Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos de cardiología de México
versión On-line ISSN 1665-1731versión impresa ISSN 1405-9940
Arch. Cardiol. Méx. vol.73 no.4 Ciudad de México oct./dic. 2003
Investigación clínica
Mechanical factors of cardiovascular risk in systemic arterial hypertension. A new sign of arterial rigidity
Factores mecánicos de riesgo cardiovascular en la hipertensión arterial sistémica. Un nuevo signo de rigidez arterial
Gustavo Sánchez-Torres*, Oscar Infante-Vázquez,* Gustavo Sánchez-Miranda,* Ángel de León-Peña,* Raúl Martínez Memije*
* Instituto Nacional de Cardiología "Ignacio Chávez".
* Corresponding author at:
'Dr. Gustavo Sánchez Torres.
Instituto Nacional de Cardiología "Ignacio Chávez"
(INCICH, Juan Badiano 1, Col. Sección XVI, Tlalpan 14080, México D.F.).
Tel. 5573 5255 ext. 1386 Fax. 5573 0926.
E-mail: gsancheztorres@yahoo.com.mx
Recibido: 11 de febrero de 2003.
Aceptado: 23 de julio de 2003.
Abstract
Antecedent: By means of sphygmokinetocardiography (SKCG) we developed and arterial rigidity index (ARI) which measure the pulse wave aortic carotid reflexion time over the left ventricular ejection time (LVET). This index, together with the pulse wave velocity (PWV) and the pulse pressure (PP) are indicators of arterial stiffness. In this paper we measured these index in 27 systemic artery hypertension. Cases (group A, GA), with and without left ventricular hypertrophy (subgroups: A1 SGA1, n = 13, and A2, SGA2, n = 14), respectively, and 28 normotensive cases (group B, GB). Protocol: In two occasions: after 3 minutes of sitting position (SP) and after 3 minute of jogging in an upright position (UP), blood pressure, ARI, PP, PWV (aortic-hand finger distance/aorto-hand finger pulse time) and R-IV interval (electrocardiographic R wave-left early ventricular kinetocardiography deflexion) were measured. Results: Demography was similar in GA and GB. Systolic, diastolic and pulse pressure were significantly higher in GA vs GB. LVET (ms) was lower in GA vs GB in SP (268 ± 42 vs 274 ± 40, p < 0.001, respectively) and higher postexercise UP (280 ± 42 vs 244 ± 46, p < 0.001). PWV m/s were higher in SP in GA vs GB (9.8 ± 2.8 vs 7.4 ± 1.2, p < 0.001, respectively) and in UP (10.1 ± 1.9 vs 7.9 ± 9, p < 0.001, respectively). ARI was lower in UP in GA vs GB (0.48 ± 0.3 vs 0.80 ± 0.3, p < 0.003). Correlation index of PP vs SBP, vs DBP and vs PWV were significant in SP and in UP. Height had a significant correlation vs ARI in SP and UP (r = 0.60, p < 0.01, and r = 0.42, p < 0.05, respectively). Conclusion: PWV is increased in GA vs GB patients. The ARI index is lower in GA vs GB cases in post exercise. PWV and PP showed a statistical significant correlation; height vs ARI had also a significant correlation: SKCG is a new method, that uses a not commercially instrument, which should have clinical application.
Key words: Aarterial mechanicsm, Arterial Hypertension, Pulse velocity, Arterial reflexion.
Resumen
Antecedentes: Mediante esfigmoquinetocardiografía (EQCG) se desarrolló un índice de rigidez arterial (IRA) que mide el tiempo aorto-onda de reflexión arterial sobre el período expulsivo. Este índice junto con la velocidad de la onda del pulso (VOP) y la presión del pulso (PP) son signos de rigidez arterial. Aquí medimos estos indicadores en 27 casos con hipertensión arterial (grupo A, GA) con y sin hipertrofia del ventrículo izquierdo: subgrupo A1, SGA1 de 13 casos y 14 individuos (subgrupo A2, SGA2) respectivamente y 28 casos normotensos (grupo B, GB). Protocolo: En 2 ocasiones: después de 3 minutos de posición sedente (PS) y después de 3 minutos de trote en posición ortostática (PO), se midió: la presión arterial (PA), el IRA, la VOP (distancia aorta-dedo-mano/tiempo de la onda del pulso aorta-dedo-mano) la PP y el intervalo R-IV (onda R del electrocardiograma -final de la deflexión ventricular temprana en el EQCG. Resultados: La demografía fue similar en ambos grupos. La PP, las presiones sistólica y diastólica fueron más altas en el GA vs el GB. El PE (ms) fue menor en el GA vs el GB en PS (268 ± 42 vs 274 ± 40, p < 0.001, respect.) y más alto en PO (280 ± 42 vs 244 ± 46, p < 0.001) en el GA vs GB. La VOP m/s, fue más alta en PS en GA vs GB (9.8 ± 2.8 vs 7.4 ± 1.2, p < 0.001, respect.) y en PO (10.1 ± 1.9 vs 7.9 ± 9, p < 0.001, respect.). El IRA fue menor en PO en el GA vs GB (0.48 ± 0.3 vs 0.8 ± 0.3, p < 0.003). El índice de correlación de la PP vs PAS, PA y VOP tuvo significancia estadística en PS y en PO. La talla correlacionó con el IRA (r = 0.6, p < 0.01 en PS y r = 0.42, p < 0.05 en PO). Conclusiones: La VOP está aumentada y el IRA más bajo en el GA vs GB en PO (lo que indica mayor rigidez arterial). La VOP y la PP tuvieron correlación significativa con la talla al igual que esta última con el IRA. La EQCG es un método con aplicación clínica. (Arch Cardiol Mex 2003; 73:261-270).
Palabras clave: Mecánica arterial, Hipertensión arterial, Velocidad del pulso, Reflexión arterial.
Introduction
There is a dynamic and structural state of blood vessel hyperigidity in arterial systemic hypertension which increases the velocity of forward and retrograde arterial pulses,1 and changes the circulatory reflection sites. This favors the early arrival of reflexive waves to the aortic root. The latter phenomenon alters the ventricular-aortic relationship, elevating the central systolic pressure and the arterial pulse pressure. It also boosts the telesystolic parietal stress of the left ventricle causing coronary circulation dysfunction.2-4
The deleterious effect of the above mentioned mechanical impairment is of epidemiological and clinical importance: population studies have revealed that increased pulse wave velocity (PWV) and increased arterial pulse pressure are risk factors for left ventricular hypertrophy (LVH),5 total cardiovascular damage6 and myocardial infarction.7 Furthermore, transversal studies have demonstrated that these indicators are associated with brain hemorrhage,8 cutaneous hypertensive microvascular changes,9 renal failure-induced cardiovascular damage10 and advanced age, to mention a few.11
Because pulse pressure can be easily measured, considerable interest has risen to apply this measurement in everyday clinical practice.12 Numerous publications have shown that increased pulse pressure is a more potent cardiovascular risk indicator than systolic or diastolic blood pressures,13 even though the latter have proven predictive value.14 Moreover, the relationshipbetween pulse pressure and arterial rigidity has been clearly established in experimental observations.15,16 A clinical physiographic method called digital sphygmokinetocardiography was recently developed at our hospital.1 This method is useful to study important hemodynamic aspects of cardio-arterial function, as the following signals can be obtained from a patient in sitting position and in standing position after exercise: electrocardiography signals, finger photoplethysmography (f-PP), carotid oscillation pulse signals, as well as vibriograms generated by the contraction of the anterior and posterior left ventricle walls registered on the precordium and the subcostal abdominal aspect respectively.1,17 Based on this method, we developed an arterial rigidity index (ARI) which measures the interference caused by the arterial reflection wave to left ventricle ejection time interval.18 In the present study we analyze ARI, pulse wave velocity and pulse pressure, all indicators of arterial stiffness, to study hypertensive patients with and without left ventricular hypertrophy (LVH), as well as normotensive individual.
Objective
1) To analyze the correlation between the arterial rigidity index and pulse wave velocity as measured by sphygmokinetocardiography, as well as with systolic, diastolic and pulse pressures, in order to assess the relevance of these parameters in detecting arterial rigidity in everyday clinical practice.
2) To study the aortic-finger pulse wave velocity, the arterial reflection index and the electrocardiographic R-IV interval in hypertensive patients with and without LVH.
Methods
This clinical study was transversal, prospective, and analyzed age and gender matched cases and controls.
Subjects. We analyzed a total of 55 individuals belonging to two different groups: group A included 27 outpatients aged > 30 years and diagnosed with essential arterial hypertension, with no acute or chronic distressing diseases (particularly any type of organ failure), and who had not received any kind of pharmacological treatment at least 2 weeks before the study (8 cases had never been treated). All standard procedures to study hypertensive patients and a 12-lead EcG were performed. Blood pressure (BP) was measured by auscultatory sphygmomanometry on 3 different days, and individuals were diagnosed as hypertensive when the average blood pressure was higher than 140/90 mm Hg.19 Group A individuals were further classified in two subgroups: SGA1 (13 cases) and SGA2 (14 cases) according to the presence or absence of left ventricle hypertrophy as assessedbythecriteriaof the Mexican School of Cardiology.20 Group B included 28 government office employees who were normal on medical examination, and with an auscultatory BP lower than 140/90 mm Hg measured both in sitting and standing positions.
Individuals from both groups were matched according to age and gender.
Protocol. BP was measured between 10:00 AM and 4:00 PM in the sitting position by auscultatory sphygmomanometry. Afterwards, the sphyg-mokinetocardiographic study was performed using a device developed by us.5,18 This device includes a personal computer (Pc) with an analog to digital converting card with 10 bits resolution, allowing to record the DII-lead ECG, the photoplethysmographic pulse on the index finger, signals generated in pneumatic detectors to register the carotid oscillation pulse (COP) from a neck segment, as well as two vibration signs (kinetocardiograms, KCG) produced by the left ventricleand registered on the anterolateral aspect of the left hemithorax (ant-KCG) and on the subcostal region below (post-KCG). Signals were captured at a rate of 350 samples per second. MPX2052 Motorola pressure transducers were used to register two simultaneous signals with two pneumatic detectors: The signals were then displayed on the PC monitor and saved to perform offline measurements.
Procedure. The following detectors were used: electrodes to register the ECG (DII), pulse photodetector placed on the indexfinger,and 3 pneumatic detectors named: no. 1 (D1), no. 2 (D2) and no. 3(D3) [4 x12 cm rubberbags connected to the pressure transducer and to a manual air pump]. These detectors were heldby inextensible cloth belts (6 cm width) positioned tightly and transversally surrounding the neck and or the thorax, as follow: no. 1 (D1)was placed on the neck from the midline to the left anterolateral side; D2 was placed on the anterolateral aspect of the thorax from the midclavicular line outwards, covering the 4th and 5th left intercostals spaces (Fig. 2); and D3 was placed on the upper abdominal aspect (left subcostal) in parallel to detector no. 2. All detectors were connected to an electronic signal adequacy box and to the PC. With the patient sitting and in sustained inspiratory breathing, the pneumatic detectors were inflated to 50 mm Hg. We then simultaneously recorded the following electronic signals in 6 to 10 consecutive cardio-arterial cycles: the DII-lead ECG signal, the finger-hand photoplethysmographic pulse (FHP), the carotid oscillation pulse (COP) (detector 1), vibrations produced by the anterior left ventricle wall (ant-KCG) (detector 2), and the vibrations produced by the posterior left ventricle wall (post-KCG) transmitted through the diaphragm (detector 3). DII ECG lead, FHP and ant-KCG signals were obtained simultaneously, as well as the following combinations: COP + ant-KCG, and COP + post-KCG. Then patient stand for 3 minutes and a complete signal recordings and BP measurements were performed after jogging in a fixed position (60 steps per minute) for 3 minutes.
Measurements. All good quality signals were printed on millimetric paper. With the aid of the ECG, the finger-hand pulse or the carotid oscillation pulse, the following cardiologic or cardiocirculatory intervals were measured in triplicate (Fig. 1): a) R-R electrocardiographic interval (R wave to R wave); b) ejection time (ET) measured by anterior sphygmokinetocardiography, and defined as the interval between the initiation of the left ventricular impulse deflexion (VID) and the aortic valve closure (AVC); c) aortic-reflection time (ar-T) defined as the interval between the VID and the reflection wave front; d) aorta-finger hand time (A-FT), defined as the interval between the beginning of the ventricular impulse and the foot of the hand-finger pulse; and e) anterior and posterior R-VI time, defined as the interval between the ECG R-wave peak and the final point of the ventricular impulse on the anterior or the posterior KGCs respectively. Based on these measurements, we obtained the following indexes and intervals: a) arterial reflection index (ARI) estimated as ar-T/ET; b)anterior and posterior R-IV time; and c) pulse wave velocity (PWV), estimated by the following equation: PWV = d/A-FT, where d) = the aorta-finger hand distance as measured with a metric tape, following the superficial projection of the arterial pathway (from the middle sternum to the distal index finger pulp),17 and A-FT is the aorta-finger hand time.
The data used for the analysis were the mean of 3 measurements.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation, while discrete variables are reported as absolute or relative (percentage) frequencies. Differences among groups were compared using non-paired Student's t test for continuous variables, and the chi square test to compare proportions.21 Statistical significance was considered when p < 0.05. The linear association between variables was assessed using Pearson's correlation coefficient.
Results
No differences in age (56.7 ± 9.98 vs 55.6 ± 11.77 years); sex ratio (male/female 13/15 vs 15/13), weight (74.6 ± 13.41 vs 76.0 ± 11.41 kg) and height (161 ± 9.81 vs 164 ± 9.33 cm) were observed between groups A and B respectively (Table I). In these groups systolic, diastolic and pulse pressures (SBP, DBP and PP) were significantly higher in hypertensive patients both in the sitting position (159 ± 15.5, 92 ± 9.2 and 64.1 ± 10.3 mm Hg respectively) and in the standing position after exercise (187 ± 24.7, 105 ± 14.0 and 86 ± 19.3 mm Hg respectively) than in non-hypertensive subjects (SBP, DBP and PP were 118 ± 10.9, 73.9 ± 6.6 and 44.1 ± 10.8 mm Hg respectively in the sitting position, and 128 ± 11, 74.2 ± 7 and .54 ± 9.2 mm Hg respectively after exercise in standing position). Mean PWV was significantly higher in group A (9.8 ± 2.8 m/s) than in group B (7.4 ± 1.2 m/s, p < 0.001), while the post-exercise arterial reflection index was significantly lower in group A (0.48 ± 0.3) than in group B (0.80 ± 0.3, p < 0.001) (Table II).
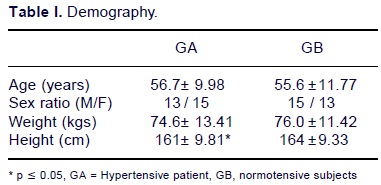
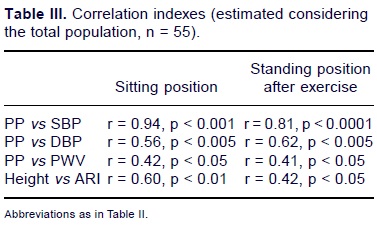
Correlation indexes were measured in the total population. Positive correlations were found for PP and SBP in the sitting position (r = 0.94, p < 0.001); for PP and DBP in the sitting position (r = 0.56, p < 0.005), for PP and SBP after exercise (r = 0.81, p < 0.0001) and for PP and DBP after exercise (r = 0.62, p < 0.005). PP vs PWV and Height vs ARI also have a positive correlation index (Table III) in both positions (Table 3).
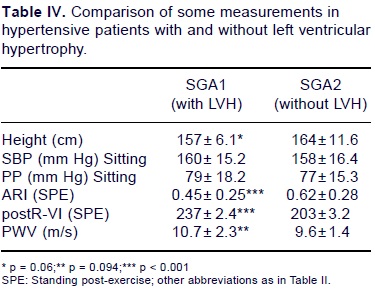
On comparing subgroups A1 (with LVH) vs A2 (without LVH), no significant differences were found on SBP in the sitting position (160 ± 15.2 vs 158 ± 16.4 mm Hg) and on pulse pressure (79 ± 18.2 vs 77 ± 15.3 mm Hg). Body height however, was significantly lower in hypertensive patients with LVH (157 ± 6.1 cm) as compared to those without LVH (164 ± 11.6 cm, p = 0.06) (Table IV). In the sitting position, the ARI, PWV and R-IV time were not significantly different in subgroups A1 (0.45 ± 0.25, 9.1 ± 2.2m/s and 222 ± 35.5 ms respectively) and A2 (50 ± 0.3, 8.9 ± 3.2, and 229 ± 30.1, respectively). However, post-exercise measurements in the standing position revealed significant differences, as the ARI was significantly lower in SGA1 (0.45 ± 0.25) than in group SGA2 (0.62 ± 0.28, p < 0.001), while R-W time was significantly higher in group SGA1 (237 ± 32.4 vs 203 ± 32, p = 0.001).
Comments
The hemodynamic impairment occurring in systemic arterial hypertension has been until recently explained applying the law of Poiseuille, which assumes that blood pressure and blood flow are constants. However, circulatory flow is not laminar and has a pulsatile character that acts against the viscous resistance and simultaneously accelerates the blood flow mass.22 For this reason, it is now considered that the arterial system represents a burden to left ventricle ejection and has two components: one is resistive, explained by the poiseullian circulatory model, and the other is pulsatile manifesting properties of unequal inertia and compliance heterogeneously distributed throughout the arterial tree.23 Increased arterial rigidity in hypertension makes the pressure wave hyperpulsatile and alterstheventricleartery relationship, leading to ventricular hypertrophy. Furthermore, it alters the viscoelastic properties of arteries favoring the development of arteriolosclerosis and aortosclerosis, which are both characteristic of this disease. Thus, the study of pulsatile mechanics is particularly relevant in everyday clinical practice.23
Arterial Reflection Index. The arterial pulse wave has two components: 1) a forward or incident component which travels from the heart to the periphery, and 2) a retrograde or reflexive component that travels in the opposite direction. The latter component is the result of the addition of several reflection waves: some are generated in bifurcations near the heart, and others of variable magnitude are reflections originated at the arteriolar region of the arterial tree.24 The pathogenic importance of these waves has been underscored in experimental studies where a hard, artificial aorta is inserted in dogs: blood flowing through this stiff aorta leads to an immediate increase of the pulse pressure, altered diastolic coronary flow24 and boost the left ventricle parietal stress.24 All these phenomena are associated with an early return of reflection waves. When the blood flows through the normal elastic aorta, all the above mentioned anomalies disappear.
At our hospital, we have developed a sphygmokinetocardiographic arterial reflection index, which measures the arrival time of arterial reflection waves in relation to the ejection time, and can disclose the interference of the retrograde flow on the forward flow at the aortic root. Moreover, this factor can distend the left ventricle during telesystole, and may cause ventricular extrasystolia by means of an electromechanical feedback phenomenon.18
In the present study, we found that the arterial reflection index was lower in hypertensive patients as compared to normal individuals (Table II), indicating that early arterial reflection interferes to a greater extent with ventricular ejection. In addition, the ARI was significantly lower in hypertensive patients with LVH as compared to those without LVH, particularly after exercise.
These findings were expected, as the rigidity of the aorta and central arteries is characteristic in hypertensive individuals, particularly in those with LVH.5 The ARI decrement after exercise is due to increased intrarterial pressure which distends the arterial wall, and according to the elastic module of the vessel, increases its rigidity.
Numerous observations in the medical literature have associated the hypertensive state per se with the early return of reflection waves, especially when hypertension coexists with left ventricle hypertrophy, advanced age or renal failure.10,26
These studies assess the effect of reflection waves by the so-called "augmentation index".27 This index measures the increase in central systolic pressure caused by reflection waves using sophisticated technologies which rebuild the intraortic pulse from an arterial (carotid or subclavian) tonometric pulse with a mathematical transference function model.28
As mentioned above, the ARI measures the precocity of reflection arrivals in relation to ventricular ejection. In fact, it indicates the percentage of time that ejection is free of retrograde influence, and when expressed inversely, indicates the percentage of time the reflexive phenomenon interacts with ejection (i.e. an index = 0.30 indicates that mesosystolic outflow is interfered with during 70% of the ejection time).18 One of the limitations of ARI or augmentation index measurements is that both infer that carotid reflections are indicators of ascending aorta rigidity, even though the viscoelastic properties of both arterial segments are not necessarily the same. However, the morphology of the aortic pressure curve rebuilt by transference function is very similar to the carotid pressure curve. Thus, the study of the latter does not represent a relevant impediment.29,30 Of course the time required for the carotid reflection to arrive to the aortic root reduces the accuracy of the temporality of events.
Pulse wave velocity (PWV). This parameter has been measured and analyzed since the last century, and is a well known indicator of arterial rigidity (because of the demonstrated inverse relationship between arterial compliance and arterial pulse wave velocity).23 As a matter of fact, over the last years PWV has been underscored as a cardiovascular risk factor in arterial hypertension with or without LVH, and has been associated with diabetes mellitus, age, renal failure and atherosclerosis.31-33 We corroborated once again the statistical association between increased PWV and hypertension and that this parameters the ARI and the pulse pressure, are arterial stiffness indicators. In general terms, pulse wave velocity is clinically measured by registering the passing fronts of the wave on two superficial sites of an accessible arterial segment.1,5
The sphygmokinetocardiograpy method developed at our department can register on the thorax the vibrations generated by left ventricle volume changes transmitted through the lung acting as a resonance box.17,18 Transmission is better perceived during sustained inspiration, as the cardiac vibration signs are registered in the absence of respiratory sounds. In this manner, the aorta-finger hand pulse time can be measured in the same cardiocirculatory cycle: from the initiation of the pulse wave at the aortic root, inferred by the initiation of the ventricular impulse (ET initiation point on the ant-KCG, Fig. 1), until it reaches the foot of the hand-finger pulse.17 This method is easy to perform, not only at rest but also in a standing position and after dynamic exercise. We have found no previous references in the medical literature describing this advantage for other procedures. This measurement provides interesting perspectives for clinical arterial mechanics studies.
R-VI time. Kinetocardiography has been used for more than 4 decades. It is a well known method used to measure systolic intervals, specially during the ejection period, and correlates well with the ejection fraction.34,35 Our version of KCG has the technological advantage of digitalization, and in addition, our mechanical receptor remains properly positioned by means of an inextensible perithoracic belt. This allows to register signals from individuals in different physiological positions (lying, sitting, standing and after exercise).
With our method, the systolic ventricular impulse is well defined and graphically expresses 2 components: one is fast, related to the initial ventricular contraction; and the other is slower, mainly expressing ventricular relaxation (Fig. 1). The analysis of high fidelity left ventricle pressure curves and the apical kine to cardiogram in humans demonstrated that point "0" on the intraventricular pressure curve and the systolic ventricular impact on the apicogram are simultaneous, and that the outlines of both graphs overlap.36,37 We thus considered that the R-VI time measures the duration of fast ventricular contractility. Experimentally, increased afterload lengthens the contractile duration of the left ventricle.11 We observed here that the R-VI time registered by post-KCG after exercise was longer in hypertensive patients with LVH as compared to those without LVH (Table IV), in accordance with the pathophysiologic assumption made above. Pulse pressure. Pulse pressure is a well established cardiovascular damage indicator.38 Information on this issue has increased considerably over the last years, and we now know that increased pulse pressure is associated with increased left ventricular mass39, carotid atherosclerosis40 and brain damage.41 In some studies, pulse pressure has been identified as an independent risk factor of prognostic value for cardiovascular damage.38
The factor underlying this phenomenon is the loss of arterial compliance, which decreases arterial pulsatility cushioning and increases pulse pressure. We found here that pulse pressure was wider in hypertensive as compared to normal subjects, and that this parameter was more significant as an independent risk factor than systolic or diastolic blood pressures, confirming previous observations made elsewhere.42-44 Thus, this parameter is a good indicator of arterial stiffness that can be applied in clinical practice. Unexpectedly, we found that PP was not significantly different in hypertensive patients with and without electrocardiographic evidence of LVH. We have no explanation for this observation, and will further analyze this issue in the future, studying a large number of cases.
Body Height. As expected,45 we observed a correlation between short stature and the post-exercise arterial reflexion index (Table III). In fact, height was significantly lower in hypertensive subjects with left ventricle hypertrophy as compared to those without LVH (Table IV). Using other arterial stiffness indicators, Marchain et al. observed the same correlation in patients with renal failure10 and suggested that this happens when renal failure onset occurs during body development leading to malnutrition and short stature. Further studies confirmed the inverse relationship between short stature and increased cardiovascular risk in all terminal renal failure patients.46 Recently, this correlation was also observed in hypertensive non-uremic patients by studying pulsatile parameters indicative of impaired arterial hemodynamics.45 As our findings are similar, we suggest that arterial reflection sites are closer to the heart in hypertensive subjects with short stature as compared to taller ones, favoring early arrival of reflection waves to the aortic root. Thus, short stature would be an independent risk factor, and when occurring in a hypertensive state would favor more severe cardiovascular damage. Perhaps this same pathogenic mechanism explains the association between cardiovascular risk (particularly coronary risk) and short stature reported since 1951.47,48
Conclusions
1. PWV is increased in sitting and orthostatic post-exercise positions in GA vs GB. Arterial reflexion index is reduced in the last position in systemic arterial hypertension as compared to normal individuals. This underscores arterial hyperigidity in hypertension, which is of particular pathogenic interest.
2. Pulse pressure shows a positive correlation with systolic blood pressure and to a lesser extent with diastolic blood pressure. PWV and PP showed a statistically significant correlation.
3. Early reflection waves correlate with short stature. This gives way to interesting pathophysiologic speculations.
4. Hypertensive patients with LVH had lower height, longer ventricular impulse and shorter post-exercise ARI as compared to those without LVH.
5. The method here described may have useful applications in everyday clinical practice. Specially it is convenient to study a large number of patients and verified changes of rigidity indexes with antihypertensive treatment.
References
1. Sánchez-Torres G, Infante VO, Martínez MR, Flores-Chávez P, Sánchez-Miranda G: Provocación y medición de la onda pulsátil retrógrada en casos normales e hipertensos sistémicos. Arch Cardiol Mex 1999; 69: 234-240. [ Links ]
2 O'Rourke MF, Kelly RP: Wave reflection in the system circulation and its implications in ventricular function. J Hypertension 1993; 11: 327-337. [ Links ]
3. Berger DS, Kimberly A, Rubrason SG: Wave propagation uncouple the left ventricular arterial system. Implications for aortic pressure. Hypertension 1996;27:1077-1089. [ Links ]
4. London GM, Guerin AP, Marcháis SS: Influence of arterial pulse and reflected waves on blood pressure and cardiac function. Am Heart J 1999; 138: 220-224. [ Links ]
5. Infante VO, Sánchez-Torres G, Martínez MR, Flores P, Sánchez-Miranda G: Sistema para la medición de la velocidad de la onda del pulso arterial en diferentes territorios vasculares. Arch Inst Cardiol Mex 1999; 69: 330-337. [ Links ]
6. Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Pede S, Porcellati C: Ambulatory pulse pressure: a potent predictor of total cardiovascular risk in hypertension. Hypertension 1988;12:983-988. [ Links ]
7. Franklin SS, Shchzad A, Nathan D, Lardón MG, Levy D: Is pulse pressure useful in predicting risk in coronary heart disease? The Framingham Heart Study. Circulation 1999; 100: 354-360. [ Links ]
8. Selker HP, Beshansky JR, Smith CH, Griffith JL, Longstreth WT Jr: Present pulse pressure predicts thrombolytic theraphy-related intracranial hemorraghe. Circulation 1994;90:1654-1661. [ Links ]
9. Nazzaro P, Triggiani R, Cianco L, Searano AM, Merlo M: Microvascular changes during laboratory stimuli and structural hemodynamic indices: the rule of pulse pressure. Clin Hemorheol Microcirc (Netherlands) 1999; 31: 225-232. [ Links ]
10. Marcháis SS, Guerin AP, Pannier BM, Safar ME, London G: Wave reflexion and cardiac hypertrophy in uremia. Influence of body size. Hypertension 1993; 22: 876-883. [ Links ]
11. Meaume S, Rudnichi A, Lynch A, Bussyc, Sebbano C: Aortic pulse wave velocity as a marker of cardiovascular disease in subjects over 70 years old. J Hypertens 2001; 19: 871-877. [ Links ]
12. O'Rourke M, Frolich ED: Pulse pressure. Is this a useful clinical risk factor? (editorial). Hypertension 1999; 34: 372-374. [ Links ]
13. Millar HA, Lever AF, Burke V: Pulse pressure as a risk factor for cardiovascular events in the MRC Mild Hypertension Trial. J Hypertens 1999; 17: 1065-1072. [ Links ]
14. Antikainen RL, Jousilahti P, Vanhnen H, Toumilehto S: Excess mortality associated with increased pulse pressure among middle age men and women is explained by high systolic blood pressure. J Hypertens 2000; 18: 417-423. [ Links ]
15. Tsoucaris-Kupfer D, Benetos A, Legrand M, Safar ME: Pulse pressure gradient along the aortic tree in spontaneous hypertensive rats: effects of nicardipine. J Hypertens 1993; 11: 135-139. [ Links ]
16. Segers P, Verdonck P, Derfck Y, Brimioulli S, Naeije R: Pulse pressure method and the area method for the estimation of total arterial compliance of dogs: sensitivity to wave reflection intensity. Ann Biomed Eng 1999; 27: 480-485. [ Links ]
17. Sánchez-Torres G, Infante VO, Martínez MR, Sánchez-Miranda G: Sistema de registro de la velocidad de la onda del pulso con ayuda del quinetocardiograma anterolateral. Estudio en sujetos normales e hipertensos. Memorias del 1er. Congreso Latinoamericano de Ingeniería Biomédica, Mazatlán, México, 1998; 2: 651-664. [ Links ]
18. Sánchez-Torres G, Infante VO, Martínez MR, Flores CHP, Rodríguez RG: Reflexión arterial y extrasistolia ventricular. Un mecanismo novel detectado por esfigmoquinetocardiografía. Arch Inst Cardiol Mex 2002; 72: 29-35. [ Links ]
19. Sánchez Torres G, Barrera GI: Seguimiento de la presión arterial por el personal de enfermería. Arch Inst Cardiol Mex 1980; 50: 709-713. [ Links ]
20. De Micheli A, Medrano G: Enfoque electrofsiológico del diagnóstico del crecimiento ventricular izquierdo. Arch Inst Cardiol Mex 1995; 65: 365-372. [ Links ]
21. Snedecor GN, Cochran WG: Statistical Methods. Ames, Ia, the Iowa State University Press, 1989: 123-249. [ Links ]
22. Lee RT, Kamm RD: Vascular mechanics for cardiologists. J Am Coll Cardiol 1994; 23: 289-295. [ Links ]
23. Darne B, Girerd X, Safar M, Camber F, Guise L: Pulsatile vs steady component of blood pressure: a cross sectional analysis and a prospective analysis of cardiovascular mortality. Hypertension 1989; 13: 329-400. [ Links ]
24. Kelly RP, Tunin R, Kass DA: Effect of reduced aortic compliance on cardiac efficiency and contractile function of in site canine left ventricle. Cir Res 1992; 71: 420-502. [ Links ]
25. Kass DA, Sacki A, Tinin RS, Rechia FA: Adverse influence of systemic vascular stiffness on cardiac function and adaptation to acute coronary occlusion. Circulation 1996; 93: 1531-1541. [ Links ]
26. London GM, Blacher J, Pannier B, Guerin AP, Marcháis SJ, Safar ME: Arterial wave reflections and survival in end-stage renal failure. Hypertension 2001; 38: 434-438. [ Links ]
27. Nichols WW, Edward DG: Arterial elastance and wave refection augmentation of systolic blood pressure: deleterious effects and implications for therapy. J Cardiovasc Pharmacol Ther 2001; 6: 5-21. [ Links ]
28. Kelly R, Daley S, Avolio A, O'Rourke M: Noninvasive registration of the arterial pressure pulse wave from using high fidelity applanation tonometry. J Vasc Med Biol 1989; 1: 142-149. [ Links ]
29. Sharir T, Marmor A, Ting CT, Chen JW Lurc P, Chang MS: Validation of a method for non-invasive measurement of central arterial pressure. Hypertension 1996; 21: 74-87. [ Links ]
30. Bramwell JC, Hill AV: Velocity of transmission of the pulse wave and elasticity of arteries. Lan* cet 1922; 1: 891-899. [ Links ]
31. Rogers WK, Hu YL, Coast D, Vido DA, Kramer CM: Age associated changes in regional aortic pulse wave velocity. J Am Coll Cardiol 2001; 38: 1123-1129. [ Links ]
32. London GM, Blacher J, Pannier B, Guerin AP, Marcháis SS: Arterial wave reflections and survival in end stage renal failure. Hypertension 2001; 38: 438. [ Links ]
33. O'Rourke ME: Wave travel and reflection in the arterial system. J Hypertens 1999; 17(Suppl 5):545-47. [ Links ]
34. Eddleman EE Jr: Kinetocardiography. In: Noninvasive Cardiology. Weissler AM, New York 1974: 275-299. [ Links ]
35. Eddleman EE, Willis F, Reever TJ, Grunner Stratton, Harrison TR: The kinetocardiogram I. Method of recording precordial movements. Circulation 1953; 8: 269-271. [ Links ]
36. Manulas J, Rutishauser W, Wirs P, Arbens V: Time relation between apex cardiogram and left ventricular events using simultaneous high fidelity tracings in man. Br Heart J 1974; 37: 1263-1267. [ Links ]
37. Reddy SP, Meno S, O'Toule JD, Curtis EL, Griff FW: High fidelity infinite constant calibrated pressure aprexcardiogram and its correlation with high fidelity left ventricular pressure. Br Heart J 1980; 44: 194-200. [ Links ]
38. Dyer AR, Stamler J, Shekelle RB, Shoenberger JA, Stanler S: Pulse pressure III. Prognostic significance in four Chicago epidemiologic studies. J Chronic Dis 1982; 35: 283-294. [ Links ]
39. Pannier B, Bruner P, El ároussy W, Lacolley P, Safar MZ: Pulse pressure and ecocardiographic findings in essential hypertension. J Hypertens 1989; 7: 121-132. [ Links ]
40. Safar ME: Pulse pressure in essential hypertension: clinical and therapeutic implications. J Hypertens 1989; 7: 766-769. [ Links ]
41. Surkula M, Agewall S, Fagerber B, Wendelhaj I, Wikstrand J: Risk intervention study (RIS) group. Ultrasound evaluation of atherosclerotic manifestation in the carotid artery in high risk hypertensive patients. Aterioscler Thromb 1994; 14: 1297-1304. [ Links ]
42. Liao D, Cooper L, Toul J. Bryan G, Shahar E: The prevalence and severity of white matter lesions their relationship with age, ethnicity, gender and cardiovascular risk factors: the ARIC study. Neuroepidemiology 1997; 16: 149-162. [ Links ]
43. Benetos A, Safar ME, Rudnichi A, Smulyan A, Richard JL: Pulse pressure: a predictor of long term cardiovascular mortality in a French population. Hypertension 1997; 30: 1410-1415. [ Links ]
44. Mitchell GF, Moye LA, Braunwald E, Rouleau JL, Bertein V: Sphygmanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. Circulation 1997; 96: 4254-4260. [ Links ]
45. Smulyan H, Marcháis SJ, Pannier B, Guerin AP, Safar ME, London GM: Influence of body height on pulsatil arterial hemodynamic data. JACC 1998; 31: 1103-1105. [ Links ]
46. London GR, Guerin AP, Pannier B, Marcháis SJ, Stimpel M: Influence of sex on arterial hemodynamics and blood pressure. Rule of body height. Hypertension 1995; 26: 514-519. [ Links ]
47. Gerfler MM, Garn SM, With PD: Young candidates for coronary heart disease. JAMA 1951; 147: 621-625. [ Links ]
48. Kannam JP, Levy D, Lauson M, Wilson PWP: Short stature and risk for mortality and cardiovascular events: the Framingham Heart Study. Circulation 1994; 90: 2241-2247. [ Links ]














