Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos de cardiología de México
versión On-line ISSN 1665-1731versión impresa ISSN 1405-9940
Arch. Cardiol. Méx. vol.76 no.3 Ciudad de México jul./sep. 2006
Revisión de temas cardiológicos
Drug–eluting stents
Stents liberadores de fármacos
Héctor M García–García,* Sophia Vaina,* Keiichi Tsuchida,* Patrick W Serruys*
* Department of Interventional Cardiology, Thoraxcenter, Erasmus Medical Center, Rotterdam, The Netherlands.
Correspondence to:
P. W. Serruys.
Professor of Interventional Cardiology. Thoraxcenter, Ba 583.
Erasmus Medical Center. Dr Mo–lewaterplein 40. 3015 GD Rotterdam, The Netherlands.
Tel: (31) 10–4635260. Fax: (31) 10–4369154.
E–mail: p.w.j.c.serruys@erasmusmc.nl
Summary
Stent implantation was developed to overcome the acute recoil and high restenosis rate of balloon angioplasty, but resulted in the development of chronic in–stent restenosis related to specific factors regarding patient, stent, lesion and procedural characteristics. Some factors are not modifiable, such as patient and lesion characteristics, whereas procedural characteristics may be improved by better implantation technique and stent design. Drug–eluting stents are a novel approach in stent technology and design with local drug delivery to inhibit intimal thickening by interfering with different pathways involved in the development of inflammation, migration, proliferation and/or secretion of the extracellular matrix. Both the drug and the delivery vehicle must fulfill pharmacological, pharmacokinetic and mechanical requirements. Current successful drug eluting stents require a polymer coating for drug delivery. Clinical trials examining several pharmaceutical agents, particularly sirolimus and paclitaxel, have demonstrated marked reduction in restenosis following stenting. Sirolimus is a natural macrocyclic lactone and paclitaxel is a cytotoxic agent against many tumors. Both compounds block cell cycle progression and thus inhibit smooth muscle cell proliferation. The development of drug–eluting stents is one of the major revolutions in the field of Interventional Cardiology. Restenosis rate has been significantly reduced, in comparison to bare metal stents. The ideal drug to prevent restenosis must have an anti–proliferative and anti–migratory effect on smooth muscle cells but on the other hand must also enhance re–endothelialization, in order to prevent late thrombosis. Additionally, it should effectively inhibit the anti–inflammatory response after balloon induced arterial injury.
Currently sirolimus, paclitaxel and more recently, ABT–578–eluting stents are commercially available, but ongoing research and clinical trials will result in new stents coming to market with novel designs loaded with a variety of compounds. As drug–eluting stent implantation becomes more liberal leading to an extensive use of this technology, the problem of restenosis in drug–eluting stents will become more common. However, for the time being, little is known regarding optimal treatment of in–stent restenosis following drug–eluting stent implantation. Future research is mandatory to further clarify, whether these patients should be treated with the same drug–eluting stent, with a different drug–eluting stent or with increased doses. Further improvements, including expansion of drug–loading capacity, coatings with programmable pharmacokinetic capacity and the discovery of new drugs may in the future further enhance the efficacy and safety of these stents. Although, drug–eluting stents have significantly reduced angiographic restenosis rate and have improved the clinical outcome, late thrombosis and restenosis remain an important subject of ongoing research. As drug–eluting stents are extensively used to treat all lesions, more efficacious agents and improved stent platforms are required. Synthetic or biological polymers can be used as matrixes for drug incorporation, but concerns have been raised regarding bio–compatibility, sterility or potential induction of inflammation. Currently, alterations on stent–backbone design (biodegradable, bioabsorbable, nanoporous etc.) are being explored. Clearly, the anti–proliferative compounds sirolimus and paclitaxel have dominated up to date clinical practice, whereas their analogues are readily emerging. In the future, however, it is likely that drugs, currently under investigation, will address additional mechanisms associated with neointimal formation leading to restenosis, either as single agents or in combination with anti–proliferative compounds.
Keywords: Drug–eluting stents. Sirolimus. Paclitaxel. ABT–578.
Resumen
Mejoras ulteriores, como la elevación de la capacidad de carga de los fármacos, su revestimiento con capacidad farmacocinética programable y la preparación de nuevos fármacos, podrían aumentar a futuro la eficacia y la seguridad de los stents liberadores de fármacos. Aunque dichos stents han reducido de manera significativa el número de reestenosis angiográficas y mejorado el resultado clínico, la trombosis tardía y la reestenosis quedan como temas importantes de investigaciones futuras. Puesto que los stents liberadores de fármacos se utilizan en toda lesión, se necesitan agentes más eficaces y mejores estructuras de tales instrumentos. Pueden utilizarse polímeros sintéticos o biológicos como matrices para incorporar los fármacos, pero han surgido preocupaciones acerca de la biocompatibilidad, la esterilidad y la capacidad potencial de inducir inflamación. Habitualmente se examinan posibles alteraciones del diseño estructural del stent (biodegradable, bioabsorbible, nanoporoso, etc.). Los compuestos antiproliferativos sirolimus y paclitaxel han dominado claramente la práctica clínica hasta la fecha, mientras que sus análogos están apareciendo prontamente. No obstante es probable que, a futuro, los fármacos actualmente sometidos a investigación, intervengan sobre mecanismos asociados con modelación de la capa neoíntima que lleva a la reestenosis. Éstos podrían actuar por sí solos o en combinación con los compuestos antiproliferativos ya mencionados.
Palabras clave: Stents liberadores de fármacos. Sirolimus. Paclitaxel. ABT–578.
PART I. CONVENTIONAL DRUG–ELUTING STENTS
Introduction
Coronary artery disease is one of the major causes of morbidity and mortality in developed countries. In addition to medical treatment, percutaneous coronary stent implantation is for many patients, the method of choice for the management of coronary atherosclerosis. This is because stents prevent acute vessel closure and early vessel recoil, and improve the long–term patency of vessels. However, restenosis still remains a problem. The pathogenesis of restenosis can be divided into four phases, which can take place from hours to weeks and months: 1) early elastic recoil, 2) mural thrombus formation, 3) neointimal proliferation with extracellular matrix formation, and 4) chronic geometric arterial changes (months).1 Some risk factors such as long, complex lesions, small vessels and diabetes have been clearly identified, and others are at present discussed.2 Local delivery of immunosuppressive agents using drug–eluting stents (DES), targets the inhibition of excessive cell growth, inflammation, migration, proliferation and/or secretion of the extracellular matrix. Several different compounds, which interfere in various sites of this vicious process, have been proposed. Currently, in clinical practice rapamycin and paclitaxel eluting stents are widely used, whereas extended research is ongoing for the evaluation of new agents and novel stent designs.
Pathogenesis of restenosis
Restenosis is a local vascular manifestation of the general biological response to injury. The response to balloon inflation and stent implantation is initially determined by the extent of the arterial injury, which is associated with endothelial cell loss exposing the underlying vessel wall.3 This leads to immediate accumulation of platelets, macrophages and polymorphonuclear neutrophils, aiming to cover the location of the injury.4 Platelets and leukocytes contain chemotactic factors and mitogens, which are released in the injured vessel wall. Chemokines increase the amount of matrix metalloproteinase, which induces remodeling of the extracellular matrix and smooth muscle cell migration.5,6 Furthermore, injury also causes stretching and lysis of some of the cells, mainly vascular smooth muscle cells of the underlying layers, which are thus activated. As a result, these cells shift from a contractile to a synthetic phenotype, leading to proliferation, migration and synthesis of extracellular matrix responsible for intimal thickening.3 Smooth muscle cells are also stimulated to increase the expression of genes involved in cell division.7 Compared to restenosis after balloon angioplasty, the pathophysiology of in–stent restenosis may be different, with more profound cellular and proliferative response and less thrombogenic potential.
Drug–eluting stents
The concept of delivering immunosuppressive agents locally to prevent in stent restenosis arose from similarities observed between tumour cell growth and tissue proliferation that characterizes intimal hyperplasia following stenting. Drug release at the site of vascular injury achieves an effective local concentration of the drug for a certain period of time, while simultaneously avoiding systemic toxicity. The safety and efficacy of DES depend on the combination of drug, delivery system, and kinetics of release.8 Two different delivery systems have been explored, with and without additional coatings. Uncoated metal stents that have a drug attached to their surface or embedded within macroscopic fenestrations or microscopic nanopores, enabling rapid drug delivery are under investigation and are not yet commercially available. Metal stents coated with an outer layer of polymer (bioabsorbable or non–bioabsorbable) canbe drug–loaded, thus providing more controlled and sustained drug delivery, which might allow more optimal drug N tissue interactions.9 Polymer coatings have been proven to be durable, and deliver drug in a uniform and controlled way.
I. Sirolimus eluting stents (SES)
The Johnson and Johnson "Cypher" stent consists of a BX VelocityTM stent (Cordis/J&J, Miami Lakes, FL, USA), and a polymer coating. The incorporation of an additional drug–free polymer matrix prolongs drug release to more than 28 days in the slow release formulation.10 Sirolimus is a natural macrocyclic lactone that binds to specific cytosolic proteins called FK506 binding protein which blocks Gl to S cell cycle progression by inhibiting the activation of a protein known as mTOR (mammalian target of rapamycin).11 This also suppresses cytokine driven T cell proliferation. The first in man (FIM) implantation study of the sirolimus eluting stent was performed in Rotterdam and Sao Paolo. Since then, four randomized trials have been conducted, and are summarized in Tables I, II and III. The first randomized trial of sirolimus eluting stent was the RAVEL study (RAndomized study with the sirolimus–coated bx VElocityTM balloon expandable stent in the treatment of patients with de novo native coronary artery Lesions). At six months, the degree of neointimal proliferation, manifested as the mean late luminal loss, was significantly lower in the SES group than the bare metal stent (BMS) group. At one year follow–up, the overall rate of major cardiac events was 5.8% in the SES group and 28.8% in the bare metal stent group (p < 0.001).12 The early RAVEL results appear to be maintained out to three years.13
The US based SIRIUS trial (Multicenter, randomized, double blind study of the SIRo1ImUS–eluting Bx–VelocityTM balloon expandable stent in the treatment of patients with de novo coronary artery lesions) randomized 1,058 patients to treatment with rapamycin–coated or bare metal Bx VelocityTM balloon expandable stents.14 At 12 months, target–lesion revascularization (TLR) was 4.9% in the sirolimus arm versus 20% in the bare metal arm (p < 0.001).15 SIRIUS was the only trial in which edge restenosis, predominantly located at the proximal margin of the stent was observed and was attributed to balloon injury occurring outside the stent.14
The C–SIRIUS (The Canadian Study of the Sirolimus–Eluting Stent in the Treatment of Patients With Long De Novo Lesions in Small Native Coronary Arteries)16 and the E–SIRIUS (the European Study)17 confirmed the effectiveness of the sirolimus–eluting stent in smaller populations (Tables I, II and III).
II. Paclitaxel–eluting stents (PES)
Paclitaxel promotes polymerization of tubulin and inhibits the disassembly of microtubules, which stabilizes microtubules and results in inhibition of cell division. Cell replication is inhibited in the G/Gj and G/M phases.18 Additionally, paclitaxel affects cell motility, shape, and transport between organelles.
Two delivery methods have been used in clinical trials, either with or without a polymer carrier. In the pivotal US trial DELIVER 1, paclitaxel was loaded without a polymer. No significant benefit was seen in the treated group and the stent was not commercialized.19
Boston Scientific Corporation pursued the development of paclitaxel stents eluted from a polymeric carrier. The randomized TAXUS I trial evaluated the safety of the TAXUSTM – NIRxTM stent system20 and was followed by TAXUS II21 and TAXUS IV. In the TAXUS IV trial, compared with the bare–metal stent the paclitaxel–eluting stent [paclitaxel–coated Express2TM stent (Boston Scientific Co., USA)] reduced the 12–month rates of TLR by 73%, the target–vessel revascularization (TVR) by 62%, and composite major adverse cardiac events (MACE) by 49%. Additionally, between 9 and 12 months, there were significantly fewer myocardial infarctions, TVR, and MACE in the paclitaxel–eluting stent than in the control stent group.22 Among patients with diabetes, compared to the bare–metal stent the TAXUSTM stent reduced the rate of 9–month binary angiographic restenosis by 81%, and reduced the 12–month rates of TLR by 65%, TVR by 53%, and composite MACE by 44%.23 The TAXUS V trial assessed the efficacy of the slow release stent compared with the bare metal stents in long lesions and small vessels. At 9–months follow–up, overall MACE rate in the TAXUS group was 15% compared with 21.2% in the control group (p = 0.0084).24 The TAXUS VI trial tested the moderate release formulation stent (not commercially available) and included complex and long lesions, treated with multiple stents. Two–year clinical results reported a TVR rate of 13.9% in the TAXUS MR group and 21.9% in the control group (p =0.0335).25 Clinical studies utilizing polymer–coated paclitaxel–eluting stents are summarized in Tables I, II and III.
Comparisons between sirolimus and paclitaxel
The 1,386 patient REALITY trial compared SES versus PES. At 8 month follow–up, there were no differences in in–stent or in–lesion binary restenosis (primary end–point) rates and in TLR rates, although the late loss was significantly less with SES.26 The SIRTAX study was also designed to compare the safety and efficacy of the SES versus the PES. Patients treated with SES stents had significantly better clinical and angiographic outcomes.27 The ISAR–DIABETES study randomized 250 patients with diabetes to SES or PES. At 9–month follow–up, there was no significant difference between the 2 groups with respect to the incidence of death or myo–cardial infarction. However, the primary end–point of the study, the in–segment late luminal loss, was significantly greater (0.24 mm 95% CI 0.09, 0.39) with PES, p = 0.002. No significant difference between the 2 groups with regard to the incidence of target lesion revascularization or angiographic restenosis was noted.28 Finally, the IS AR–DESIRE trial (The Intracoronary Stenting or Angioplasty for Restenosis Reduction –Drug–Eluting Stents for In–Stent REstenosis) was designed to investigate the effectiveness of SES and PES compared with balloon angioplasty in patients with in–stent restenosis. Late lumen loss was significantly lower in the SES group with no difference in restenosis rates although TVR was lower in the SES group.29
Drug–eluting stents in the "Real World"
I. Rapamycin and paclitaxel registries
In the RESEARCH registry the one–year clinical outcomes of the first 508 consecutive patients treated exclusively with SES were compared to 450 patients who received bare stents in the period immediately prior to the introduction of SES; demonstrated an overall reduction of 38% in the composite risk of death, myocardial infarction, or re–interventions.30 Following on, the T–SEARCH registry compared the first 576 patients exclusively treated with PES against the 508 patients treated with SES from the RESEARCH registry. At one year, the unadjusted MACE rate was 13.9% in the PES group and 10.5% in the SES group (p = 0.1) and following multivariate adjustment had a hazard ratio of 1.16 (95% CI 0.81 to 1.64, p = 0.4). The one–year incidence of clinically driven TVR was 5.4% vs 3.7%, respectively (p = 0.3).31 These results suggest a similar efficacy between the two devices.
II. Complex lesions
Angiographic restenosis after DES implantation in complex patients is an infrequent event, occurring mainly in association with lesion–based characteristics and diabetes mellitus.32 The effectiveness of drug–eluting stent implantation for left main stenoses has been investigated. Despite the higher–risk patients and lesion profiles in the DES compared to the BMS group, the incidence of MACE at a 6–month clinical follow–up was lower.33 The short– and long–term clinical outcome of left main percutaneous coronary intervention in 181 patients was recently reported. With a median follow–up of 503 days, the cumulative incidence of MACE was lower in the DES than in the BMS cohort with significantly lower rates of both myocardial infarction and TVR in the drug–eluting stent group.34
Percutaneous treatment of coronary bifurcation lesions is considered a technical challenge. Follow–up data demonstrates a high technical success rate. However, with conventional stents restenosis rates higher than 30% have been reported in most studies. Introduction of DES has resulted in a lower event rate and reduction of main vessel restenosis in comparison with historical controls.35,36
Treatment of chronic total occlusion is limited by high rates of subacute reocclusion and late restenosis. Preliminary results from two studies investigating SES implantation in patients with chronic total occlusion demonstrated less restenosis rates and TLR after 6 months compared with BMS.37,38 Similar promising results were observed after PES implantation in a non–randomized study assessing the efficacy of PES in chronic total coronary occlusions.39
Treatment of saphenous vein graft disease is an emerging problem, due to the increasing number of patients undergoing bypass surgery. Stent implantation has better long–term outcome compared to balloon angioplasty.40 In a preliminary study, SES were implanted in 19 patients with saphenous vein graft lesions and reported an in–hospital MACE of 11%, whereas over a mean 12.5 ± 2.6 month follow–up, TLR was 5% and event–free survival 84%.41 Improved midterm and long–term efficacy of DES implantation compared to BMS implantation in saphenous vein graft lesions was confirmed in two studies, which included 61 and 40 patients, respectively.42,43
The treatment of in–stent restenosis remains a therapeutic dilemma, since many pharmacological and mechanical approaches have shown disappointing results. In a recent study sixteen patients with severe, recurrent in–stent restenosis were treated with SES.44 Four months follow–up demonstrated that in–stent late lumen loss averaged 0.21 mm and the volume obstruction of the stent was 1.1%. At nine months clinical follow–up, three patients had experienced four MACE.44 Similar results were reported in Sao Paulo, Brazil, where no patient had in–stent or stent margin restenosis at 4 months, and only one patient developed in–stent restenosis at 1 –year follow–up.45 In a subgroup of 44 patients in RESEARCH with complex in–stent restenosis, the data showed at one year that the incidence of repeat intervention due to re–restenosis was 11.6%, with no stent thromboses or deaths.46 The TROPICAL study, a prospective multicenter registry, assessed the effectiveness and safety of SES in the treatment 162 patients with in–stent restenosis. The 9–month rate of death was 1.2% and that of nonfatal myocardial infarction was 1.2%.47 These findings confirm the efficacy of SES in the treatment of in–stent restenosis.47 The TAXUS III trial investigated the feasibility and safety of PES for the treatment of in–stent restenosis. No subacute stent thrombosis occurred up to 12 months and MACE rate at was 29%.48 The ISAR–DESIRE randomized trial showed that DES are superior to balloon angioplasty.29 However, the results were less remarkable than those for de novo lesions. The TAXUS VISR pivotal trial, designed to compare PES with intra–coronary brachytherapy for in–stent restenosis, has completed enrolment and the results are pending.
III. Stent thrombosis
There is concern that DES implantation results in subsequent thrombosis. Early stent thrombosis is a complication observed within the first 30 days post–procedurally, and is due to the arterial injury caused by the stent struts,49 while delayed endothelialization associated with DES implantation may result in late stent thrombosis (> 30 days). In sequential consecutive cohorts of 506 patients with BMS, 1,017 with SES, and 989 with PES, the incidence of angiographically proven early stent thrombosis was 1.2%, 1.0%, and 1.0% respectively.50 Including possible stent thrombosis, the respective incidences were 1.4%, 1.5% and 1.6%, indicating a similar risk profile in all 3 groups. Early stent thrombosis was associated with bifurcation stenting and was more prominent in the acute myocardial infarction setting. Additionally, a meta–analysis of eight trials in 3,817 patients with coronary artery disease who were randomized to either PES or BMS suggested that standard dose paclitaxel–eluting stents do not increase the hazard of stent thrombosis compared to bare metal stents.51 Similar results were reported in a pooled analysis including 10 randomized studies, where 2,602 were allocated to drug–eluting stents and 2,428 patients to bare metal stents.52 In <<Real World>> patients increased late thrombosis after drug–eluting stent implantation has been observed and has been associated with premature antiplatelet therapy discontinuation, renal failure, bifurcation lesions, diabetes, and low ejectionfraction.53,54 Late angiographic stent thrombosis has been reported with an incidence of 0.35% with a mean follow–up of 1.5 years,55 and using a broader definition to include sudden death, between 0.5 N 0.8% with 9–month follow–up.53 Importantly, patients with DES should remain on antiplatelet therapy lifelong following DES implantation unless an absolute contraindication supervenes.
IV. Drug–eluting stents versus coronary artery bypass grafting
ARTS II compared SES in multivessel disease with the outcomes in ARTS I. The results clearly showed that use of drug–eluting stents in this study was better than the bare metal stent arm results in ARTS I, and although overall events were similar to the CABG arm of ARTS I, the need for repeat revascularization remained lower with surgery.56 The SYNTAX randomized, multi–centre clinical trial is designed to compare 12–month event free survival of percutaneous coronary intervention using the PES with coronary artery bypass grafting for 3 vessel disease or left main disease.57 In the trial, 1,500 patients are being randomized to either PES implantation or CABG at up to 90 sites in Europe and the United States, with nested registries of CABG and PCI. The FREEDOM multi–centre trial (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) will randomize 2,400 diabetes patients with multi–vessel coronary disease to PCI with DES or CABG; with the primary outcome of 5–year mortality.
Conclusions
Drug eluting stents have undoubtedly improved the outcomes following stenting. Sirolimus and paclitaxel eluting stents have lived up to early promise with consistent results in increasingly complex trial lesions and in worldwide registries. New drugs and stent designs are being tested in clinical trials and if successful, will add to the current generation of devices available.
BEYOND "CONVENTIONAL" DRUG–ELUTING STENTS
Sirolimus and paclitaxel–eluting stents have been proven to be a significant novel tool for the treatment of coronary artery disease.14,22 Although, DES have significantly reduced angiographic restenosis rate and have improved the clinical outcome, late thrombosis and restenosis remain an important subject of ongoing research. As DES are extensively used to treat all lesions, more efficacious agents and improved stent platforms are required. Synthetic or biological polymers can be used as matrixes for drug incorporation, but concerns have been raised regarding biocompatibility, sterility or potential induction of inflammation. Currently, alterations on stent–backbone design (biodegradable, bioabsorbable, nanoporous etc.) are being explored. Clearly, the anti–proliferative compounds sirolimus and paclitaxel have dominated up to date clinical practice, whereas their analogues are readily emerging. In the future, however, it is likely that drugs, currently under investigation, will address additional mechanisms associated with neointimal formation leading to restenosis, either as single agents or in combination with anti–proliferative compounds.
A. Bioabsorbable stents
Although stenting has improved outcomes in patients undergoing percutaneous coronary interventions, permanent metallic implants have specific drawbacks that limit their unrestricted use. These include thrombogenicity, permanent physical irritation, mismatches in mechanical behaviour between stented and non–stented vessel areas, long term endothelial dysfunction, and chronic inflammatory local reactions.58 Additionally, their inability to adapt to growth could be an obstacle for later surgical revascularization (Fig. 1). In respect to the aforementioned limitations, degradable implants seem to offer some advantages (Table IV). At present, their major downside is their great bulk and the induced exaggerating inflammatory response during degradation.58
I. Tailored magnesium alloys
Experimental studies about magnesium alloys, which contain small amounts of aluminum, manganese, zinc, lithium, and rare earth elements, have been reported.58 The magnesium based alloy AE21 (containing 2% aluminum and 1% rare earth elements) stent, which expected to have 50% loss of mass within six months, was placed in eleven domestic pigs. During follow–up, there were no thromboembolic events observed. Between the 10th and 35th day, histological analysis demonstrated a 40% loss of lumen diameter (p < 0.01), corresponding to neointima formation. On the other hand, intravascular ultrasound between the 35th and 56th day showed a 25% of positive remodeling (p < 0.05), which was attributed to the loss of mechanical integrity of the stent.
In humans, a different magnesium alloy (WE43) has been implanted in peripheral vessels. A total of 20 patients with critical limb ischaemia were treated. No adverse effects were reported and at one month follow–up a 90% patency was observed by Doppler imaging.59 The PROGRESS study is a prospective, non–randomized, multicenter trial designed to evaluate the safety and efficacy of the magnesium alloy stents in patients with coronary artery disease. At four–months follow–up the study reached its primary end–point (MACE<30%), with an ischaemically driven TLR of 23.8%. During this follow–up period no deaths, stent thrombosis or acute myocardial infarction were observed.60
II. Biodegradable Poly–1–Lactic acid coronary stents
Although, many different polymers have been evaluated as candidate materials for stents and stent coatings, most materials induced a marked inflammatory and thrombogenic response. Polylactide polymers are widely and safely used as suture and osteosynthesis materials. Accordingly, polylactic acid has been shown to combine good mechanical stability, biodegradability, and sufficient blood compatibility.60 However, an exaggerated inflammatory response by the resulting acidic degradation products has been described.
To evaluate scaffolding properties and local drug release, new stents have been examined in animal models. A poly (D, L)–lactic acid (PDLLA) double helical paclitaxel–loaded stent that can release the drug over a period of at least 4 weeks, has been evaluated in a porcine restenosis model.61 Twelve paclitaxel–loaded polylactide stents, 12 unloaded polylactide stents, and 12 stainless steel, bare metal stents were deployed in 36 animals. Histological analysis showed early complete endothelialization. A significant cut down on restenosis was seen in animals that received paclitaxel–eluting stents. Nevertheless, as expected a local inflammatory response due to the polymer absorption process was observed.
The Igaki–Tamai stent (Igaki Medical Planning Co, Ltd) is a coil stent made of Poly–1–Lactic Acid (PLLA) monofilament with a zigzag helical design.62 The stent strut thickness is 0.17 mm. The length of the stent is 12 mm and, in its expanded state, the stent covers 24% of the vessel area. The stent has 2 radiopaque gold markers to facilitate the identification of both ends of the prosthesis. Deployment of the stent is currently achieved with a balloon–expandable system through an 8 French guiding catheter. A total of 25 stents (15 patients) were successfully implanted in 19 lesions, and the angiographic success was achieved in all procedures. No stent thrombosis and no major cardiac event occurred within 30 days. At 6 months, both the restenosis rate and target lesion revascularization rate per lesion were 10.5% and the rates per patient were 6.7%. Intravascular ultrasound, performed the first day after stent implantation, revealed no significant stent recoil. The PLLA stents used in this study seemed to maintain their scaffolding properties at 6 months. One of the major limitations stated in the report was the impossibility to identify signs of biodegradation. Although, PLLA biodegradable stent implantation is feasible, safe, and effective in humans, long–term follow–up with more patients will be required to validate their long–term efficacy.
B. Porous stents
I. Ceramic–coated stents and ceramic drug–coated stents (Nanoporous Stent)
A new inorganic ceramic nanoporous aluminum oxide stent (Jomed, Rangendingen, Germany) has been recently investigated as a carrier for immunosuppressive drugs such as tacrolimus. The stent consists of a stainless steel stent with a length of 16 mm, a surface area of 91.81 mm2, and a strut diameter of 0.12 mm. A two–step process is required for the coating of the stent. First, a thin layer of aluminum is used to coat inside and outside the stent. In the second step, the metallic layer is electrochemically converted into a nanoporous ceramic using a bath of 2% oxalic acid in 0°C water. The pore size is adjusted by this process. Thus, when using pores between 5 and 15 nm, pore density is approximately 109 pores/ cm2. Drug loading is achieved by dipping the stents into a defined solution of 3 mg tacrolimus (Fujisawa Pharmaceutical, Japan). In the experimental setting, this ceramic nanolayer loaded with tacrolimus was implanted in the common carotid artery of New Zealand rabbits. Ceramic coating of coronary stents with a nanoporous layer of aluminum oxide in combination with tacrolimus resulted in a significant reduction in neointima formation and inflammatory response.63 However, subsequent studies have shown that major particle debris from a nanoporous stent coating counteracting the inhibitory effect of tacrolimus.64
II. Dose–adjustable and multiple drug–eluting stent system (Microsporous stent)
In specific patient and lesion subsets, such as insulin–dependent diabetic subjects, bifurcation stenting and chronic total occlusion, the stenosis rate remains higher. Therefore, dose adjustments according to the clinical and lesion characteristics are desirable. Furthermore, experience has taught us that polymeric coating may lead to localized hypersensitivity reactions and even late coronary thrombosis. To address these issues, a new drug–eluting stent system that consists of 2 components, the mobile coating device and the sandblasted 316L stainless steel microporous stent (Yukon DES; Translumina, Hechingen, Germany) has been introduced. Porosity allows drug deposition and retards drug release without obligating application of a polymer. The loading process is performed on–site by a positionable ring containing 3 jet units, which allow for uniform delivery of the drug onto the stent surface (Fig. 2). The microporous stent loaded with a 0.75% sirolimus solution has been compared to standard sirolimus–eluting stent in porcine coronary arteries. At 30 days, in–stent neointima formation was similar in both groups.65

The microporous stent has been evaluated in a clinical study involving a high proportion of patients with acute coronary syndromes, multi–vessel disease and complex lesions.66 A total of 602 patients were randomized to four groups, to the microporous bare metal stent and to the microporous stent loaded with different concentrations of sirolimus solution (0.5%, 1.0%, and 2.0% sirolimus solution, respectively). Compared with the microporous bare metal, in–segment restenosis and 1–year target lesion revascularization were significantly reduced in the SES.
C. New stent design
I. The Conor MedStentTM
The Conor MedStentTM (Conor Medsystems, Manlo Park California, USA) is the first coronary stent specifically engineered for vascular drug delivery and differs significantly from the first generation of DES. The first ConorTM stents were made of 316L stainless steel, whereas the current generation is made of Cobalt–Chrome alloy and offers lower profiles and greater flexibility, while maintaining excellent radiopacity. The Conor MedStentTM has a unique stent design with several hundred laser–cut holes along its struts and connecting bridges. These wells can be individually filled with a combination of polymer and drug. Within any given well, the drug–polymer combination can differ, resulting in the creation of a variety of controllable pharmacokinetic profiles. The Conor MedStentTM uses an erodable polymer, which releases drug by a combination of diffusion and erosion. The use of slowly eroding barrier layers at the lumen surface enables elution of the drug towards the vessel wall. The addition of a cap polymer slows the initial 24–hour burst release. The particular characteristics of the stent allow multi–target chemotherapy, releasing one agent to the vessel wall to prevent local restenosis and another agent into the lumen to treat diffuse disease (Fig. 3).

The PISCES study (Paclitaxel In–Stent Controlled Elution Study) was a multi–center dose optimization registry (Table II).67 The registry evaluated high and low paclitaxel dose delivery in mural and bi–directional profiles with slow and fast release kinetics. Overall, 244 patients were enrolled with approximately 30 patients in each release profile group. At 4–month follow–up the best efficacy was observed in the long–release groups.67 In the two long release formulations the TLR and MACE rates remained low at 12 months.67 These results indicate that the most important predictor of efficacy is the release rate rather than the dose.
The COSTAR trial applied the Cobalt–Chrome metal platform. The study was designed to evaluate several doses, in order to define the dose response curve for paclitaxel (Table II).68,69 The COSTAR II pivotal trial (CObalt chromium STent with Antiproliferative for Restenosis) will include approximately 1,700 patients at up to seventy US sites and fifteen international sites. The COSTAR II trial will be a study comparing the Co StarTM stent with the TAXUSTM Express2TM drug–eluting stent (Boston Scientific, USA) for the treatment of coronary artery disease (Table V).

Finally, the EUROSTAR (EUROpean cobalt chromium STent with Antiproliferative for Restenosis) trial was looking at 10 and 30µg, long release formulations of the Co StarTM cobalt chromium stent with 6–month angiographic and IVUS follow–up and 6–month and one year clinical follow–up (Table V).70 A total of 145 patients were treated with the 10µg of paclitaxel release formulation and 125 patients were treated with the 30µg. At twelve–month follow–up in the 10µg group, the TLR rate was 2.9% and the rate of MACE was 7.6%. There were no reported cases of stent thrombosis between the cessation of anti–platelet therapy at six months and twelve–month follow–up.70
II. The millennium matrix coronary stentTM
The Millennium MatrixTM (Sahajanand Medical Technologies Pvt Ltd, Surat, India) coronary stent is made of stainless steel and is characterized by serpentine radial and longitudinal struts that cross each other in a unique junction that undergoes rotation during expansion. The InfinimumTM stent is a fourth generation of the Millennium stent series, which uses the Millennium MatrixTM stent as the platform for paclitaxel release. The binding agent in the InfinimumTM stent is a bio–compatible and biodegradable polymer. The stent is coated with three different layers of polymers (Fig. 4). The outermost protective layer does not contain any drug and dissolves immediately after the deployment of the stent. The middle layer is a moderate drug release layer and the final basal layer is a slow drug release layer. The pivotal safety and efficacy study for the InfinimumTM stent was the SIMPLE I trial. SIMPLE I trial was multi–center, non–randomized study, which included 282 patients. Event free survival at 22.5 months was 90.43%. Follow–up quantitative angiography at 6 months in 85 patients demonstrated an in–stent late loss of 0.19 ± 0.68 mm and a mean percentage diameter stenosis of 18.8 ± 18.7%.73 SIMPLE II is a multi–center, prospective study, aiming to investigate the safety and the efficacy of the InfinimumTM paclitaxel–eluting stent. One hundred and three patients were treated with the InfinimumTM paclitaxel–eluting stent (paclitaxel concentration of 2.0 mg/mm2, cumulative release of the drug from the polymer at 38 days) at eight centres in India, South America, and Europe. At 30 days four patients had stable angina and only one patient had a major adverse cardiac event, which was a non–q wave myocardial infarction. Six–month angiographic results are pending. These preliminary results are encouraging suggesting that the InfinimumTM stent is safe with efficacy comparable to that of other drug–eluting stents.

III. The titanium–nitride–oxide N coated stent
Titanium is a material used for biomedical implants. Animal studies demonstrated that titanium–nitride–oxide coating significantly reduces neointimal hyperplasia in stainless steel stents.72 The safety and efficacy of titanium–nitride–oxide for stent coating has been investigated in a prospective randomized trial, which used a commercially available stainless steel stent with a tubular slotted design (OMEGA, Qualimed Inc).73 The titanium–nitride–oxide coating was achieved by physical vapor deposition of titanium in a gas mixture of nitrogen and oxygen in a vacuum chamber. At 30 days, no stent thromboses or other adverse events were reported in either group. At 6 months angiographic follow–up, lower late loss (0.55 ± 0.63 versus 0.90 ± 0.76 mm, P = 0.03) and percent diameter stenosis (26 ± 17% versas 36 ± 24%, P = 0.04) were observed in lesions treated with titanium–nitride–oxide–coated than in control stents.73 Moreover, binary restenosis was reduced from 33% in the control group to 15% in the titanium–nitride–oxide N coated stent group (P = 0.07).73
D. Sirolimus analogues
I. ABT–578–Eluting stent
The ABT–578 (zotarolimus) is a tetrazole–containing macrocyclic immunosuppressant and potent antiproliferative agent. Zotarolimus inhibits the protein phosphorylation events associated with translation on mRNA and cell cycle control. The Endeavor Drug N Eluting Stent System consists of a Medtronic's DriverTM coronary stent, a phosphorylcholine polymer coating and zotarolimus. In the pilot ENDEAVOR I trial (Table VI), at four–month follow–up, angiographic binary restenosis rate was 2.1%, with no restenosis observed at either edge, whereas IVUS measurements at baseline and at four months exhibited nearly complete preservation of lumen volume. The 12–month cumulative restenosis rate was 5.4% and MACE rate was 2%.74 The ENDEAVOR II clinical trial (Table III) was a randomized, double–blind pivotal trial designed to evaluate the safety and efficacy of the EndeavorT Drug Eluting Coronary Stent compared to the Driven cobalt alloy coronary stent in patients. The study enrolled 1,197 patients from 17 countries. The primary endpoint of the trial was target vessel failure, which includes death, myocardial infarction and TVR at nine months. The study successfully met its primary and secondary endpoints. The study also demonstrated a 62% reduction in TLR between the Endeavor arm (4.6%) and the control group (12.1%).75 Based on these results, the Endeavor Zotarolimus–eluting stent received CE approval for sale in Europe. The results of the ENDEAVOR III study (Table III) (Endeavor drug–eluting stent vs the CypherTM Sirolimus–eluting stent) are still expected. The ENDEAVOR IV will be a comparison with the TAXUSTM stent and will involve about 1,000 patients (Table III). ENDEAVOR V is a registry.
The ZoMaxxTM drug–eluting stent consists of the TriMaxxTM stent (Abbott Laboratories, Illinois, USA), a polymer carrier and zotarolimus. The TriMaxxTM stent has a tri–layer composite of 316L stainless steel and tantalum (middle layer) (Fig. 5). ZOMAXX I conducted in Europe, Australia and New Zealand and ZOMAXX II in North America (Table VII) are prospective and randomized clinical trials designed to evaluate the safety and efficacy of ZoMaxxTM drug–eluting stent system in comparison to Taxus Express2TM drug–eluting stent.

II. Everolimus N eluting stent
Everolimus, is a sirolimus derivative with chemical substitution at C–43. Everolimus, like rapamycin, blocks growth factor derived cell proliferation, arresting the cell cycle at the Gl to S phase (Fig. 6). More specifically, in the cellular level, everolimus forms a complex with the cytoplasmic protein FKBP12. The complex inhibits the growth factor–stimulated phosphorylation of two proteins involved in the initiation of protein synthesis, the p70 s6 kinase and 4E–BP1. The phosphorylation of those two proteins is controlled by the mammalian Target of Rapamycin (mTOR). This indicates that everolimus interferes in the same pathway as sirolimus. However, the everolimus effect is not restricted to T–lymphocytes, but potentially affects other cells, such as smooth muscle cells.

The FUTURE I trial (Table V) evaluated both safety and feasibility of the everolimus–eluting stent. The trial utilized an S–stentTM and bioab–sorbable polymer system (Biosensors International, Singapore). The binary in–segment restenosis rate at 6 months follow–up was 4.3% in the everolimus–eluting stent group versus 36.4% in the controls (p = 0.01).76 Furthermore, MACE rate was 7.7%, with no late thrombosis and no late malapposition.76 The 12–month results demonstrated sustained safety and efficacy with no additional MACE.77 FUTURE II was designed to demonstrate safety and feasibility of the everolimus–eluting stent in a small patient population with focal de novo coronary lesions (Table VIII). At follow–up, an acceptable safety profile without evidence of stent thrombosis or late stent malapposition was observed. Moreover, this study revealed a remarkable reduction of neointimal proliferation with everolimus–eluting stent implantation versus bare–metal stents.78 The SPIRIT I multi–center randomized trial investigated the safety and effectiveness of the everolimus–eluting XIENCETM V coronary stent system (Guidant Corporation, Indianapolis, Indiana) (Table VIII)79 The XIENCETM V coronary stent system consists of the MULTI–LINK VISIONTM stent (Guidant Corporation, Indianapolis, Indiana), a serpentine configured, thin strut cobalt chromium stent, and a nonerodable polymer. The stent is designed to release approximately 70% of the drug within 30 days after implantation. At 6 months follow–up, compared with the MULTI–LINK VISIONTM bare metal stent, in–stent late loss, diameter stenosis and binary restenosis were significantly lower in the everolimus eluting stent. Accordingly, IVUS analysis revealed significantly less neointimal hyperplasia and less volume obstruction in the everolimus group compared with the bare metal stent group. MACE rate in the everolimus–eluting group was 7.7% and 21.4% in the control group (p = NS).79 This was the first study to show that everolimus released from a durable polymer on a cobalt chromium stent can effectively inhibit neointimal proliferation at 6 months compared with the conventional bare metal stent. The SPIRIT II clinical trial will evaluate the XIEN–CETM V stent compared to the TAXUSTM Express 2TM paclitaxel–eluting stent. The SPIRIT III, a large–scale pivotal clinical trial conducted in USA, will also compare the XIENCEt V with the TAXUSTM Express 2TM stent (Table V).

III. Biolimus A9TM–eluting stent
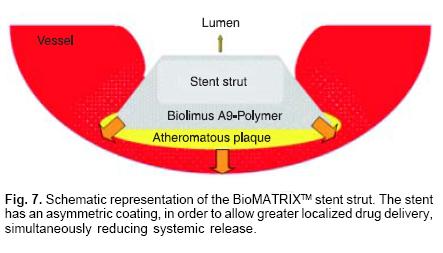
Biolimus A9TM is also a sirolimus derivative that inhibits growth factor–driven cell proliferation, such as T–cells and vascular smooth muscle cells. The STEALTH trial (STent Eluting A9 BioLimus Trial in Humans) was the first in man study to investigate the safety and efficacy of a biolimus A9–eluting stent, the BioMATRIXTM stent (Biosensors International, Singapore) (Table VIII).80 The BioMATRIXTM stent comprises a stainless steel, corrugated ring, quadrature–link design S–stent and a bioabsorbable polylactic polymer/ biolimus A9TM coating. The stent delivers 15.6 µg of drug/mm of stent and its coating is asymmetric to allow greater localized drug delivery, simultaneously reducing systemic release (Fig. 7). The STEALTH study randomized 80 patients to the BioMATRIXTM stent and 40 patients to the bare metal S–stent. The 6–month in–lesion and in–stent late loss was significantly reduced in the drug–eluting stent compared with the control, whereas event–free survival was similar in both groups.80 These results suggest that the BioMATRIXTM stent is superior in reducing late loss compared with the respective bare metal stent, with similar clinical safety.
Recently, a new trial with a biolimus A9TM–eluting stent has been initiated. The NOBORI 1 clinical trial will compare the NoboriTM biolimus A9TM–eluting coronary stent system (TERUMO Europe NV) with the TaxusT stent and plans to prospectively randomize approximately 400 patients in up to 30 centers in Europe, Australia and Asia (Table VIII). The NoboriT drug–eluting stent system uses Biosensors' bare–metal S–Stent as its platform and is coated with biodegradable polymer and polylactic acid.
IV. Tacrolimus–eluting stent
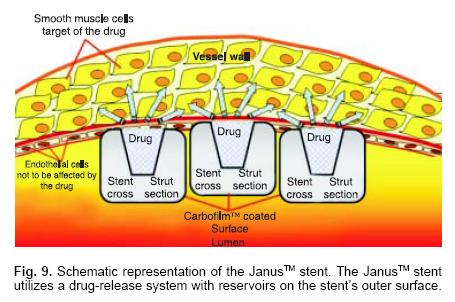
Tacrolimus (FK506) is a water–insoluble macrolide immunosuppressant (Fig. 8).81 It has been widely used to reduce the incidence and severity of allograft rejection after organ transplantation and to treat other inflammatory conditions such as atopic dermatitis. The JUPITER II trial investigated the safety and the efficacy of the JanusTM tacrolimus–eluting carbostent (Sorin Group) compared to the bare metal TecnicTM carbostent (Sorin Group). The JanusTM stent requires no polymer matrix to carry the drug, but utilizes a drug–release system with reservoirs on the stent's outer surface (Fig. 9). A total of 332 patients were enrolled in 17 European centers and randomized to either the JanusTM tacrolimus–eluting carbostent (166 patients) or the bare metal TecnicTM carbostent (166 patients). Early clinical outcomes demonstrated low MACE rate in both groups. These results were sustained up to 6 months follow–up82 (Table VIII).

E. Endothelial protective agents
I. The GenousTM Bio–engineered R stentTM
Endothelial progenitor cells have been identified as a key factor for re–endothelialization. The GenousTM Bio–engineered R stentTM (Orbus–Neich, Fort Lauderdale, Florida) is developed to enhance accumulation of endothelial progenitor cells at the site of arterial injury after stent implantation, in order to rapidly create a functional endothelial layer and thus reduce potential thrombosis and restenosis. Endothelial progenitor cell recruitment is achieved through surface immobilized antibodies directed toward endothelial progenitor cell N surface antigens (Fig. 10). The HEALING–FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth–First In Man) registry was the first clinical investigation using this technology. The nine–month composite major adverse cardiac and cerebrovascular events rate was 6.3%.83 This preliminary study demonstrated that GenousTM Bio–engineered R stentTM implantation is safe and feasible. The HEALING II trial is a multi–center, prospective, registry study, which aims to evaluate the safety and the effectiveness of the GenousTM Bio–engineered R stentTM in single de novo native coronary lesions. In the study 63 patients were included in total from 10 European centers, and the primary endpoint is MACE rate at 30 days.

F. Other anti–restenotic agents
I. Dexamethasone–eluting stent
Dexamethasone crosses target cell membranes and binds to the cytoplasmic glucocorticoid receptor, resulting to its activation. The activated receptor–dexamethasone complex then migrates to the nucleous, where it binds to Glucocorticoid Response Elements in the DNA causing modification of protein synthesis and inhibition of inflammatory responses. A Transcription Activator Protein can interact with the activated receptor–dexamethasone complex to modify collagenase and interleukins. During this process cytokines may also be affected through a similar process. Glucocorticoids also exert an effect on the prostaglandin synthesis pathway, which is responsible for the production of the lipid–in–flammatory mediators.
In the clinical setting, the STRIDE (STudy of anti–Restenosis with the BIodivYsio Dexamethasone–Eluting stent) trial evaluated the safety and the efficacy of the BiodivYsio Matrix LO stentTM loaded with dexamethasone (0.5 µg/mm2 of stent). In the study were enrolled 71 patients. During the 30–day follow–up, one patient developed a non–Q–wave myocardial infarction. At 6–month follow–up, two patients presented with recurrence of symptoms. Both showed a significant restenosis in the study stent, treated with balloon angioplasty. Six–months restenosis rate was 13.3% with a late loss of 0.45 mm.84 The preliminary STRIDE study demonstrated relatively low MACE and revascularization rate.
II. Terumo statin release stent
Preliminary experiments have shown that statins have a potent anti–proliferative effect on smooth muscle cells.85 Additionally, simvastatin appears to reduce neointimal hyperplasia, while enhancing re–endothelialization.86 Currently, animal studies explore the utilization of the TsumaniTM stent (Terumo Corporation, Tokyo, Japan) as the platform for local simvastatin delivery.
G. Experimental agents
I. Antisense oligonucleotide–eluting stent
Antisense oligomers are polymers designed to interfere with the information transfer from the gene to the protein. Thus, they may target specific genetic sequences that potentially play an important role at particular stages during disease progress. However, currently, clinical applicability of this technology remains limited mainly due to a relative lack of target specificity, slow uptake across the cell membrane, and rapid intracellular degradation of the oligonucleotide.
A new antisense oligomer was recently introduced. The AVI–4126 belongs to the Phosphorodiamidate Morpholino Oligomers, which are capable of binding to RNA in a sequence–specific fashion with sufficient avidity to be useful for the inhibition of the translation of the of mRNA into protein in vivo, a result referred to as an <<antisense>> effect. The AVI–4126 antisense oligomer blocks the expression of c–myc protein, which is responsible for many of the pathologies, associated with restenosis.87 In the experimental setting, animal studies have shown that AVI–4126 can efficiently reduce neo–intimal formation, without inducing aneurysm formation.87
The AVAIL trial evaluated the safety and efficiency of local AVI–4126 delivery. Forty–six patients were randomized into a low dose group (3 mg), a high dose group (10 mg) and a control group. Procedural success was 80.8%, with no MACE in any group. At 6 months follow–up, 6 patients from the low dose group and 3 patients from the control group underwent TVR, whereas only one patient from the high dose group was submitted to TVR. Angiographic and IVUS follow–up demonstrated 44.12 ± 7.75% restenosis rate in the low dose group, 39.50 ± 7.34% in the control group and only 18.55 ± 5.41% in the high dose group.88
Conclusions
Although conventional drug–eluting stents have shown remarkable effectiveness in reducing both angiographically and clinically defined restenosis, there are still several caveats and concerns such as late thrombosis, hypersensitivity, abnormal vasomotion, etc.
Following the first generation of sirolimus and paclitaxel–eluting stents, a variety of new stent designs, coatings and locally delivered agents are emerging and most likely will become available in a few years time.
Elimination of neointimal hyperplasia is no longer the ultimate goal, but also the maintenance of a functional endothelial lining has been identified as an important factor in controlling the growth of the underlying vascular tissue. Development of more biocompatible and bioabsorbable stents facilitating enhanced endothelialization, is expected in the near future and may offer an alternative therapeutic approach to optimally abolish restenosis.
References
1. Dangas G, Fuster V: Management of restenosis after coronary intervention. Am Heart J 1996; 132(2 Pt l): 428–36. [ Links ]
2. Kastrati A, Mehilli J, Dirschinger J, Pache J, Ulm K, Schuhlen H, et al : Restenosis after coronary placement of various stent types. Am J Cardiol 2001; 87(1): 34–9. [ Links ]
3. Van Belle E, Bauters C, Asahara T, Isner JM: Endothelial regrowth after arterial injury: from vascular repair to therapeutics. Cardiovasc Res 1998; 38(1): 54–68. [ Links ]
4. Liu MW, Roubin GS, King SB, 3rd: Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation 1989; 79(6): 1374–87. [ Links ]
5. Filippatos G, Parissis JT, Adamopoulos S, Kardaras F: Chemokines in cardiovascular remodeling: clinical and therapeutic implications. Curr Mol Med 2003; 3(2): 139–47. [ Links ]
6. Galis ZS, Khatri JJ: Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 2002; 90(3): 251–62. [ Links ]
7. Winslow RD, Sharma SK, Kim MC: Restenosis and drug–eluting stents. Mt Sinai J Med 2005; 72(2): 81–9. [ Links ]
8. Regar E, Sianos G, Serruys PW: Stent development and local drug delivery. Br Med Bull 2001; 59: 227–48. [ Links ]
9. Rogers CD: Optimal stent design for drug delivery. Rev Cardiovasc Med 2004; 5 Suppl 2: S9–S15. [ Links ]
10. Serruys PW, Regar E, Carter AJ: Rapamycin eluting stent: the onset of anew era in interventional cardiology. Heart 2002; 87(4):305–7. [ Links ]
11. Gingras AC, Raught B, Sonenberg N: mTOR signaling to translation. Curr Top Microbiol Immunol 2004; 279: 169–97. [ Links ]
12. Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, et al: A randomized comparison of a sirolimus–eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002; 346(23): 1773–80. [ Links ]
13. Fajadet J, Morice MC, Bode C, Barragan P, Serruys PW, Wijns W, et al : Maintenance of long–term clinical benefit with sirolimus–eluting coronary stents: three–year results of the RAVEL trial. Circulation 2005; 111(8): 1040–4. [ Links ]
14. Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, et al: Sirolimus–eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003; 349(14): 1315–23. [ Links ]
15. Holmes DR, Jr., Leon MB, Moses JW, Popma JJ, Cutlip D, Fitzgerald PJ, et al: Analysis of 1–year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus–eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation 2004; 109(5):634–40. [ Links ]
16. Schampaert, Cohen EA, Schluter M, Reeves F, Traboulsi M, Title LM, et al: The Canadian study of the sirolimus–eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C–SIRIUS). J Am Coll Cardiol 2004; 43(6): 1110–5. [ Links ]
17. Schofer J, Schluter M, Gershlick AH, Wijns W, Garcia E, Schampaert E, et al: Sirolimus–eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double–blind, randomized controlled trial (E–SI–RIUS). Lancet 2003; 362(9390): 1093–9. [ Links ]
18. Giannakakou P, Robey R, Fojo T, MV B: Low concentrations ofpaclitaxel induce cell type–dependent p53, p21 and G1/G2 arrest instead of mitotic arrest: molecular determinants of paclitaxel–induced cytotoxicity. Oncogene 2001; 20(29): 3806–13. [ Links ]
19. Lansky AJ, Costa RA, Mintz GS, Tsuchiya Y, Mdei M, Cox DA, et al: Non–polymer–basedpa–clitaxel–coated coronary stents for the treatment of patients with de novo coronary lesions: angiographic follow–up of the DELIVER clinical trial. Circulation 2004; 109(16): 1948–54. [ Links ]
20. Grube E, Silber S, Hauptmann KE, Mueller R, Buellesfeld L, Gerckens U, et al: TAXUS I: six–and twelve–month results from a randomized, double–blind trial on a slow–release paclitaxel–eluting stent for de novo coronary lesions. Circulation 2003; 107(1): 38–42. [ Links ]
21. Serruys PW, Degertekin M, Tanabe K, Russell ME, Guagliumi G, Webb J, et al: Vascular responses at proximal and distal edges ofpaclitaxel–eluting stents: serial intravascular ultrasound analysis from the TAXUS II trial. Circulation 2004; 109(5): 627–33. [ Links ]
22. Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, et al: One–year clinical results with the slow–release, polymer–based, paclitaxel–eluting TAXUS stent: the TAXUS–IV trial. Circulation 2004; 109(16): 1942–7. [ Links ]
23. Hermiller JB, Raizner A, Cannon L, Gurbel PA, Kutcher MA, Wong SC, et al: Outcomes with the polymer–based paclitaxel–eluting TAXUS stent in patients with diabetes mellitus: the TAXUS–IV trial. J Am Coll Cardiol 2005; 45(8): 1172–9. [ Links ]
24. Stone GW, Ellis SG, Cannon L, Greenberg JD, Tift Mann JT, Spriggs D, et al: Outcomes of the Polymer–Based, Paclitaxel–Eluting Taxus Stent In Complex Lesions: Principal Clinical and Angiographic Results From the Taxus–V Pivotal Randomized Trial. 54th Annual Scientific Session 2005. [ Links ]
25. Grube E: Randomized Trial of Moderate–Rate Release Polymer–Based Paclitaxel–Eluting TAXUS stent for the Treatment of Longer Lesions. EUROPCR 2005, Paris, France 2005. [ Links ]
26. Morice MC, Serruys PW, Colombo A, Meier B, Tamburino C, Guagliumi G, et al: Prospective Randomized Multi–Center Head–to–Head Comparison of the Sirolimus–Eluting Stent (Cypher) and the Paclitaxel–Eluting Stent (Taxus) (REALITY). Sunday, Mar 06, 2005. 2005; 54th ACC Annual Scientific Session, Orlando, USA. [ Links ]
27. Windecker S: SIRTAX: Randomized Comparison of a Sirolimus– vs a Paclitaxel–Eluting Stent for Coronary Revascularization. In: 54th ACC Annual Scientific Session; 2005; Orlando, USA; 2005. [ Links ]
28. Dibra A, Kastrati A, Mehilli J, Pache J, Schuhlen H, von Beckerath N, et al: Paclitaxel–eluting or sirolimus–eluting stents to prevent restenosis in diabetic patients. N Engl J Med 2005; 353(7): 663–70. [ Links ]
29. Kastrati A, Mehilli J, von Beckerath N, Dibra A, Pache J, Schuhlen H, et al: ISAR–DESIRE: Drug–Eluting Stents for In–Stent Restenosis. In: European Society of Cardiology Congress 2004; 2004; Munich, Germany; 2004. [ Links ]
30. Lemos PA, Serruys PW, van Domburg RT, Saia F, Arampatzis CA, Hoye A, et al : Unrestricted utilization of sirolimus–eluting stents compared with conventional bare stent implantation in the <<real world>>: the Rapamycin–Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) registry. Circulation 2004; 109(2): 190–5. [ Links ]
31. Ong AT, Serruys PW, Aoki J, Hoye A, Van Meghem CA, Rodríguez–Granillo GA, et al: The unrestricted use of paclitaxel– versus sirolimus–eluting stents for coronary artery disease in an unselected population: one–year results of the Taxus–Stent Evaluated at Rotterdam Cardiology Hospital (T–SEARCH) registry. J Am Coll Cardiol 2005; 45(7): 1135–41. [ Links ]
32. Lemos PA, Hoye A, Goedhart D, Arampatzis CA, Saia F, van der Giessen WJ, et al: Clinical, angiographic, and procedural predictors of angiographic restenosis after sirolimus–eluting stent implantation in complex patients: an evaluation from the Rapamycin–Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) study. Circulation 2004; 109(11): 1366–70. [ Links ]
33. Chieffo A, Stankovic G, Bonizzoni E, Tsagalou E, Iakovou I, Montorfano M, et al: Early and mid–term results of drug–eluting stent implantation in unprotected left main. Circulation 2005; 111(6): 791–5. [ Links ]
34. Valgimigli M, Van Meghem CA, Ong AT, Aoki J, Granillo GA, McFadden EP, et al: Short– and long–term clinical outcome after drug–eluting stent implantation for the percutaneous treatment of left main coronary artery disease: insights from the Rapamycin–Eluting and Taxus Stent Evaluated At Rotterdam Cardiology Hospital registries (RESEARCH and T–SEARCH). Circulation 2005; 111(11): 1383–9. [ Links ]
35. Colombo A, Moses JW, Morice MC, Ludwig J, Holmes DR Jr, Spanos V, et al: Randomized study to evaluate sirolimus–eluting stents implanted at coronary bifurcation lesions. Circulation 2004; 109(10): 1244–9. [ Links ]
36. Sharma SK: Simultaneous kissing drug–eluting stent technique for percutaneous treatment of bifurcation lesions in large–size vessels. Catheter Cardiovasc Interv 2005; 65(1): 10–6. [ Links ]
37. Nakamura S, Muthusamy TS, Bae JH, Cahyadi YH, Udayachalerm W, Tresukosol D: Impact of sirolimus–eluting stent on the outcome of patients with chronic total occlusions. Am J Cardiol 2005; 95(2): 161–6. [ Links ]
38. Ge L, Iakovou I, Cosgrave J, Chieffo A, Montorfano M, Mchev I, et al: Immediate and mid–term outcomes of sirolimus–eluting stent implantation for chronic total occlusions. Eur Heart J 2005; 26(11): 1056–62. [ Links ]
39. Werner GS, Krack A, Schwarz G, Prochnau D, Betge S, Figulla HR: Prevention of lesion recurrence in chronic total coronary occlusions by paclitaxel–eluting stents. J Am Coll Cardiol 2004; 44(12): 2301–6. [ Links ]
40. Hanekamp CE, Koolen JJ, Den Heijer P, Schalij MJ, Piek JJ, Bar FW, et al: Randomized study to compare balloon angioplasty and elective stent implantation in venous bypass grafts: the Venestent study. Catheter Cardiovasc Interv 2003; 60(4): 452–7. [ Links ]
41. Hoye A, Lemos PA, Arampatzis CA, Saia F, Tanabe K, Degertekin M, et al: Effectiveness of the sirolimus–eluting stent in the treatment of saphenous vein graft disease. J Invasive Cardiol 2004; 16(5): 230–3. [ Links ]
42. Ge L, Iakovou I, Sangiorgi GM, Chieffo A, Melzi G, Cosgrave J, et al: Treatment of saphenous vein graft lesions with drug–eluting stents: immediate and midterm outcome. J Am Coll Cardiol 2005; 45(7): 989–94. [ Links ]
43. Tsuchida K, Ong AT, Aoki J, Van Meghem CAG, Rodríguez–Granillo GA, Valgimigli M, et al: Immediate and one–year outcome of percutaneous intervention of saphenous vein graft disease with paclitaxel–eluting stents. Am J Cardiol 2005; (in press). [ Links ]
44. Degertekin M, Regar E, Tanabe K, Smits PC, van der Giessen WJ, Carlier SG, et al: Sirolimus–eluting stent for treatment of complex in–stent restenosis: the first clinical experience. J Am Coll Cardiol 2003; 41(2): 184–9. [ Links ]
45. Sousa JE, Costa MA, Abizaid A, Sousa AG, Feres F, Mattos LA, et al: Sirolimus–eluting stent for the treatment of in–stent restenosis: a quantitative coronary angiography and three–dimensional intravascular ultrasound study. Circulation 2003; 107(1): 24–7. [ Links ]
46. Saia F, Lemos PA, Arampatzis CA, Hoye A, Degertekin M, Tanabe K, et al: Routine sirolimus eluting stent implantation for unselected in–stent restenosis: insights from the rapamycin eluting stent evaluated at Rotterdam Cardiology Hospital (RESEARCH) registry. Heart 2004; 90(10): 1183–8. [ Links ]
47. Neumann FJ, Desmet W, Grube E, Brachmann J, Presbítero P, Rubartelli P, et al: Effectiveness and safety of sirolimus–eluting stents in the treatment ofrestenosis after coronary stentplacement. Circulation 2005; 111(16): 2107–11. [ Links ]
48. Tanabe K, Serruys PW, Grube E, Smits PC, Selbach G, Van Der Giessen WJ, et al TAXUS III Trial: in–stent restenosis treated with stent–based delivery of paclitaxel incorporated in a slow–release polymer formulation. Circulation 2003; 107(4): 559–64. [ Links ]
49. Komatsu R, Ueda M, Naruko T, Kojima A, Becker AE: Neointimal tissue response at sites of coronary stenting in humans: macroscopic, histological, and immunohistochemical analysis. Circulation 1998; 98(3): 224–33. [ Links ]
50. Ong AT, Hoye A, Aoki J, van Mieghem CA, Rodriguez–Granillo GA, Sonnenschein K, et al: Thirty–day incidence and six–month clinical outcome ofthrombotic stent occlusion after bare–metal, sirolimus, orpaclitaxel stent implantation. J Am Coll Cardiol 2005; 45(6): 947–53. [ Links ]
51. Bavry AA, Kumbhani DJ, Helton TJ, Bhatt DL: What is the risk of stent thrombosis associated with the use of paclitaxel–eluting stents for percutaneous coronary intervention?: a meta–analysis. J Am Coll Cardiol 2005; 45(6): 941–6. [ Links ]
52. Moreno R, Fernandez C, Hernandez R, Alfonso F, Angiolillo DJ, Sabate M, et al: Drug–eluting stent thrombosis: results from a pooled analysis including 10 randomized studies. J Am Coll Cardiol 2005; 45(6): 954–9. [ Links ]
53. Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, et al: Incidence, predictors, and outcome of thrombosis after successful implantation of drug–eluting stents. JAMA 2005; 293(17): 2126–30. [ Links ]
54. McFadden EP, Stabile E, Regar E, Cheneau E, Ong AT, Kinnaird T, et al: Late thrombosis in drug–eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 2004; 364(9444): 1519–21. [ Links ]
55. Ong AT, McFadden EP, Regar E, de Jaegere PP, van Domburg RT, Serruys PW: Late angiographic stent thrombosis (LAST) events with drug–eluting stents. J Am Coll Cardiol 2005; 45(12): 2088–92. [ Links ]
56. Serruys PW, Ong AT, Colombo A, Dawkins K, de Bruyne B, Fajadet J, et al: Arterial Revascularization Therapies Study Part II: Sirolimus–Eluting Stents for the Treatment of Patients With Multivessel De Novo Coronary Artery Lesions. Euro Intervention 2005; 2: 146–157. [ Links ]
57. Ong AT, Serruys PW, Mohr FW, Morice MC, Kappetein AP, Holmes DR, et al: The SYNergy between Percutaneous Coronary Intervention with TAXust and Cardiac Surgery (SYNTAX) Study: Design, Rationale and Run–In Phase. Am Heart J 2005; (in press). [ Links ]
58. Heublein B, Rohde R, Kaese V, Niemeyer M, Hartung W, Haverich A: Biocorrosion of magnesium alloys: a new principle in cardiovascular implant technology? Heart 2003; 89(6): 651–6. [ Links ]
59. Di Mario C, Griffiths H, Goktekin O, Peeters N, Verbist J, Bosiers M, et al: Drug–eluting bioabsorbable magnesium stent. J Interv Cardiol 2004; 17(6): 391–5. [ Links ]
60. Agrawal CM, Haas KF, Leopold DA, Clark HG: Evaluation of poly (L–lactic acid) as a material for intravascularpolymeric stents. Biomaterials 1992; 13(3): 176–82. [ Links ]
61. Vogt F, Stein A, Rettemeier G, Krott N, Hoffmann R, vom Dahl J, et al: Long–term assessment of a novel biodegradable paclitaxel–eluting coronary polylactide stent. Eur Heart J 2004; 25(15): 1330–40. [ Links ]
62. Tamai H, Igaki K, Kyo E, Kosuga K, Kawashima A, Matsui S, et al: Initial and 6–month results of biodegradable poly–l–lactic acid coronary stents in humans. Circulation 2000; 102(4): 399–404. [ Links ]
63. Wieneke H, Dirsch O, Sawitowski T, Gu YL, Brauer H, Dahmen U, et al : Synergistic effects of a novel nanoporous stent coating and tacrolimus on intima proliferation in rabbits. Catheter Cardiovasc Interv 2003; 60(3): 399–407. [ Links ]
64. Kollum M, Farb A, Schreiber R, Terfera K, Arab A, Geist A, et al: Particle debris from a nanoporous stent coating obscures potential antiproliferative effects of tacrolimus–e luting stents in a porcine model ofrestenosis. Catheter Cardiovasc Interv 2005; 64(1): 85–90. [ Links ]
65. Wessely R, Hausleiter J, Michaelis C, Jaschke B, Vogeser M, Milz S, et al: Inhibition ofneoin–tima formation by a novel drug–eluting stent system that allows for dose–adjustable, multiple, and on–site stent coating. Arterioscler Thromb Vase Biol 2005; 25(4): 748–53. [ Links ]
66. Hausleiter J, Kastrati A, Wessely R, Dibra A, Mehilli J, Schratzenstaller T, et al: Prevention ofrestenosis by a novel drug–eluting stent system with a dose–adjustable, polymer–free, on–site stent coating. Eur Heart J 2005; 26(15): 1475–81. [ Links ]
67. Serruys PW, Sianos G, Abizaid A, Aoki J, den Heijer P, Bonnier H, et al: The effect of variable dose and release kinetics on neointimal hyperplasia using a novel paclitaxel–eluting stent platform: the Paclitaxel In–Stent Controlled Elution Study (PISCES). J Am Coll Cardiol 2005; 46(2): 253–60. [ Links ]
68. Kaul U: Erodablepolymer–reservoir Paclitaxel–drug eluting stents II: Cobalt chromium platforms in complex lesions. In: TCT; 2004; Washington, USA; 2004. [ Links ]
69. Serruys PW: The new CoStarTM stent: 12 month results with the new DES technology. In: EuroPCR 2005; 2005; Paris, France; 2005. [ Links ]
70. Dawkins K: The Eurostar trial. In: EuroPCR 2005; 2005; Paris, France; 2005. [ Links ]
71. Gambhir DS: The Infinnium polymere–based paclitaxel drug–eluting stent: Results from real world clinical trials SIMPLE 1 and SIMPLE 2. In: TCT2004; 2004; Washington, USA; 2004. [ Links ]
72. Windecker S, Mayer I, De Pasquale G, Maier W, Dirsch O, De Groot P, et al: Stent coating with titanium–nitride–oxide for reduction of neointimal hyperplasia. Circulation 2001; 104(8): 928–33. [ Links ]
73. Windecker S, Simon R, Lins M, Klauss V, Eberli FR, Roffi M, et al: Randomized comparison of a titanium–nitride–oxide–coated stent with a stainless steel stent for coronary revascularization: the TiNOX trial. Circulation 2005; 111(20): 2617–22. [ Links ]
74. Meredith IT, Ormiston J, Whitbourn R, Kay P, Muller D, Popma JJ, et al: ENDEAVOR I <<first–in–human>> safety and efficacy study. 12–month clinical, angiographic, and intravascular ultrasound results. Circulation 2004; 110: III–563–III–564. [ Links ]
75. Wijns W, Fajdet J, Kuntz RE: A randomized comparison of the endeavor ABT–578 drug Eluting Stent With a Bare Metal Stent for Coronary Revascularization: Results of the Endeavor II trail. In: 54th ACC Annual Scientific Session; 2005; Orlando USA; 2005 [ Links ]
76. Costa RA, Lansky AJ, Mntz GS, Mehran R, Tsuchiya Y, Negoita M, et al: Angiographic results of the first human experience with everolimus–eluting stentsfor the treatment of coronary lesions (the FUTURE I trial). Am J Cardiol 2005; 95(1): 113–6. [ Links ]
77. Grube E, Sonoda S, Ikeno F, Honda Y, Kar S, Chan C, et al: Six– and twelve–month results from first human experience using everolimus–eluting stents with bioabsorbable polymer. Circulation 2004; 109(18): 2168–71. [ Links ]
78. Grube E: FUTURE II: Multicenter evaluation of the bioabsorbable polymer–based everolimus–eluting stent. In: TCT; 2003; Washington, USA; 2003. [ Links ]
79. Serruys PW, Ong ATL, Piek JJ, Neumann FJ, van der Giessen WJ, Wiemer M, et al: A randomized comparison of a durable polymer Everolimus–eluting stent with a bare metal coronary stent: The SPIRIT first trial. Eurolntervention 2005; 1(1): 58–69. [ Links ]
80. Grube E, Hauptmann KE, Buellesfeld L, Lim V, Abizaid A: Six–month results of a randomized study to evaluate safety and efficacy of a Biolimus A9t eluting stent with a biodegradable polymer coating. Euro Intervention 2005; 1: 53–7. [ Links ]
81. Stepkowski SM: Molecular targets for existing and novel immunosuppressive drugs. Expert Rev Mol Med 2000; 2000: 1–23. [ Links ]
82. Morice MC: JUPITER II Trial. Six–month interim clinical data. In: EuroPCR 2005; 2005; Paris, France; 2005. [ Links ]
83. Aoki J, Serruys PW, van Beusekom H, Ong AT, McFadden EP, Sianos G, et al: Endothelial progenitor cell capture by stents coated with antibody against CD34: the HEALING–FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth–First In Man) Registry. J Am Coll Cardiol 2005; 45(10): 1574–9. [ Links ]
84. Liu X, Huang Y, Hanet C, Vandormael M, Legrand V, Dens J, et al: Study ofantirestenosis with the BiodivYsio dexamethasone–eluting stent (STRIDE): a first–in–human multicenter pilot trial. Catheter Cardiovasc Interv 2003; 60(2): 172–8; discussion 179. [ Links ]
85. Lefer AM, Scalia R, Lefer DJ: Vascular effects of HMG CoA–reductase inhibitors (statins) unrelated to cholesterol lowering: new concepts for cardiovascular disease. Cardiovasc Res 2001; 49(2): 281–7. [ Links ]
86. Tanabe K, Ishiyama H, van der Giessen W, Serruys PW: Terumo statin releasing stent (preclinical results). In: Serruys PW, Gershlick A, editors. Drug–Eluting Stents. London: Taylor and Francis Group; 2005: 313–321. [ Links ]
87. Kipshidze NN, Iversen P, Kim HS, Yiazdi H, Dangas G, Seaborn R, et al: Advanced c–myc antisense (AVI–4126)–eluting phosphorylcholine–coated stent implantation is associated with complete vascular healing and reduced neointimal formation in the porcine coronary resteno–sis model. Catheter Cardiovasc Interv 2004; 61(4): 518–27 [ Links ]
88. Kipshidze N, Overlie P, Dunlap T, Titus B, Lee D, Lauer M: First human experience with local delivery of novel antisense AVI–4126 with infiltrator catheter in de novo native and restenotic coronary arteries: Six–month clinical and angiographic follow–up from AVAIL study. In: American Heart Association, Scientific Sessions 2004; 2004; New Orleans, USA; 2004. [ Links ]
89. Regar E, Serruys PW, Bode C, Holubarsch C, Guermonprez XL, Wijns W, et al: Angiographic findings of the multicenter Randomized Study With the Sirolimus–Eluting Bx Velocity Balloon–Expandable Stent (RAVEL): sirolimus–eluting stents inhibit restenosis irrespective of the vessel size. Circulation 2002; 106(15): 1949–56. [ Links ]
90. Colombo A, Drzewiecki J, Banning A, Grube E, Hauptmann K, Silber S, et al: Randomized study to assess the effectiveness of slow– and moderate–release polymer–based paclitaxel–eluting stents for coronary artery lesions. Circulation 2003; 108(7): 788–94. [ Links ]
91. Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, et al: Apolymer–based, paclitaxel–eluting stent in patients with coronary artery disease. N Engl J Med 2004; 350(3): 221–31. [ Links ]
92. Morice MC, Serruys PW, CostantiniC,Wuelfert E, Wijns W, Fajadet J, et al: Two–Year Follow –Up of the RAVEL Study: A Randomized Study With the Sirolimus–Eluting Bx VELOCITYt Stent in the Treatment of Patients With De Novo Native Coronary Artery Lesions. J Am Coll Cardiol 2003; 41(6(Suppl A)): 32A [ [ Links ]abstract].
93. Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, et al: A randomized comparison of a sirolimus–eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002; 346(23): 1773–80. [ Links ]
94. Meredith I: Endeavor DES Program. Endeavor I–IV summary update. In: TCT2004; 2004; Washington, USA; 2004. [ Links ]
95. Chevalier B: ZoMaxx: global and clinical programme development. In: Euro PCR 2005; 2005; Paris, France; 2005. [ Links ]
96. Grube E: FUTURE Clinical Trials. ChampionTM Everolimus Eluting Coronary Stent System. In: TCT2004; 2004; Washington, USA; 2004. [ Links ]














