Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Archivos de cardiología de México
On-line version ISSN 1665-1731Print version ISSN 1405-9940
Arch. Cardiol. Méx. vol.76 n.4 Ciudad de México Oct./Dec. 2006
Investigación clínica
Impaired myocardial perfusion score and inflammatory markers in patients undergoing primary angioplasty for acute myocardial infarction
Alteraciones de la perfusión miocárdica y marcadores de inflamación en pacientes con infarto agudo al miocardio tratados con angioplastía primaria
J Emilio Exaire,* Robert B Fathi,** Sorin J Brener,** Juhana Karha,** Stephen G Ellis,** Deepak L Bhatt**
1 Instituto Nacional de Cardiología, Mexico City, Mexico.
2 The Department of Cardiovascular Medicine, Cleveland Clinic Foundation, Cleveland, OH.
Correspondence to:
Dr. J. Emilio Exaire.
Adjunto Departamento de Hemodinámica y Cardiología Intervencionista.
Instituto Nacional de Cardiología "Ignacio Chávez". (INCIH, Juan Badiano Núm. 1 Col. Sección XVI, Tlalpan
14080 México, D.F.)
E–mail: jeexaire@yahoo.com
Recibido: 15 de junio de 2005
Aceptado: 27 de Julio de 2006
Summary
Background: Microcirculatory dysfunction during acute myocardial infarction is mediated by various mechanisms including inflammation, thrombus, or plaque embolization. We hypothesize that patients with acute myocardial infarction and admission Thrombolysis in Myocardial Infarction (TlMl) myocardial perfusión grade (TMP) < 2 had increased inflammatory status as measured by high sensitivity C–reactive protein (hs–CRP).
Methods: From January 2002 to December 2003, 166 patients (178 lesions) were referred for primary percutaneous coronary intervention. Patients were stratified based on pre–PCI TMP < 2 or TMP 3 2. Univariate and multivariate predictors of in–hospital and 30–day death were determined with logistic regression.
Results: Pre–PCI TMP < 2 was found in 66% vs 34% with TMP 3 2 (P < .001). Hs–CRP levels were high in both groups but not significantly different (37.9 ± 6 vs 33.7 ± 6 mg/L, P = .63). Patients with TMP < 2 had higher WBC (12.83 ± 4.55 * 103 vs 10.83 ± 3.00 * 103, P = .04), lower ejection fraction (40 ± 11% vs 46 ± 12%, P < .001), and higher admission CK–MB levels (116 ± 13 ng/mL vs 55 ± 13 ng/mL, P = .006). Death occurred in 12% in the poor TMP group vs 1.8% in the good TMP group (P = .03). Advanced age, use of an intra–aortic balloon pump, and elevated admission WBC were independently associated with in–hospital and 30–day death.
Conclusions: High hs–CRP levels were not associated with impaired myocardial perfusión score. Microcirculatory impairment may be related to an increased inflammatory process, independent from high hs–CRP levels.
Key words: Percutaneous intervention. Myocardial infarction. Inflammation.
Resumen
Objetivos: La disfunción de la microcirculación coronaria durante el infarto agudo al miocardio es mediada por varios mecanismos incluyendo inflamación y embolización de placa y/o trombo. La hipótesis del presente estudio es que los pacientes con infarto agudo al miocardio que se presentan con niveles bajos de perfusión microcirculatoria (definidos como grado de perfusión Thrombolysis in Myocardial Infarction (TIMI) (TMP) < 2) tienen un aumento en los marcadores inflamatorios, tales como proteína C–reactiva de alta sensibilidad (hs–CRP) y glóbulos blancos, y la correlación de estos niveles con la mortalidad de esta cohorte.
Métodos: De enero de 2002 a diciembre de 2003, 166 pacientes (178 lesiones) fueron referidos para intervención percutánea. Los pacientes fueron estratificados para este análisis en TMP < 2 o TMP 3 2. Los factores asociados a mortalidad intrahospitalaria fueron determinados con análisis de regresión logística.
Resultados: Un TMP < 2 preintervención fue encontrado en 66% vs 34% de los pacientes (P < .001). Los niveles de hs–CRP se encontraron elevados en ambos grupos pero no significativamente diferentes (37.9 ± 6 vs 33.7 ± 6 mg/L, P = 0.63). Los pacientes con TMP < 2 tuvieron cuentas de glóbulos blancos mayores (12.83 ± 4.55 * 103 vs 10.83 ± 3.00 * 103, P = .04), menor fracción de expulsión (40 ±11% vs 46 ± 12%, P < .001), y mayores niveles de CK–MB a la admisión (116 ± 13 ng/mL vs 55 ± 13 ng/mL, P = .006). La muerte ocurrió en 12% del grupo TMP <2 vs 1.8% en el grupo con TMP > 2 (P = .03). La edad avanzada, el uso de balón de contrapulsación y los niveles elevados de glóbulos blancos al momento de la admisión se relacionaron independientemente con la muerte intrahospitalaria y a 30 días.
Conclusiones: Los niveles elevados de hs–CRP no se asociaron con disfunción de la microcirculación. La disfunción microcirculatoria puede estar relacionada a un proceso inflamatorio, independiente de los niveles elevados de hs–CRP.
Palabras clave: Intervención percutánea. Infarto del miocardio. Inflamación.
Inflammation plays a central role in cardiovascular disease. Elevated levels of C–reac–tive protein and white blood cell count (WBC) have been unequivocally associated with adverse outcome in patients with stable and unstable coronary syndromes as well as stroke.1,17 However, the impact of an increased inflammatory milieu, such as acute myocardial infarction (AMI),4,18–30 on a microcirculation surrogate such as Thrombolysis in Myocardial Infarction (TIMI) perfusión (TMP) grade has not been entirely elucidated.31–34 Thus, we sought to analyze the potential role of inflammation markers on the microcirculation, as measured by admission TMP, as well as its impact on post–percutaneous coronary intervention (PCI) short–term mortality.
Methods
From January 2002 to December 2003, 166 patients (178 lesions) were referred to the Cleveland Clinic Foundation for primary PCI. Demographic and clinical information, procedural technique, and major adverse cardiac events (MACE) (including death or re–infarction during hospitalization and 30–day follow–up), pre–and post–procedural creatine kinase (CK), and CK–MB, high sensitivity CRP levels (hs–CRP), and WBC were recorded prospectively. All patients had clinical and electrocardiographic criteria of acute ST–segment elevation MI. All patients received aspirin, loading doses of Clopidogrel (300 mg) and 75 mg thereafter, and GP Ilb/IIIa inhibitors (abciximab 88%; other 12%). Univariate and multivariate predictors of in–house and 30–day death were determined with logistic regression.
Two independent experienced blinded operators assessed the pre– and post–PCI TMP score according to the established TIMI grading system34,35 obtaining an interobserver correlation of 98%. TMP 0 was defined as failure of dye to enter the microvasculature (no ground–glass appearance). TMP 1 was defined as dye slowly entering the microvasculature and failing to exit. TMP 2 was defined as delayed entry and/or exit from the microvasculature (persisting after 3 cardiac cycles). TMP 3 was defined as normal entry and exit of dye from the microvasculature. Patients were stratified based on pre–PCI TMP < 2 orpre–PCITMP > 2.
Continuous variables were compared using Student's t test if normally distributed and Wilcox–on rank–sum if not. Binary variables were compared using 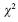 with normal approximation or Fisher's exact test when appropriate. A 2–tailed P value of 0.05 was considered significant. In order to analyze the variables associated with post–PCI death a stepwise logistic regression model was performed.
with normal approximation or Fisher's exact test when appropriate. A 2–tailed P value of 0.05 was considered significant. In order to analyze the variables associated with post–PCI death a stepwise logistic regression model was performed.
Results
Both groups were similar regarding most admission characteristics (Table I). However, patients with pre–PCI TMP < 2 had higher WBC (12.83 ± 4.55 * l0–3 vs 10.83 ± 3.00 * l0"3, P = .04), lower ejection fraction as measured by echocardiography (40 ± 11% vs 46 ± 12%, P = .001), and higher CK–MB levels (116 ± 13 ng/mL vs 55 ± 13 ng/mL, P = .006). Hs–CRP levels were high in both groups but not statistically different (37.9 ± 6 vs 33.7 ± 6 mg/ L, P = .63). Death occurred in 12% (N = 13) in the pre–PCI TMP < 2 group vs 1.8% (N = 1) in the pre–PCI TMP > 2 group (P = 0.03) (Fig. 1).
Univariate predictors of death included advanced age (P = .005), admission Troponin T (P = .04), final visual TDVII flow (P < .001), Admission TMP grade (P < .001), post–PCI TMP grade (P < .001), glucose levels (P = .002), WBC (P < .001), left ventricular ejection fraction (P < .001), peripheral vascular disease (P < .001), systolic blood pressure (P = .01), left anterior descending artery territory AMI (P = .01), and use of intraaortic balloon pump (P < .001) (Table II).
The multivariate logistic regression analysis found that advanced age (P < .001), use of intra–aortic balloon pump (P < .001), and admission white blood cell counts (P = .01) were independently associated with in–hospital and 30–day death (Beta 0.333) (Table II).

Discussion
Impaired myocardial blood flow after AMI is a result of multiple insults including platelet/ thrombus microaggregate embolization combined with humoral factors derived from fibrinolysis (increased thrombin) and platelets (adenosine, serotonin, thromboxane A2). Inflammation and reperfusion injury may also exacerbate the problem by increasing neutrophil capillary plugging, liberating free radicals, and increasing capillary permeability, resulting in tissue edema and further deterioration of myocardial perfusión.36,37 Ischemic injury secondary to microvascular obstruction may be amplified in patients with an enhanced inflammatory milieu. In patients undergoing fibrinolysis as primary treatment for AMI, increased levels of CRP were associated with either death or post–infarction complications.21,23–25 Our results suggest that patients with AMI have very high levels of hs–CRP on admission, reflecting an increased inflammatory status. However, this did not translate into lower admission TMP levels.
Elevated WBC levels constitute another unspecific marker of inflammation.17,38 WBCs make a major contribution to the rheologic properties of blood by altering its adhesive properties under stress (including the stress of ischemia) and participate in endothelial injury, both acutely and chronically, by adhering to endothelium and damaging it with toxic oxygen compounds and proteolytic enzymes.39 Our results confirm the previously observed link of elevated WBC and death. The mechanism of this association remains uncertain. It suggests that other inflammatory stimuli, beyond CRP, are present. Various mechanisms may be perpetuating the inflammatory response including interleukin (IL)–l, IL–6, TNF–α, adhesion molecules, and platelets.4042 These factors have a pro–inflammatory capacity that may up regulate and amplify a multitude of interactions that result in an increase in substances such as fibrinogen and PAI–1, promoting adhesion of neutrophils and ultimately regulate gene expression of key proteins that potentiate the inflammatory cascade.43,44
Age has been related to poor outcome in AMI patients undergoing PCI.45 Our results confirm this observation. Patients that needed an intraaortic balloon pump had worse outcome suggesting that this cohort possibly was hemodynamically unstable, and therefore, at higher risk. As previously described, admission glucose was also associated with death as univariate variable. This has been associated to low myocardial perfusión, impairing left ventricular function thus worsening the outcome.30,46 Interestingly, the presence of peripheral vascular disease was also associated to death, reflecting the extent of cardiac and extracardiac atherosclerotic disease.47
Limitations
The present study should be interpreted in the light of the following limitations. This is not a randomized trial, with all the inherent potential confounding effects of an observational study. TMP grade is a subjective assessment of microcirculation, and the inter–observer variation may bias the results, although the rate of concordance between observers in this study was 98%. The monitoring of other inflammatory markers, such as IL–6, could have added more robust evidence of other inflammatory pathways associated with poor outcome. The time of onset and size of the myocardial infarction may also increase the CRP levels; these factors were not available in our database, therefore we were not able to include them in our analysis.
Conclusions
Hs–CRP levels were not significantly different between patients with or without a patent microcirculation before undergoing PCI. An increased inflammatory milieu, not reflected by CRP elevation alone, possibly mediates microcirculation impairment.
References
1. Yen MH, Bhatt DL, Chew DP, Harrington RA, Newby LK, Ardissino D, et al: Association between Admission White Blood Cell Count and One–Year Mortality in Patients with Acute Coronary Syndromes. Am J Med 2003; (115): 318–321. [ Links ]
2. Yen MH, Topol EJ: Atheromatous Showers, Periprocedural Myocardial Infarction, and Fatality. Am Heart J 2003; (145): 933–935. [ Links ]
3. Kuller LH, Tracy RP, Shaten J, Meilahn EN: Relation of C–Reactive Protein and Coronary Heart Disease in the Mrfit Nested Case–Control Study. Multiple Risk Factor Intervention Trial. Am J Epidemiol 1996; (144): 537–547. [ Links ]
4. Morrow DA, Rifai N, Antman EM, Weiner DL, McCabe CH, Cannon CP, et al: C–Reactive Protein Is a Potent Predictor of Mortality Independently of and in Combination with Troponin T in Acute Coronary Syndromes: A Timi 11a Substudy. Thrombolysis in Myocardial Infarction. J Am Coll Cardiol 1998; (31): 1460–1465. [ Links ]
5. Abdelmouttaleb I, Danchin N, Ilardo C, Aimone–Gastin I, Angioi M, Lozniewski A, et al: C–Reactive Protein and Coronary Artery Disease: Additional Evidence of the Implication of an Inflammatory Process in Acute Coronary Syndromes. Am Heart J 1999; (137): 346–351. [ Links ]
6. Albert MA, Ridker PM: The Role of C–Reactive Protein in Cardiovascular Disease Risk. Curr Cardiol Rep 1999; (1): 99–104. [ Links ]
7. Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, et al: C–Reactive Protein, a Sensitive Marker of Inflammation, Predicts Future Risk of Coronary Heart Disease in Initially Healthy Middle–Aged Men: Results from the Monica (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation 1999; (99): 237–242. [ Links ]
8. Gussekloo J, Schaap MC, Frolich M, Blauw GJ, Westendorp RG: C–Reactive Protein Is a Strong but Nonspecific Risk Factor of Fatal Stroke in Elderly Persons. Arterioscler Thromb Vase Biol 2000; (20): 1047–1051. [ Links ]
9. Mendall MA, Strachan DP, Butland BK, Ballam L, Morris J, Sweetnam PM, et al: C–Reactive Protein: Relation to Total Mortality, Cardiovascular Mortality and Cardiovascular Risk Factors in Men. Eur Heart J 2000; (21): 1584–1590. [ Links ]
10. Strandberg TE, Tilvis RS: C–Reactive Protein, Cardiovascular Risk Factors, and Mortality in a Prospective Study in the Elderly. Arterioscler Thromb Vase Biol 2000; (20): 1057–1060. [ Links ]
11. Guijarro C: High–Sensitivity C–Reactive Protein: Potential Adjunct for Global Risk Assessment in the Primary Prevention of Cardiovascular Disease. Circulation 2001; (104): E127. [ Links ]
12. Kanda T: C–Reactive Protein (Crp) in the Cardiovascular System. Rinsho Byori 2001; (49): 395–401. [ Links ]
13. Rifai N, Ridker PM: High–Sensitivity C–Reactive Protein: A Novel and Promising Marker of Coronary Heart Disease. Clin Chem 2001; (47): 403–411. [ Links ]
14. Ridker PM: High–Sensitivity C–Reactive Protein: Potential Adjunct for Global Risk Assessment in the Primary Prevention of Cardiovascular Disease. Circulation 2001; (103): 1813–1818. [ Links ]
15. Auer J, Berent R, Lassnig E, Eber B: C–Reactive Protein and Coronary Artery Disease. Jpn Heart J 2002; (43): 607–619. [ Links ]
16. Speidl WS, Graf S, Hornykewycz S, Nikfardjam M, Niessner A, Zorn G, et al: High–Sensitivity C–Reactive Protein in the Prediction of Coronary Events in Patients with Premature Coronary Artery Disease. Am Heart J 2002; (144): 449–455. [ Links ]
17. Barron HV, Harr SD, Radford MJ, Wang Y, Krumholz HM: The Association between White Blood Cell Count and Acute Myocardial Infarction Mortality in Patients > or = 65 Years of Age: Findings from the Cooperative Cardiovascular Project. J Am Coll Cardiol 2001; (38): 1654–1661. [ Links ]
18. Kroop IG, Shackman NH: Level of C–Reactive Protein as a Measure of Acute Myocardial Infarction. Proc Soc Exp Biol Med 1954; (86): 95–97. [ Links ]
19. Kushner I, Broder ML, Karp D: Control of the Acute Phase Response. Serum C–Reactive Protein Kinetics after Acute Myocardial Infarction. J Clin Invest 1978; (61): 235–242. [ Links ]
20. Voulgari F, Cummins P, Gardecki TI, Beeching NJ, Stone PC, Stuart J: Serum Levels of Acute Phase and Cardiac Proteins after Myocardial Infarction, Surgery, and Infection. Br Heart J 1982; (48): 352–356. [ Links ]
21. Pietila K, Harmoinen A, Poyhonen L, Koskinen M, Heikkila J, Ruosteenoja R: Intravenous Strep–tokinase Treatment and Serum C–Reactive Protein in Patients with Acute Myocardial Infarction. Br Heart J 1987; (58): 225–229. [ Links ]
22. Pietila K, Harmoinen A, Teppo AM: Acute Phase Reaction, Infarct Size and in–Hospital Morbidity in Myocardial Infarction Patients Treated with Streptokinase or Recombinant Tissue Type Plasminogen Activator. Ann Med 1991; (23): 529–535. [ Links ]
23. Pietila K, Harmoinen A, Hermens W, Simoons ML, Van de Werf F, Verstraete M: Serum C–Reactive Protein and Infarct Size in Myocardial Infarct Patients with a Closed Versus an Open Infarct–Related Coronary Artery after Thrombolytic Therapy Eur Heart J 1993; (14): 915–919. [ Links ]
24. Liuzzo G, Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, et al: The Prognostic Value of C–Reactive Protein and Serum Amyloid a Protein in Severe Unstable Angina. N Engl J Med 1994; (331): 417–424. [ Links ]
25. Pietila KO, Harmoinen AP, Jokiniitty J, Pasternack AI: Serum C–Reactive Protein Concentration in Acute Myocardial Infarction and Its Relationship to Mortality During 24 Months of Follow–up in Patients under Thrombolytic Treatment. Eur Heart J 1996; (17): 1345–1349. [ Links ]
26. Gheno G, Libardoni M, Zeppellini R, Cucchini F: C–Reactive Protein on Admission as a Predictor of in–Hospital Death in the Elderly with Acute Myocardial Infarction. Cardiología 1999; (44): 1023–1028. [ Links ]
27. Tommasi S, Carluccio E, Bentivoglio M, Buccolieri M, Mariotti M, Politano M, et al: C–Reactive Protein as a Marker for Cardiac Ischemic Events in the Year after a First, Uncomplicated Myocardial Infarction. Am J Cardiol 1999; (83): 1595–1599. [ Links ]
28. Zairis MN, Manousakis SJ, Stefanidis AS, Papadaki OA, Andrikopoulos GK, Olympios CD, et al: C–Reactive Protein Levels on Admission Are Associated with Response to Thrombolysis and Prognosis after St–Segment Elevation Acute Myocardial Infarction. Am Heart J 2002; (144): 782–789. [ Links ]
29. Auer J, Berent R, Eber B, Tanaka A, Sano T, Namba M, et al: C–Reactive Protein in Patients with Acute Myocardial Infarction. Circulation 2004; (109): E20. [ Links ]
30. Ishihara M, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, Nishioka K, et al: Impact of Acute Hyperglycemia on Left Ventricular Function after Reperfusion Therapy in Patients with a First Anterior Wall Acute Myocardial Infarction. Am Heart J 2003; (146): 674–678. [ Links ]
31. Kirtane AJ, Bui A, Murphy SA, Barron HV, Gibson CM: Association of Peripheral Neutrophilia with Adverse Angiographic Outcomes in St–Elevation Myocardial Infarction. Am J Cardiol 2004; (93): 532–536. [ Links ]
32. Wong CK, French JK, Gao W, White HD: Relationship between Initial White Blood Cell Counts, Stage of Acute Myocardial Infarction Evolution at Presentation, and Incidence of Thrombolysis in Myocardial Infarction–3 Flow after Streptokinase. Am Heart J 2003; (145): 95–102. [ Links ]
33. Marfella R, Siniscalchi M, Esposito K, Sellitto A, De Fanis U, Romano C, et al: Effects of Stress Hyperglycemia on Acute Myocardial Infarction: Role of Inflammatory Immune Process in Functional Cardiac Outcome. Diabetes Care 2003; (26): 3129–3135. [ Links ]
34. Gibson CM, Cannon CP, Murphy SA, Ryan KA, Mesley R, Marble SJ, et al: Relationship of Timi Myocardial Perfusion Grade to Mortality after Administration of Thrombolytic Drugs. Circulation 2000; (101): 125–130. [ Links ]
35. Gibson CM, Cannon CP, Murphy SA, Marble SJ, Barron HV, Braunwald E: Relationship of the Timi Myocardial Perfusion Grades, Flow Grades, Frame Count, and Percutaneous Coronary Intervention to Long–Term Outcomes after Thrombolytic Administration in Acute Myocardial Infarction. Circulation 2002; (105): 1909–1913. [ Links ]
36. Mukherjee D, Moliterno DJ: Achieving Tissue–Level Perfusion in the Setting of Acute Myocardial Infarction. Am J Cardiol 2000; (85): 39C–46C. [ Links ]
37. Mehta JL, Nichols WW, Mehta P: Neutrophils as Potential Participants in Acute Myocardial Ischemia: Relevance to Reperfusion. J Am Coll Cardiol 1988; (11): 1309–1316. [ Links ]
38. Brown DW, Giles WH, Croft JB: White Blood Cell Count: An Independent Predictor of Coronary Heart Disease Mortality among a National Cohort. J Clin Epidemiol 2001; (54): 316–322. [ Links ]
39. Ernst E, Hammerschmidt DE, Bagge U, Matrai A, Dormancy JA: Leukocytes and the Risk of Ischemic Diseases. JAMA 1987; (257): 2318–2324. [ Links ]
40. Furman MI, Becker RC, Yarzebski J, Savegeau J, Gore JM, Goldberg RJ: Effect of Elevated Leukocyte Count on in–Hospital Mortality Following Acute Myocardial Infarction. Am J Cardiol 1996; (78): 945–948. [ Links ]
41. Furman MI, Benoit SE, Barnard MR, Valeri CR, Borbone ML, Becker RC, et al: Increased Platelet Reactivity and Circulating Monocyte–Platelet Aggregates in Patients with Stable Coronary Artery Disease. J Am Coll Cardiol 1998; (31): 352–358. [ Links ]
42. Rus HG, Niculescu F, Vlaicu R: Tumor Necrosis Factor–Alpha in Human Arterial Wall with Atherosclerosis. Atherosclerosis 1991; (89): 247–254. [ Links ]
43. Miyao Y, Yasue H, Ogawa H, Misumi I, Masuda T, Sakamoto T, et al: Elevated Plasma Interleukin–6 Levels in Patients with Acute Myocardial Infarction. Am Heart J 1993; (126): 1299–1304. [ Links ]
44. Kushner I, Ganapathi M, Schultz D: The Acute Phase Response Is Mediated by Heterogeneous Mechanisms. Ann NY Acad Sci 1989; (557): 19–29; discussion 29–30. [ Links ]
45. Klein LW, Block P, Brindis RG, McKay CR, McCallister BD, Wolk M, et al: Percutaneous Coronary Interventions in Octogenarians in the American College of Cardiology–National Cardiovascular Data Registry: Development of a Nomogram Predictive of in–Hospital Mortality. J Am Coll Cardiol 2002; (40): 394–402. [ Links ]
46. Iwakura K, Ito H, Ikushima M, Kawano S, Okamura A, Asano K, et al: Association between Hyperglycemia and the No–Reflow Phenomenon in–Patients with Acute Myocardial Infarction. J Am Coll Cardiol 2003; (41): 1–7. [ Links ]
47. Cotter G, Cannon CP, McCabe CH, Michowitz Y, Kaluski E, Charlesworth A, et al: Prior Peripheral Arterial Disease and Cerebrovascular Disease Are Independent Predictors of Adverse Outcome in Patients with Acute Coronary Syndromes: Are We Doing Enough? Results from the Orbofiban Thrombolysis in Myocardial Infarction (Opus–Timi) 16 Study. Am Heart J 2003; (145): 622–627. [ Links ]














