Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Archivos de cardiología de México
On-line version ISSN 1665-1731Print version ISSN 1405-9940
Arch. Cardiol. Méx. vol.76 n.4 Ciudad de México Oct./Dec. 2006
Investigación clínica
Clinical variables related with in–stent restenosis late regression after bare metal coronary stenting
Regresión tardía de la estenosis intrastent
Alejandro Alcocer,* Raúl Moreno,* Rosana Hernández,* María–José Pérez–Vizcayno,* César Conde,* Fernando Alfonso,* Manel Sabaté,* Javier Escaned,* Camino Bañuelos,* Luis Azcona,* Carlos Macaya*
* From the Division of Interventional Cardiology, Cardiovascular Institute. Hospital Clínico San Carlos, Madrid, Spain.
Correspondence to:
Alejandro Alcocer Chauvet.
Cir. Fuentes del Pedregal Num. 789, Col. Fuentes del Pedregal Tlalpan,
México, D,F.
14140. Tel. 56521910
E mail: chovei@lycos.com
Recibido: 8 de junio de 2005
Aceptado: 24 de agosto de 2006
Summary
In–stent restenosis (ISR) has an incidence between 20% and 30% using bare metal stents. ISR late regression phenomenon (ISRLR) has been previously described, but clinical variables related with this phenomenon remain unclear. The aim of the study was to identify the variables related with ISRLR.
Methods: We identified from our data base 30 patients between November 1995 and September 2002 that fulfilled the following criteria: 1) Documented ISR at follow–up angiography (CA–1); 2) treated medically; and 3) Referred for a second follow–up angiography (CA–2) at least 3 months after CA–1. ISRLR was defined as a > 0.2 mm increase in MLD between CA–1 and CA–2, calculated as the 2–fold of our inter–observer variability. ISR late progression was defined as a > 0.2 mm decrease in minimum lumen diameter (MLD) between CA–1 and CA–2.
Results: At the time of CA–2 only 2 patients (6.7%) had symptoms related with the previously stented vessel. We found a mean MLD of 1.03 ± 0.34 mm and 1.54 ± 0.48 mm at CA–1 and CA–2 respectively (ΔMLD = 0.51 ± 0.34 mm; p < 0.001). Twenty four patients (80.0%) had ISRLR. Two variables were related to the presence or absence ISRLR: Current smoking at the time of coronary stenting (70.8% vs 20.0% respectively, p = 0.026) and acute coronary syndrome as clinical indication for coronary stenting (and 83.5% vs 40.0% respectively, p = 0.029).
Conclusion: ISRLR is afrequent phenomenon in patients with ISR treated medically, probably contributing to the benign long–term clinical outcome that has been previously described in patients with asymptomatic or mildly symptomatic ISR. Current smoking at the time of coronary stenting and acute coronary syndrome as clinical indication for coronary stenting are associated with this phenomenon.
Key words: Coronary stenting. In–stent restenosis. Late regression.
Resumen
La reestenosis intrastent (RIS) tiene una incidencia del 20 al 30% cuando se utilizan stents convencionales. El fenómeno de regresión tardía de (RTRIS) ha sido descrito previamente, pero no se han identificado variables relacionadas con dicho fenómeno. El objetivo del estudio fue identificar variables relacionadas con la RTRIS.
Métodos: Identificamos en nuestra base de datos 30 pacientes con los siguientes criterios: 1) RIS identificada durante el seguimiento angiográfico (AC–1); 2) sujetos que permanecieron con tratamiento médico; 3) referidos para una angiografía de seguimiento (AC–2) al menos 3 meses posteriores a la AC–1. RTRIS fue definida como un incremento del diámetro lumi–nal mínimo (DLM) > 0.2 mm entre la AC–1 y AC–2, calculado a partir del doble del valor de nuestra variabilidad interobservador. La progresión tardía de la RIS, se definió como un decremento en el DLM 0.2 mm entre AC–1 y AC–2.
Resultados: Al momento de la AC–2 sólo 2 pacientes (6.7%) tenían síntomas relacionados con el vaso blanco del tratamiento. Encontramos una media de DLM de 1.03 ± 0.34 mm y 1.54 ± 0.48 mm en AC–1 y AC–2 respectivamente (p < 0.001; ΔDLM = 0.51 ± 0.34 mm). Del total de 30 pacientes, 24 (80.0%) tenían RTRIS. Existieron dos variables relacionadas con la presencia o ausencia de RTRIS: Fumar concordantemente con el tiempo de la implantación del stent (70.8% vs 20.0% respectivamente, p = 0.026) y la existencia de un síndrome coronario agudo (SICA) como indicación para el tratamiento inicial (83.5% vs 40.0% respectivamente, p = 0.029).
Conclusión: La RTRIS es un fenómeno frecuente en pacientes con RIS que permanecen con tratamiento médico, hecho que probablemente contribuye con la evolución clínica benigna que ha sido previamente descrita, en pacientes con RIS asintomáticos o con escasa sintomatología. Sujetos fumadores al momento de la implantación del stent así como SICA como indicación al tratamiento inicial, son situaciones asociadas con la presentación de RTRIS.
Palabras clave: Stent convencional. Reestenosis intra–stent. Regresión tardía.
Introduction
In–stent restenosis (ISR) is still the Achilles heel of the coronary intervention, with an estimated incidence between 20% and 30% of the procedures using bare metal stents (BMS);1,2 mainly within 3 to 6 months after the stent implantation.3,4 ISR late regression (ISRLR) is a previously described phenomenon.5,6 The mechanisms involved, however, are not fully understood. Kimura et al7 demonstrated a late improvement in the luminal diameter after Palmaz–Schatz coronary stenting, with an increase in minimum lumen diameter after 6 months and 3 years following stent implantation. Hermiller et al8 also observed ISR regression after 3 years using Gianturco Roubin stents. More recently Miereles et al,9 demonstrated that using thick strut stent in de novo lesions, resulted in significantly increased MLD from 6 to 12 months; and also observed that binary restenosis were reduced from 17% at 6 vs 11% at 12 months although non statistically significant. In contrary Sadamatsu K et al.10 demonstrated that older age, diabetes mellitus, hyperlipidemia, smoking and small stent diameter (< 3.0 mm) were associated with late luminal loss beyond 6 months after implantation of thicker strut stents.
Although we are in era of drug eluting stents11 and intracoronary brachytherapy,12 there are still many patients treated with BMS with ISR, and thus knowledge about factors influencing ISRLR remains an issue of clinical relevance.
The aim of this study was to elucidate which clinical and angiographic variables are related with ISRLR.
Methods
Study population
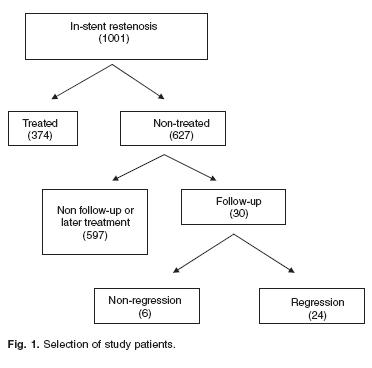
Out of 1,001 patients with angiographically documented ISR (stenosis severity > 50%) at follow–up angiography (CA–1: 6.0 ± 2.3 months after stent implantation), we selected from our data base 30 cases that fulfilled the following two additional inclusion criteria: 1) Treated medically; 2) Referred for a second follow–up angiography > 3 months after CA–1 (CA–2: 24.8 ± 21.6 months after stent implantation). Figure 1 shows the flow–chat for the selection of the patients.
Stenting procedure13
Cardiac catheterization was performed by femoral approach in most patients. All of them were pre–treated with aspirin, and heparin was administered as an intra–coronary bolus at the beginning of the procedure, and additional boluses were given when necessary to maintain an activated clotting time > 300 seconds (200–250 seconds when Ilb/IIIa inhibitors were administered). Provisional or direct stenting was performed at the discretion of the operator, and balloon–to–artery ratio was 1.0–1.1/1.
Coronary angiography analysis
Quantitative coronary angiographic analysis was performed using a validated, automatic edge–detection algorithm (MEDIS, CMS 4.0, Leiden, the Netherlands).14 Similar angiographic views demonstrating maximal stenosis were reviewed for the same vessel segment in CAI and CA2 studies. The minimal lumen diameter (MLD) was defined angiographically as the minimal vessel diameter within the stent. ΔMLD was defined as the difference between MLD at CA2 and CAI. ASP was calculated as the difference between in–stent stenosis percentage (SP) at CA–2 and CA–1.
Two different, technically trained investigators analyzed the 2 angiograms of each individual patient, blinded to the order of follow–up cine–angiograms (CA–1 or CA–2). The inter–observer variability was 0.1 ± 0.11 mm and 4.23 ± 4.27% in measuring MLD and SP respectively. ISRLR was defined as a > 0.2 mm increase in MLD between CA–1 and CA–2, which was calculated as the 2–fold of the inter–observer variability. ISR late progression was defined as a > 0.2 mm decrease in MLD between CA–1 and CA–2.
Statistical analysis
Statistical analysis was performed using the SPSS package, version 10.0 (Chicago, Illinois, USA). Continuous variables were presented as mean ± standard deviation, and discrete variables as proportions (percentages). Student's t, Pearson's chisquare, and Fisher's exact test were performed as indicated. A p value < 0.05 was considered statistically significant.
Results
Baseline clinical and angiographic characteristics
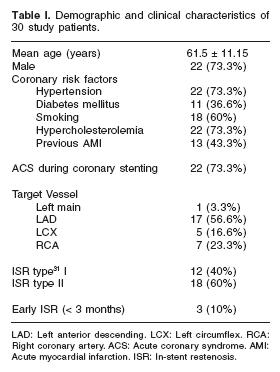
Baseline characteristics are presented in Table I. Mean age was 61.5 ± 11.2 years, and 73.3% of patients were male gender. The indication for coronary stenting was acute coronary syndrome in 22 (73.3%), and chronic ischaemic heart disease in 8 (26.7%) patients. Only 3 patients (10%) had an early (< 3 months since stent implantation) ISR.
Elective, bailout and direct stenting was performed in 21 (70.0%), 6 (20.0%), and 3 (10.0%) patients, respectively. Mean stent diameter was 3.1 ± 0.5 mm and mean stent length was 21.2 ± 8.8 mm. Relatively high pressures were used (12.6 ± 3.1 ami). The type of stent was Multi–Link in 7 (23.3%), NIR in 7 (23.3%), Velocity in 6 (20.0%), and other types in 10 (33.3%) patients, therefore the great majority of stents used were thick strut devices (> 100 µm: Multi–Link, NIR and Velocity).
At the time of CA–1, 11 patients (36.7%) were symptomatic, and 19 (63.3%) asymptomatic. At the time of CA–2, most patients (n = 28; 93.3%) were symptomatic, although only in 2 cases symptoms were related with the previously stented segment: one of them had unstable angina due to ISR late progression and was referred for coronary artery bypass grafting, and the other had stable angina and was treated medically.
Long–term angiographic outcome
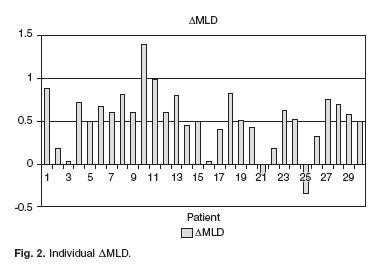
Mean time from CA–1 to CA–2 was 18.7 ± 20.8 months (range 3.2–113.4). Mean MLD was 1.03 ± 0.34 mm and 1.54 ± 0.48 mm at CA–1 and CA–2, respectively (p < 0.001; AMLD = 0.51 ± 0.34 mm). Mean Stenosis percentage was 59.1 ± 5.8% and 37.7 ± 16.0% at CA–1 and CA–2, respectively (p < 0.001; ASP = 21.4 ± 15.5%). Mean reference vessel diameter did not vary significantly between CA–1 and CA–2 (2.6 ± 0.8, and 2.5 ± 0.7 mm respectively, p = 0.49). Twenty–four patients (80.0%) had ISRLR, whereas ISR late progression occurred in one patient (3.3%). In the remaining 5 patients (16.7%), there was neither ISRLR nor ISR late progression. At CA–2, only 4 patients (13.3%) had > 50% SP. The individual AMLD are displayed in Figure 2.
Characteristics associated with ISRLR (Table II)
For patients with ISRLR, smoking at the time of stent implantation is related to ISRLR (70.8% vs 20.0%, p = 0.026).
Acute coronary syndrome as indication for coronary stenting was also related with ISRLR (83.5% vs 40.0%, p = 0.029).
Discussion
Late regression of ISR.
ISRLR is a well–documented phenomenon, although there is still debate whether it is a constant or a random finding in the evolution of ISR. The underlying mechanisms of ISRLR are not clear. According to the study by Asakura et al,15 neointimal thickening and subsequent thinning may be the main responsible mechanisms. Other groups have proposed the absence of continuous vascular injury during the time (which initially explains the relatively early hyperplastic response after the stent delivery), as the mechanism of neointima late remodelling.5 Kimura et al16 demonstrated late improvement in the lumi–nal diameter in patients who received Palmaz–Schatz stents. They found that MLD was significantly larger at 3 years in comparison with that after 6 months of stent implantation, and proposed a mechanism of fibrotic maturation of intimal hyperplasia similar to wound healing.
Our results show that ISRLR may occur in a high proportion of patients with ISR that are treated conservatively. In our study, ISRLR was documented in 80% of patients, and 86.6% of patients had a < 50% SP at long–term follow–up. This proportion is high, although not out of the range found in previous studies: Mehta et al5 reported an ISRLR of 78% in their study population. However, due to the selection criteria, patients with ISR that comprised our study population, as also occurred in other similar studies, are within the range of less severe ISR (–60% by quantitative coronary analysis) and more benign clinical situation. In patients with more severe ISR the proportion of patients with ISRLR could be lower.
Current smoking at the time of coronary stenting was significantly associated with higher frequency of ISRLR. Historically, controversy has existed regarding the relation of smoking and ISR, since Arora et al17 found non–significantly lower restenosis rates in patients who smoked at the time of percutaneous coronary intervention. Melkerk et al18 showed that a history of cigarette smoking was aprotective factor of restenosis at six months. Kotakami et al19 also observed a lower restenosis rate in current smokers, although they were younger and had less severe lesions than non–smokers. On the contrary, Galan et al20 found higher restenosis rates in patients who continued smoking in comparison with that in those who ceased smoking. Finally, Violaris et al21 found no significant differences in the rate of ISR at 6 months among current smokers, ex–smokers and non–smokers. We have not found any previous study relating smoking with ISRLR.
Another relevant finding of our study was the higher frequency of ISRLR among patients that presented with an acute coronary syndrome at the time of coronary stenting. Several studies have demonstrated high rates of restenosis in patients with unstable ischemic heart disease.22–24 In fact we found a high percentage (73%) of patients with acute coronary syndrome at the time of coronary stenting in our population of patients with ISR. To the best of our knowledge, there is no previously published information showing an association between ISRLR and the clinical situation at the time of coronary stenting. A retarded apoptosis phenomena could be involved.25,26
Conclusion
There seems to exist consensus about the benign prognosis of asymptomatic restenosis, both after balloon angioplasty27,28 and after coronary stent implantation.29,30 The phenomenon of ISRLR probably contributes to the benign long–term clinical outcome of asymptomatic patients with ISR that are managed conservatively.
According to our results, conservative management could be considered in asymptomatic or mildly symptomatic patients with ISR, especially in those who were smokers and those who presented with an acute coronary syndrome at the time of coronary stenting.
Nevertheless conclusions of the study are limited by its retrospective nature and short sample size, therefore we can only make hypothesis regarding probably related clinical variables and treatment of ISRLR.
References
1. Serruys PW, de Jaegere P, Kiemened F, Macaya C, Rutsch W, Heyndrickz G, et al: A comparison of balloon–expandable–stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med 1994; 331: (8): 489–495. [ Links ]
2. Moreno R, Fernandez C, Alfonso F, Hernandez RA, Perez–Vizcaino MJ, Escaned J, et al: Coronary stenting versus balloon angioplasty in small vessels: a meta–analysis from 11 randomized studies. J Am Coll Cardiol 2004; 43(11): 1964–1972. [ Links ]
3. Serruys PW, Luijten HE, Beatt KJ, Geuskens R, de Feyter PJ, van den Bran M, et al: Incidence of restenosis after successful coronary angioplasty: a time related phenomenon. A quantitative angiographic study in 342 consecutive patients at 1, 2, 3 and 4 months. Circulation 1988; 77: 361–371. [ Links ]
4. Nobuyoshi M, Kimura T, Nosaka H, Mioka S, Ueno K, Yokoi H, et al: Restenosis after successful percutaneous transluminal coronary angioplasty: serial angiographic follow up of 229 patients. J Am Coll Cardiol 1988; 12: 616–623. [ Links ]
5. Mehta VY, Jorgensen MB, Raizner AE, Wolde–Tsadik G, Mhrer PR, Mansukhani P: Spontaneous Regression of Restenosis: An Angiographic Study. J Am Coll Cardiol 1995; 26: 696–702. [ Links ]
6. Ormiston JA, Stewart FM, Roche HG, Webber BJ, Whitlock RM, Webster MW: Late Regression of the Dilated Site After Coronary Angioplasty: A 5–year Quantitative Angiographic study. Circulation 1997; 96: 468–474. [ Links ]
7. Kimura T, Yokoi H, Yoshihisa N, Tamura T, Kaburagi S, Sawada Y, et al : Three year follow–up after implantation of metallic coronary artery stents. N Engl J Med 1996; 334: 561–566. [ Links ]
8. Hermiller JB, Fry ET, Peters TF, Orr CM, Van Tassel J, Waller B: Late coronary artery stenosis regression within the Gianturco Roubin intra–coronary stent. Am J Cardiol 1996; 77: 247–251. [ Links ]
9. Meireles GC, Lemos PA, Ambrose JA, Ribeiro E, Horta PE, Perin M, et al: Luminal recovery from six to twelve months after implantation of "thicker strut" coronary stents. Am J Cardiol 2004; 93(2): 210–213. [ Links ]
10. Sadamatsu K, Tashiro H, Tanaka E, Yamamoto K: Clinical and angiographic predictors of luminar changes beyond 6 months alter implantation of thicker strut coronary stents. Circ J 2005; 69(1): 35–38. [ Links ]
11. Morice MC, Serruys P, Sousa J, Fajadet J, Ban Hayashi E, Perin M et al: A randomized comparison of a sirolimus– eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002; 346(23): 1773–1780. [ Links ]
12. Waksman R, Raizner A, Yeung AC, Lanski AJ, Vandertier L: Use of localized intracoronary bd radiation in the treatment of in–stent restenosis: the INHIBIT randomized controlled trial. Lancet 2002; 359: 551–557. [ Links ]
13. Alfonso F, Hernandez R, Goicolea J, Segovia J, Perez–Vizcayno MJ, Banuelos C, et al: Coronary stenting for acute coronary dissection after coronary angioplasty: implications of residual. J Am Coll Cardiol 1994; 24: 989–995. [ Links ]
14. Keane D, Haase J, Slager CJ, Mountanban van Swinjdregt, Lehmann KG, Ozaki, et al: Comparative validation of quantitative coronary angiography systems. Results and implications from a multicenter study using a standardized approach. Circulation 1995; 91: 2174–2183. [ Links ]
15. Asakura M, Ueda Y, Nanto S, Hirayama A, Adachi T, Kitakaze M, et al: Remodeling of In–stent Neointima, Which Became Thinner and Transparent Over 3 Years. Serial Angiographic and Angioscopic Follow–up. Circulation 1998; 97: 2003–2006. [ Links ]
16. Kimura T, Yokoi H, Yoshihisa N, Tamura T, Kaburagi S, Sawada Y, et al : Three y ear follow–up after implantation of metallic coronary artery stents. N Engl J Med 1996; 334: 561–566. [ Links ]
17. Arora RR, Konrad K, Badhwar K, Hollman J: Restenosis after transluminal coronary angioplasty: a risk factor analysis. Catheterization and Cardiovascular Diagnosis 1990; 19: 17–22. [ Links ]
18. Melkert R, Violaris AG, Serruys PW: Luminal narrowing after percutaneous transluminal coronary angioplasty. A multivariate analysis of clinical, procedural and lesion related factors affecting long term angiographic outcome in the PARK study. J Invasive Cardiology 1994; 6: 160–171. [ Links ]
19. Kotamaki M, Laustiola K, Syvanne M, Heikkila J: Influence of continued smoking and some biological risk factors on restenosis after percutaneous transluminal coronary angioplasty. J Intern Med 1996; 240: 293–301. [ Links ]
20. Galan KM, Deligonul U, Morton MJ, Chaitman BR, Vandormael MJ: Increased frequency of restenosis in patients continuing to smoke cigarettes after percutaneous transluminal coronary angioplasty. Am J Cardiol 1988; 61: 260–263. [ Links ]
21. Violaris AG, Thury A, Regar E, Melker R, Serruys PW: Influence of a history of smoking on short term (six month) clinical and angiographic outcome after successful coronary angioplasty. Heart 2000; 84: 299–306. [ Links ]
22. Lemgruber PP, Roubin G, Hollman J, Cotsonis GA, Meir B, Douglas JS, et al: Restenosis after successful coronary angioplasty in patients with single vessel disease. Circulation 1986; 73: 710–717. [ Links ]
23. Thus Plokker HW, Ernst SM, Bal ET, Peeremboom PJ, Mast EJ, van den Berg EG, et al: Percutaneous transluminal coronary angioplasty in patients with unstable angina pectoris refractory to medical therapy: long–term clinical and angiographic results. Cathet Cardiovasc Diagn 1988; 14: 15–18. [ Links ]
24. De Groote P, Bauters Ch, McFadden E, Lablanch JM, Leroy F, Bertrand ME: Local Lesion–Related Factors and Restenosis After Coronary Angioplasty: Evidence From a Quantitative Angiographic Study in Patients UIT Unstable Angina Undergoing Double–Vessel Angioplasty. Circulation 1995; 91: 968–972. [ Links ]
25. Isner JM, Kearney M, Bortman S, Passeri J: Apoptosis in human atherosclerosis and restenosis. Circulation 1995; 91: 2703–2711. [ Links ]
26. Bauriedel G, Schluckebier S, Hutter R, Welsch U, Kandolf R, Luderitz B, et al: Apoptosis in restenosis versus stable–angina atherosclerosis: implications for the pathogenesis of restenosis. Arterioscler Thromb Vasc Biol 1998; 18: 1132–1139. [ Links ]
27. Hernández RA, Macaya C, Iñiguez A, Alfonso F, Goicolea J, Fernandez–Ortiz A, et al: Midterm outcome of patients with asymptomatic restenosis after coronary balloon angioplasty. J Am Coll Cardiol 1992; 19: 1402–1409 [ Links ]
28. Chenu PC, Schroeder E, Krémer R, Marhandise B: Long–term outcome of patients with asymptomatic restenosis after percutaneous transluminal coronary angioplasty. Am J Cardiol 1993; 72: 1209–1211. [ Links ]
29. Ruygrok PN, Webster MW, Valk V, Van Es GA, Ormiston JA, Morel MA, et al: Clinical and angiographic factors associated with asymptomatic restenosis after percutaneous coronary intervention. Circulation 2001; 104: 2289–2294. [ Links ]
30. Lee JH, Lee CW, Park SW, Hong MK, Kim JJ, Rhee KS, et al: Long–term follow–up after deferring angioplasty in asymptomatic patients with moderate non critical in–stent restenosis. Clin Cardiol 2001; 24: 551–555. [ Links ]
31. Mehran R, Dangas G, Abizaid AS, Mintz GS, Lansky AJ, Satler LF, et al: Angiographic patterns of in–stent restenosis: classification and implications for long–term outcome. Circulation 1999; 100:1872–1878. [ Links ]














