Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos de cardiología de México
versión On-line ISSN 1665-1731versión impresa ISSN 1405-9940
Arch. Cardiol. Méx. vol.75 no.4 Ciudad de México oct./dic. 2005
Investigación clínica
Risk factors for prolonged mechanical ventilation after surgical repair of congenital heart disease
Factores de riesgo por ventilación mecánica prolongada tras cirugía cardíaca por cardiopatía congénita
José A. García–Montes,* Juan Calderón–Colmenero,* Miguel Casanova,* Ernesto Zarco,* Guillermo Fernández de la Reguera,* Alfonso Buendía*
* Departamento de Cardiología Pediátrica, INC "Ignacio Chávez".
Correspondence to:
Dr. José A. García–Montes.
Instituto Nacional de Cardiología "Ignacio Chávez"
(INCICH, Juan Badiano Núm. 1 Col. Sección XVI Tlalpan,
14080 México, D.F.)
E–mail: pepegamon@yahoo.com
Recibido: 18 de octubre de 2005
Aceptado: 16 de noviembre de 2005
Summary
Objective: The purpose of this study was to determine factors contributing to prolonged mechanical ventilation in children following surgery for congenital heart defects.
Design: Prospective cohort trial.
Setting: Critical Care Unit. "Ignacio Chavez" National Heart Institute, México; from January to December 2000.
Patients: A total of 318 consecutive patients <18 years old who underwent cardiovascular surgical procedures for congenital heart defects were enrolled in this study. Of these, 297 patients were admitted to the intensive care unit with respiratory support and 2.8% required prolonged mechanical ventilation (MV) > 120 hours.
Measurements and main results: Patients with cardiac failure had MVt ime of 214 ± 200 hours, whereas those without it had MV time of 33 ± 73 hours (p > 0.001). Patients with severe pulmonary hypertension had MV time of 160 ± 176 hours, while those who did not had MV time of 47 ± 105 hours (p < 0.001). Twenty–four patients (8.5%) had extubation failure, in 79% them due to hemodynamic alteration during the respiratory support time of 277 ± 188 hours versus the rest of the group with MV time of 41 ± 92 hours (p < 0.001). Factors associated with prolonged MV(> 120 hours) were: patients ages of < 1 year, pulmonary hypertension, and cardiac failure, and represented the greatest risk (90%, Cl 58 to 99) of prolonging MV. Mortality rate was 34% for patients with prolonged MV. Times of aortic clamping and cardiopulmonary bypass were not significant risk factors for prolonged respiratory support.
Conclusion: Patients ages of < 1 year old, pulmonary hypertension, and cardiac failure were significant risk factors for prolonged respiratory support.
Key words: Mechanical ventilation. Cardiac surgery. Congenital heart disease.
Resumen
Objetivo: El propósito del estudio fue establecer los factores de riesgo para ventilación mecánica prolongada en pacientes menores de 18 años sometidos a cirugía cardíaca.
Tipo de estudio: Prospectivo. Unidad de Cuidados Intensivos Postoperatorios. Instituto Nacional de Cardiología "Ignacio Chávez". Del 1º de enero al 31 de diciembre de 2000.
Material y métodos: Fueron analizados 318 pacientes consecutivos menores de 18 años con cardiopatía congénita sometidos a cirugía cardíaca paliativa o correctiva.
Resultados: Los pacientes con insuficiencia cardíaca tuvieron un tiempo de ventilación mecánica de 214 ± 200 horas, mientras los pacientes sin insuficiencia cardíaca sólo requirieron 33 ± 73 horas (p > 0.001). En presencia de hipertensión pulmonar severa el tiempo de asistencia ventilatoria fue de 160 ± 176 horas, en comparación con los que no la tenían, que fue de 47 ± 105 (p < 0.001). En 24 pacientes (8.5%) fue la extubación fallida siendo por factor hemodinámico en 79% y requirieron de ventilación mecánica de 277 ±188 horas versus el resto del grupo con 41 ± 92 horas (p < 0.001). Los factores asociados con ventilación mecánica prolongada (> 120 horas) fueron: edad < 1 año, hipertensión pulmonar severa e insuficiencia cardíaca (90%, LC 58 a 99). La mortalidad, en los pacientes que requirieron ventilación mecánica prolongada, fue del 34%. El tiempo de circulación extracorpórea o de pinzamiento aórtico no fueron factores de riesgo para la necesidad de ventilación mecánica prolongada. Conclusiones: En nuestra institución los factores de riesgo para ventilación mecánica prolongada, en pacientes menores de 18 años operados de cirugía cardíaca paliativa o correctiva fueron: pacientes menores de 1 año, hipertensión pulmonar severa e insuficiencia cardíaca.
Palabras clave: Cardiopatías congénitas. Ventilación mecánica. Cirugía cardíaca.
Introduction
Progress in surgical techniques, anesthesia, and intensive care has diminished the need for prolonged respiratory support. Early extubation decreases the likelihood of pulmonary complications and the number of day in the intensive care unit.1–4 Prolonged respiratory support may be necessary for any patient after surgical management of congenital heart defects. The factors associated with prolonged respiratory support are still unclear. Risk factors that have been thought to prolonged mechanical ventilation (MV) in children patients include: cardiopulmonary bypass and aortic clamping times, hemodynamic disturbances, respiratory disorders and infection, among others.
Extubation failure, defined as the need for rein–tubation and restart of respiratory support, has been reported with a frequency from 17% for adult patients, 6 to 16% for infants and children, to 22 to 28% of newborn patients undergoing surgical procedures for congenital heart defects.2–6
This study was performed to determine factors contributing to prolonged mechanical ventilation in children patients after surgical management, either corrective or palliative, for congenital heart defects.
Materials and methods
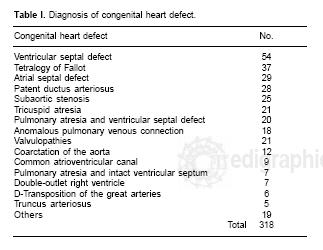
This retrospective study was approved by local ethics committee. We did not consent for the study was observational. Between January and December 2000,318 consecutive patients with congenital heart defects < 18 years of age underwent surgical management. When surgery required cardiopulmonary bypass, the surgical procedure was a median sternotomy, but when cardiopulmonary bypass was not required, a right or left thoracotomy was used depending on the pathology, such as, patent ductus arteriosus or coarctation of the aorta, systemic to pulmonary shunt procedure or bandage of the pulmonary artery. Patient pathologies are listed in Table I. Anesthetic management in the induction phase included: fentanyl, midazolam, pancuronium and the maintenance phase with sevoflorane, fentanyl, midazolam and pancuronium.
Mechanical ventilation was performed with cycling by time or by volume, with Vt 10 – 20 mL/kg, inspiratory time 0.5–1 sec, respiratory frequency 20 – 60 min, FiO2100% and PEEP 3–6 cmH20. Sedative and paralytic agents were administered as needed with intensive respiratory support, management of hemodynamic instability, pulmonary hypertension management, and, when necessary, avoiding, psychomotor agitation. MV was discontinued and patients were extubated with to the following criteria: a) hemodynamic stability; b) lack of pulmonary hypertensive crisis; c) adequate respiratory function and arterial blood gas measurements; d) lack of alteration in thoracic radiographic findings. Prolonged respiratory support was defined as the need for more than 120 hours of mechanical ventilation, and early extubation when it was possible to eliminate the MV before 8 post surgical hours. Severe pulmonary hypertension was defined as Rp/Rs > 0.65; ventricle systemic dysfunction was defined as ejection fraction < 60% or diastolic end pressure in the systemic ventricle > l0 mmHg.
Statistical analysis
Qualitative variables are presented as frequencies, proportions, and reasons. When appropriate, data are expressed as mean (SD). Numerical data were analyzed with the unpaired Student's t test, and categorical data were by using X2 analysis. If the distribution was abnormal, a non–parametric Mann– Whitney U test was used or odds ratio were calculated. Kruskal–Wallis analysis of variance was used to determine significant trends. Pearson correlation method with linear regression analysis was used to test for significant relationships between continuous variables. Significance was defined as a p value < .05.
Results
Of the 318 patients, 226 (71%) underwent open cardiovascular surgical procedures, and in 92 patients (29%) such procedures were performed without cardiopulmonary bypass. The mean patient age was 5 ± 5, with a range of 15 days to 18 years. The mortality was 9% (27 patients), mean age on patients who died was 1 ± 1.6 years, with a range of 15 days to 6 years. A total of 21 patients (6%) were taken to at the intensive care unit extubated. Of the remaining 297 patients, MV was discontinued within the first 8 hours of admission in 97 of them (32%). Thirty–eight patients (12%) required prolonged MV (< 120 hours) with a range of 144 to 720 hours; 28 of these patients (74%) underwent open heart surgery, with a mortality of 34% (13 patients).
The duration of cardiopulmonary bypass, aortic clamping and, type of anesthesia did not influence the duration of MV. On the other hand, age, cardiac failure, pulmonary hypertension, and reintubation were the factors most associated with prolonged MV. Patients younger than 1 year old required a longer period of mechanical ventilation than did older patients (Fig. I). Patients with cardiac failure had MV time of 214 ± 200 hours, whereas those patients without it had MV time of 33 ± 73 hours (p > 0.001) (Fig. 2). Presurgical pulmonary hypertension was documented in 87 patients (27%). Post–surgically, severe pulmonary hypertension was noted in 34 patients (10.6%). Patients with severe pulmonary hypertension had MV time of 160.41 ± 176.32 hours, while those who did not had MV time of 47 ± 105 hours (p < 0.001) (Fig. 3).



Twenty–four patients (8.5%) had extubation failure in 79% in whom it was due to hemodynamic alteration with respiratory support for 277 ± 188 hours versus the rest of the group with MV for 41 ± 92 hours (p < 0.001) (Table II). With an average age of 1.7 ± 2.2 years of the reintubated versus not reintubated of 5 ± 5 years (p < 0.001). Various factors were examined for likelihood of prolonging MV (> 120 hours). Age younger than 1 year, pulmonary hypertension, and cardiac failure were the principal factors increasing the likelihood for need the prolonged respiratory support in a 91% (CI58 to 99%) (Table III). The risk of death patients with MV lasting more than 120 hours who had, pulmonary hypertension, and cardiac failure was 40% (CI 12 to 73%) (Table IV). A total of 34% of patients with prolonged MV died versus 5% of patients who did not have prolonged MV (p > 0.001), with 6.8 times relative related risk (Table V).



Discussion
Among the benefits of mechanical ventilation following heart surgery with cardiopulmonary bypass the following are outstanding: improvement of pulmonary distensibility, increase in oxygen arterial content, reduction of respiratory work, and prevention of complications such as atelectasis, which can increase cardiac failure and pulmonary hypertension after a heart surgery especially in newborns and infants.7 All groups agree that extubation should be performed as early as possible, since early extubation diminishes pulmonary complications, number of days in the intensive care unit and cost of treatment as well. Heard and collaborators8 reported 67% early extubation in the first 6 hours and prolonged mechanical ventilation in 33%. Kloth9 reported that 48 of 102 patients (47%) could be extubated in the surgical room or when arriving at the intensive care unit. Neither of these two authors reported reintubation in these patients. In our study, 37% of patients were extubated early, 32% was in the intensive care unit, and the remainder in the surgical room.
Extubation failure occurred in 24 patients (8%), 20 of whom required prolonged ventilation (83%). In the literature, there are reports of extubation failure in 4.9% to 23%. In patients who need respiratory support for short periods failure of extubation is < 6%, and in those who required mechanical ventilation longer than 48 hours, the failure rate was increased more the 20%, especially in infant patients. The reintubation causes were: obstruction of the upper airway, hypoxia, respiratory acidosis, bronchoaspiration, arrhythmia, encephalopathy, phrenic nerve paralysis, infectious processes, accidental extubation, and cardiac failure. All these conditions contribute to the need for prolonged mechanical ventilation, and as a result, longer stay in the intensive care unit.10–14
For patients undergoing corrective surgery for congenital heart disease who were < 2 years old, Bandla and collaborators15 reported that in 41% of cases, it was necessary to maintain mechanical ventilation for more than 7 days, of these cases, 43% had respiratory alterations such as airway obstruction, pulmonary hypertension, phrenic nerve paralysis, and pleural effusion. In our study, the reasons for reintubation causes were cardiac failure in 79% of the cases, and infection, pneumothorax or hemothorax, and upper airway obstruction in the rest of the cases. Pulmonary hypertension can be the cause of morbidity, and mortality in children patients after heart surgery and could be a reason for prolonged respiratory support. Lindberg16 reported a 2% incidence of severe pulmonary hypertension, while our results were an incidence of 10% pulmonary hypertension, it being one of the relevant causes of prolonged mechanical ventilation.
Age, especially in the newborn or infant patients, is an important contributor to the need for prolonged mechanical ventilation. In addition to this, other factors are present, such as cardiac failure and/or pulmonary hypertension, in which cases, the possibilities of requiring postoperative prolonged mechanical ventilation in the postoperatory in congenital heart defects are increased. Furthermore, the 34% of our patients with prolonged mechanical ventilation died.21 Pereira and collaborators reported an incidence of 2.3% for subglotic stenosis in 300 open heart surgeries with an average mechanical ventilation period of 7 days (range 3 to 17 days), while we have experienced only one patient with upper airway obstruction who required reintubation.17–23 The duration of respiratory support following surgical correction of congenital heart defects must be determined individually for each patient and established in each institution in accordance with its own experience. A wrong inadequate decision regarding when to stop the respiratory support, will have a systemic repercussion with increase in morbidity and mortality, the need for a long stay in the intensive care unit and increased costs. A large, prospective multiple institution trial is necessary to clearly identify the causes and risks of prolonged mechanical ventilatory in young children after congenital heart surgery.
References
1. Heinle JS: Early extubation after cardiac operations in neonates and young infants. J Thorac Cardiovasc Surg 1997; 114: 413–418. [ Links ]
2. Kanter RK, Bove EL, Tobin JR, Zimmereman JJ: Prolonged mechanical ventilation of infants after open heart surgery. CritCareMed 1986; 14: 211–214. [ Links ]
3. Schuller JL, Bovill JG, Nijveld A, Patrick MR, Marcelletti C: Early extubation of the trachea after open heart surgery for congenital heart disease. J Anaesth 1984; 56: 1101–1107. [ Links ]
4. Barash PG, Lescovich F, Katz JD, Talner NS, Stansel JrHC: Early extubation following pedia–tric cardiothoracic operation: A viable alternative. An Thorac Surg 1980; 29: 228–233. [ Links ]
5. Venkataraman ST, Khan N, Brown A: Validation of predictors of extubation success and failure in mechanically ventilated infants and children. Crit Care Med 2000; 28: 2991–2996. [ Links ]
6. Kurachek SC, Newth CJ, Quasney MW, Rice T, Sachdeva RC, Patel NE, et at: Extubation failure inpediatric intensive care: A multiple–center study of risk factors and outcomes. Crit Care Med 2003; 31:2657–2664. [ Links ]
7. Jenkins J, Lynn A, Edmonds J, Barker G: Effects of mechanical ventilation on cardiopulmonary function in children after open–heart surgery. Crit Care Med 1985; 13:77–80. [ Links ]
8. Heard GG, Lamberti JJ, Mn–Park S, Waldman D, Waldman J: Early extubation after surgical repair of congenital heart disease. Crit Care Med 1985; 13: 830–832. [ Links ]
9. Kloth RL, Baum VC: Very early extubation in children after cardiac surgery. Crit Care Med 2002; 30:787–791. [ Links ]
10. Edmunds S, Weiss I, Harrison R: Extubation failure in largepediatric ICU population. Chest 2001; 119:897–900. [ Links ]
11. Beckmann U, Gillies DM: Factors associated with reintubation in intensive care. Chest 2001; 120: 538–542. [ Links ]
12. Epstein SK: Decision to extubate. Intensive Care Med 2002; 28: 535–546. [ Links ]
13. Chevron V, Ménard JF, Riuchard JC, Girault C, Leroy J, Bonmarchand G: Unplanned extubation: Risk factors of development and predictive criteria for reintubation. Crit Care Med 1998; 26:1049–1053. [ Links ]
14. Campbell RS: Extubation and the consequences of reintubation. Respir Care 1999; 44: 799–803. [ Links ]
15. Bandla HP, Hopkins RL, Beckerman RC, Gozal D: Pulmonary risk factors compromising postoperative recovery after surgical repair for congenital heart disease. Chest 1999; 116: 740–747. [ Links ]
16. Lindberg L, Olsson AK, Jogi P, Jonmarker C: How common is severe pulmonary hypertension after pediatric cardiac surgery. J Thorac Cardiovasc Surg 2002; 123: 1155–1163. [ Links ]
17. Marianeschi SM, Seddio F, McElhinney DB,Colagrande L, Abella RF, De la Torre T, et al: Fast–Track congenital heart operations: A less invasive technique and early extubation. Ann Thorac Surg 2000; 69: 872–876. [ Links ]
18. Mok Q, Ross–Russell R, Mulvery D, Green M, Shinebourne EA: Phrenic nerve injury in infants and children undergoing cardiac surgery. Br Heart J 1991; 65: 287–292. [ Links ]
19. Tonz M, von Segesser LK, Mihalijevic T, Arbenz U, Stauffer UG, Turina MI: Clinical implications of phrenic nerve injury after pediatric cardiac surgery. J Pediatr Surg 1996; 31: 1265–1267. [ Links ]
20. Hong–Xu Z, D'Agostino RS, Pitlick PT, Shumway NE, Miller DC: Phrenic nerve injury complicating closed cardiovascular surgical procedures for congenital disease. Ann Thorac Surg 1985; 39:445–449. [ Links ]
21. Davis S, Worley S, Mee R BB, Harrison M: Factors associated with early extubation after cardiac surgery inyoung children. Ped Crit Care Med 2004 5(1): 63–68. [ Links ]
22. Watanabe T, Trusler GA, Williams WG, Edmonds JF, Coles JG, Hosokawa Y: Phrenic nerve paralysis after pediatric cardiac surgery. J Thorac Cardiovasc Surg 1987; 94: 383–388. [ Links ]
23. Pereira KD, Mitchell RB, Younis RT, Lazar RH: Subglottic stenosis complicating cardiac surgery in children. Chest 1997; 111: 1769–1772. [ Links ]














