudden inspiration induces phasic activation of sympathetic skin nerves (C fibers) accompanied simultaneously by cutaneous vasoconstriction.10,11 Surgical sympathectomy abolishes the vasoconstrictor reflexes.12–9 Hence, it is clear that the decrease skin blood flow during deep inspiration depends on the integrity of the efferent sympathetic nerve fibers. Furthermore patients with small fiber neuropathy have decreased skin blood flow, measured with a laser Doppler flowmeter, which indicates that the vasoconstrictor responses are mediated by small sympathetic C fibers.11–13
Since 1939 it has been observed that the skin blood flow (SBF) has some variability but the type and source of this variability has been a source of controversy.14–16 Some authors have argued that the high SBF variability hampers its use as a diagnostic tool.10 It has recently been shown that during spontaneous breathing there is a low frequency (LF) non respiratory variability of the SBF.14–18 We undertook a systematic appraisal of the variability of the SBF during normal natural breathing and compared it to rhythmic respiration at 6 cycles per minute in order to evaluate the respiratory and the non respiratory variability of the SBF. This may be of importance for the use of the SBF as a diagnostic tool for the evaluation of the sympathetic innervation of the skin blood vessels.
Subjects and methods
Subjects
We studied 30 normal subjects without history of peripheral or central neurological disease, 18 females (60%) and 12 males (40%), the age ranged from 18 to 45 years.
Inclusion criteria
No history of vascular or peripheral o central nervous system diseases. The subjects had no coffee, tobacco and had a light breakfast. The studies were performed between 8 and 11 a.m. They did no ingest any medications in particular anticholinergics or antiadrenergics.
Objective
The main objective of the study was to determine if rhythmic oscillations similar to those observed in the heart rate and blood pressure could be observed in the pulsatile skin blood flow during rhythmic breathing and also if non respiratory oscillations in the low frequency could also be observed.
Methods
The subjects were reclined in an armchair. They were asked to be alert to the indications and not to move. The temperature of the fingers was monitored and kept between 30 and 35 degrees Celsius. The room was semidarkened and silenced. The temperature of the room was kept between 25 and 30 degrees Celsius. The temperature of the extremities and the ambient temperature were recorded at the onset of each maneuver. Other confounding factors that were excluded were the veno–arteriolar reflex. This factor was limited by keeping the hand at the heart level during the procedure. As the SBF may also be sensitive to emotional factors14 we maintained the patients relaxed in a dim lighted room without noise.
Measurement of the variability of the SBF with a digital polygraph
The physiological recording had a duration of 30 minutes. We recorded the basal activity during 5 to 10 minutes and at the end of this period we recorded three epochs of 6 respiratory cycles per minute (respiratory frequency 0.1 Hz, 5 seconds of inspiration and 5 seconds of expiration, total duration of each rhythmic breathing 10 seconds) separated each by 5 minutes.
For recordings we used a digital polygraph machine with an A/D card of 16 bits of A/D conversion (Cadwell, Easy EEG VI.5) with a sampling rate of 400 Hz. The sweep speed was adjusted at 2.5 to 10 mm per second but sometimes we also used 20 to 150 mm/s. We measured the following variables: 1) heart rate; 2) respiratory frequency; 3) skin blood flow. The skin blood flow (SBF) was measured with a high resolution infrared photoplethysmograph with a LED in the wavelength band of 640 ± 20 nm and an infrared LED in the band of 960 ±.20 nm. The photoplethysmograph was placed in the pad of the forefinger and the hand was kept at the heart level throughout the study. The signal of the photoplethysmograph was fed into the digital amplifier of the polygraph. The systolic amplitude of the signal was measured in μV. The amplitude was measured before, during and after the maneuvers were performed. The signal was filtered at 75 Hz and we used a time constant of 0.32 seconds as these filters gave the most clear signal free of artifacts. The signal we obtained was of high quality. The impedance was kept below 5 K Ohms. The EKG signal was measured with two electrodes located over the sternum. The respiratory movements were measured with a neumograph adapted with a piezo–electric crystal that was placed in the thoraco–abdominal junction. The time constant was kept at 1 second. We did not measure ambient humidity because the relative humidity in our city is consistently dry during the whole year. As the experiments were done indoors we did not measure wind velocity.
We measured the following variables on the polygraph: 1) respiratory frequencies at rest and during rhythmic respirations in Hz; 2) plethys–mographic signal amplitude changes in μY; 3) EKG and plethysmographic signals changes in RR intervals and pulse intervals in ms. We measured the RR intervals and the amplitude changes of the SBF during three minutes for natural breathing and rhythmic breathing in each subject. The RR intervals and the SBF amplitudes were measured manually at 150 mm/s. The calculated manual measurement error for RR intervals was 2 ms and 3 μV for SBF amplitudes. We measured the RR intervals at high sweep speeds of 150 mm/s.
We correlated the respiratory frequency with the amplitude and frequency of the plethysmograph signal at rest and during rhythmic breathing.
Statistical analysis
We used the Excel and the Statistica software (USA, 1998) to analyze the short term variability in the time domain of the RR and pulse amplitudes of the SBF. The mean RR interval and mean pulse amplitudes, the standard deviations and the coefficients of variation were obtained. We also obtained the index of the maximum RR interval/minimum RR interval, the total duration of the variability (maximum minus minimum RR intervals in ms) and the percent of the variability (total duration of the variability/mean RR interval). We obtained the frequency data using the Fourier program of Statistica using the Hamming window at 5. Then we resampled the data using Excel at a frequency of 0.5 Hz. We also used the Mathlab program to generate the spectral analysis of the RR and amplitude variability of the skin blood flow using the Hamming window at 5. The value considered to be significant in the cross correlation between the amplitude of the SBF and the RR intervals was > 0.5.
Results
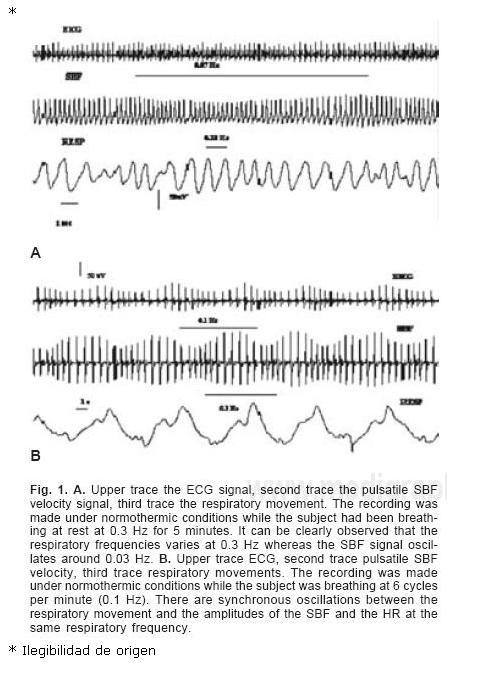
1. Under normothermic condition and during natural breathing at rest in all healthy subjects there was no clear modulation, on visual inspection of the traces, of the amplitude of the SBF by respiration. Most normal people breathed at a frequency of 0.3 Hz (range 0.2–0.4 Hz) (respiratory movements every 2–4 seconds, 12–24 cycles per minute). Using Fourier analysis we found a weak respiratory modulation of the SBF (around 13%). The modulation we found most frequently was at 0.03 ± 0.01 Hz (every 30 seconds). The dissociation between the frequency of the respiratory movements and the amplitude of the oscillations of the SBF is clearly visible in Figure 1. The ratio between respiratory frequencies and SBF amplitude oscillations was 10:1. In Figure 2 it can be clearly observed that the respiratory frequencies varies at 0.3 Hz whereas the SBF signal oscillates around 0.03 Hz.<
Frequency modulation of the RR intervals while breathing at rest was found at HF, LF and VLF frequencies (Fig. 2). Spectral analysis of amplitudes of SBF showed a modulation at LF and predominantly VLF (Fig. 2). The HF peak that is present with the RR interval oscillations was absent in most subjects or highly decreased in the SBF. We used a cospectral density technique to see the cross correlation between the variability of the heart rate and the amplitudes of the skin blood flow and found there was a correlation at LF and VLF frequencies but not at HF frequencies (Fig. 2).
2. Under normothermic condition and during rhythmic breathing at 6 cycles per minute (0.1 Hz of respiratory frequency, every 10 seconds, 5 seconds inspiration, 5 seconds expiration, 10 per minute) in all healthy subjects we found synchronous oscillations in frequencies and amplitudes of the HR and the SBF (Fig. 1). There was a strong modulation of the amplitude and frequency of the SBF by rhythmic breathing at 0.1 Hz that was characterized by a decrease SBF during inspiration and increase SBF during expiration. The decreased SBF coincided with the tachycardia period and the increased SBF with the period of bradycardia (Fig. 1). The photople–tysmographic tracings have a characteristic appearance: waxing and waning in amplitude and frequency synchronous to the respiratory movements (Figs. 1 y 3) and the two have the same frequency of 0.1 Hz. Fourier spectral analysis of RR intervals showed a peak at LF at RR intervals and LF and VLF in SBF amplitudes (Fig. 3). Spectral analysis of amplitudes of SBF showed a modulation at LF at 0.1 Hz and VLF. We used a cospectral density technique to see the cross correlation between the variability of the heart rate and the amplitudes of the skin blood flow and found there was a significant correlation at 0.1 Hz that was the paced respiratory frequency.
Table I shows the analysis in the time domain for RR intervals and SBF amplitudes. The coefficient of variation (CV)( Standard deviation/ mean RR interval) of the RR interval during normal breathing was found to be 6.9, whereas the CV during rhythmic breathing was found at 11.2. A coefficient of variation lower than 10 is usually found to be reliable for a test. The standard deviation (SD) was lower during normal breathing (70.2) as compared with rhythmic breathing (107.3). The total duration of variability and the percent of variability was also lower during normal breathing as compared to rhythmic breathing. This suggests that during rhythmic breathing the variability is higher than at default breathing. The SBF had similar standard deviations and coefficients of variations during natural and rhythmic breathing. The ratio of maximum/minimum amplitudes was higher for the SBF than the RR intervals (1.9 during natural breathing and 1.8 during rhythmic breathing).
Discussion
In 1939 Burton discovered that the SBF had spontaneous fluctuations in frequency and amplitude.17 He attributed them to changes in skin temperature. Since that time the mechanism of these rhythmic fluctuations has been a matter of controversy.15 The oscillations have been postulated to be a consequence of: a) local regulation; b) changes in systemic blood pressure and c) changes in sympathetic outflow to the blood vessels.15–17 Recently Bernardi et al. have demonstrated that during breathing at rest the oscillations of the SBF are mostly in the low frequency (LF; 0.03–0.15 Hz) and very low frequency (0.001 and 0.03 Hz) range and are synchronous to the fluctuations observed in the heart rate (HR), systolic blood pressure (SBP) and muscle sympathetic nerve activity (MSNA).14 The syn–chronicity to these parameters is highly suggestive of a central autonomic control and strongly argues against an important local regulation under normothermic conditions.14 The HF (0.15–0.40 Hz) respiratory component accounts for a small fraction (18 percent) of the regulation of SBF during normal breathing at rest. Cogliati et al. addressed the question of whether the oscillatory profile of the SBF while breathing at rest was secondary to oscillations in arterial pressure or to oscillations in skin sympathetic nerve activity (SSNA). They found that the oscillations were correlated with discharges in SSNA.16 They also found that the discharges in SSNA were synchronous with changes in HR, SBP and MSNA.
The observations of Bernardi et al.14 and Cogliati et al.16 were done in the frequency domain using auto–regression techniques and during spontaneous breathing at rest. We have also observed with non invasive techniques that during spontaneous breathing at rest there is no clear relationship between the respiratory movements and the SBF oscillations of the fingers. During spontaneous breathing at rest the SBF amplitudes oscillates mostly at low frequencies as was described by Bernardi et al.14 in contrast we have observed that during rhythmic breathing at 0.1 Hz there is a strong respiratory modulation of the SBF amplitudes at LF and VLF. The SBF decreases in amplitude during the inspiratory phase and is time locked with the tachycardia. It increases in amplitude during the expiratory phase and is also time locked with the bradycardia. This gives the tracing a typical waxing and waning appearance. These oscillations are similar to those reported to be present in the HR and the SBP at these respiratory frequencies and are also suggestive of a central modulation of the skin blood flow. During breathing at rest HF oscillations time–locked with respirations are observed using spectral analysis although they are small.14,16 It remains for future studies to see if the respiratory drive is effective at other frequencies than the one studied by us. It is most likely that the respiratory modulation will exist at several frequencies.
What are the origins of the oscillations of the SBF? As the peripheral arteries do not have vagal innervation, as the heart does, it is to be expected that the rapid changes observed at the heart level would not be observed in the blood vessels. This is in fact what we found. On the other hand the presence of a LF spectra (between 0.04–0.15 Hz) is quite consistent with vasomotion probably related to baroreceptor activity and also to the intrinsic constricting–dilating properties of the arterial wall.18–19 This is also consistent with the works of Stauss et al. who found that stimulation of the skin sympathetic fibers of the median nerves at 0.075 to 0.10 Hz induced high power peaks of SBF vasoconstriction at those frequencies but not at higher frequencies.20 This suggests that sympathetic modulation of vasomotor tone is strongest in the frequency band centered around 0.1 Hz. They concluded that peripheral sympathetic transmission to the blood vessels behave as a low–pass filter with a cut–off frequency above 0.1 Hz.20 It could also be said that the time constant of the activation of the blood vessels is much longer than the activation of the HF of the heart. Whether the optimal response at this frequency is due to some intrinsic property of the vessel wall, or to the very long conduction time of the sympathetic C fibers or to the properties of the neuro–effector synapses (multiple varicosities) is not clear.20 Wesseling has proposed that the spectral peak at 0.1 Hz is due to «resonance» of the baro–reflex loop.21
It should be of interest to see if the SBF spectra changes with other type of stimuli such as standing. A more prominent peak of the LF spectra would be expected as the sympathetic activity increases while a subject stands (16). The VLF are probably related to temperature changes because the SBF is highly related to temperature control. It is also possible that some of the LF changes be related to temperature control. However more studies are needed in this respect. In any case the oscillations of the amplitude of the SBF do not seem to be just passively transmitted from the heart because in this case the HF changes would appear in the blood vessels. In summary respiratory and non respiratory modulation of SBF amplitude probably reflects central changes while breathing at rest and during rhythmic breathing. During respirations at rest the oscillations appear mostly at non respiratory frequencies (LF and VLF) and during rhythmic breathing the oscillations appear at LF although they are also present at VLF. However with rhythmic breathing the SBF is strongly driven by the respiration. It remains to be studied whether other respiratory frequencies also entrain the SBF. The peak at LF of the SBF is suggestive of baroreceptor action modulated by respiration. The blood vessels contract and dilate relatively slowly. It may take 10 to 20 seconds to contract and dilate.18–19 This is consistent with the findings in this study.
The high variability of the amplitude of the SBF has been considered a deterrent for its use in clinical neurophysiological diagnosis. The precise knowledge of the normal variability in amplitude and frequency of the SBF may allow its use for the diagnosis of autonomic disorders and in particular to the clinical diagnosis of damage to the sympathetic C fibers to the skin blood vessels. It may also serve as a window or aprobé to the function of sympathetic activity to the skin blood vessels. We need a clinical non invasive method to study the innervation of the skin blood vessels. This type of study may prove to be useful in different types of disorders of autonomic innervation of the skin blood vessels including peripheral neuropathies and central autonomic disorders.
References
1. JÄNIG W: Functions of the sympathetic innervation of the skin. In: Lowey AD, Speyer KM, eds: Central Regulation of Autonomic Functions. Oxford: Oxford University Press; 1990:334–348. [ Links ]
2. BRODAL A: Neurological Anatomy. 3rd Ed Oxford: Oxford University Press; 1981:698–787. [ Links ]
3. PICK J: The Autonomic Nervous System. Philadelphia: JB Lippincott; 1970:351–358. [ Links ]
4. KHURANA R: Acral sympathetic dysfunction and hyperhydrosis. In: Clinical Autonomic Disorders 2nd ed. P.A. Low editor. Philadelphia. 1997:809–818. [ Links ]
5. GILLIAT RW: Vasoconstriction in the finger after a deep inspiration. J Physiol (London) 1948;107:70–88. [ Links ]
6. GILLIAT RW: Inspiratory vasoconstriction in patients after spinal injuries. J Physiol (London) 1948;107:67–69. [ Links ]
7. HAGBARTH KE, HALLIN RG, HONGELL A, TOREBJORK HE, WALLIN BG: General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand 1972;84:16–176. [ Links ]
8. DELIUS DW, HAGBARTH KE, HONGELI A, WALLIN BG:Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand 1972c;84:82–94. [ Links ]
9. HALLIN RG, TOREBJORK HE: Single unit sympathetic activity in human skin nerves during rest and various manoeuvres. Acta Physiol Scand 1974;92:303–317. [ Links ]
10. LOW PA, NEUMANN C, DYCK PJ, FEALEY CD, TUCK RR: Evaluation of skin vasomotor reflexes by using laser Doppler velocimetry. Mayo Clin Proc 1983;14:573–580. [ Links ]
11. WALLIN BG, FAGIÜS J: Peripheral sympathetic neural activity in conscious humans. Annual Rev Physiol 1988M;50:565–576. [ Links ]
12. BARON R, MAIER C: Reflex sympathetic dystrophy: skin blood flow, sympathetic vasoconstrictor reflexes and pain before and after surgical sympathectomy. Pain 1996;67:317–326. [ Links ]
13. SCHÜLLER TB, HERMANN K, BARON K: Quantitative assessment and correlation of sympathetic, para–sympathetic, and afferent small fiber function in peripheral neuropathy. J Neurol 2000;247:267–272. [ Links ]
14. BERNARDI L, HAYOS D, WENZEL R, PASSINO C, CALCIATTI A, WEBER R, NOLL G: Synchronous and baroreceptor–sensitive oscillations in skin microcirculation: evidence for central autonomic control. AJP–(Heart and Circulatory Physiology) 1997;274:H1867–H1878. [ Links ]
15. BINI G, HAGBARTH KE, WALLLIN BG: Cardiac rhythmicity of skin sympathetic activity recorded from peripheral nerves in man. J Auton Nerv Syst 1981;4:17–24. [ Links ]
16. COGLIATI C, MAGATELLI R, MONTANO N, NARKIEWICZK, SOMMERS V: Detection of low and high frequency rhythms in the variability of skin sympathetic nerve activity. AJP–(Heart and Circulatory Physiology) 2000;278:H1256–H1260. [ Links ]
17. BURTON AC: The range and variability of the blood flow in the human fingers and the vasomotor regulation of body temperature. Am J Physiol 1939;127:437–453. [ Links ]
18. MALLIANI A, PAGANI M, LOMBARDI F, CERUTTI F: Cardiovascular neural regulation explored in the frequency domain. Circulation 1991;84:482–492. [ Links ]
19. MANCIA G, PARATI G, DI RIENZO M, ZANCHETTI A: Blood pressure variability. In: Handbook of hypertension. (Eds. Zanchetti A, Mancia G). Vol. 17, 1997,pp 117–169, Elsevier Science. [ Links ]
20. STAUSS HM, ANDERSON EA, HAYNES WG, KREGEL KC: Frequency response characteristics of sympathetically mediated vasomotor waves in humans. Am J Physiol Heart Ore Physiol 1998;274:H1277–H1238. [ Links ]
21. WESSELING KH, SETTELS JJ: Baromodulation explains short term blood pressure variability. In: Orlebeke TF, Mudder G, Van Doochen JJP: Psychophysiology of Cardiovascular control. Model, methods and data, Editors. Plenum, New York, 69–97. [ Links ]














