Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
Compartir
Archivos de cardiología de México
versión On-line ISSN 1665-1731versión impresa ISSN 1405-9940
Arch. Cardiol. Méx. vol.72 no.2 Ciudad de México abr./jun. 2002
Investigación básica
A subpopulation of large granular von Willebrand Ag negative and CD105 positive endothelial cells, isolated from abdominal aortic aneurysms, overexpresses ICAM-1 and Fas antigen
Una subpoblación de células endoteliales grandes y granulares, antígeno von Willebrand negativas y CD105 positivas, aisladas de aneurismas aórticos abdominales, sobreexpresan ICAM-1 y antígeno Fas
Araceli Páez* Abel Archundia,** René Méndez Cruz,***,**** Emma Rodríguez,* Rebeca López Marure,* Felipe Masso,* José Luis Aceves,** Leopoldo Flores,*** Luis F Montaño*
* Depto. Biología Celular, Instituto Nacional de Cardiología "Ignacio Chávez", México, D.F.
** División Cirugía Cardíaca, Servicio de Cirugía Cardiovascular, CMN "20 de Noviembre", ISSSTE; México.
*** Depto. Patología Experimental, CINVESTAV, IPN, México.
**** Facultad de Estudios Superiores Iztacala, UNAM, México.
Correspondence:
Luis F Montaño,
Ph.D. Depto. Biología Celular,
Instituto Nacional de Cardiología "Ignacio Chávez"
(INCICH, Juan Badiano No. 1,
Col. Sección XVI, Tlalpan, 14080 México, D. F.).
Tel. (52)(55) 5573-2911 Ext. 1337
Fax (52)(55) 5573-0926.
E-mail: lfmontmx@yahoo.com
Recibido: 14 de enero de 2002
Aceptado: 16 de abril de 2002
Summary
The aim of this work was to determine whether there is a pre-established basal condition of the endothelial cells isolated from aortic abdominal aneurysm that might augment immune effector mechanisms and thus provide us an insight into the possible causes of aneurysm rupture. Endothelial cells isolated from saccular aortic aneurysm fragments were analyzed by cytofluorometry for the expression of different immune response-related molecules. Our results showed that there is a subpopulation of granule-rich, CD105 positive and von Willebrand antigen negative endothelial cells that have an enhanced basal expression of ICAM-1, and Fas antigen, but, interestingly, no apoptotic bodies were detected. Control endothelial cells derived from healthy areas of the same abdominal aortas did not show such enhanced expression. We conclude that in the endothelium that lines abdominal aorta aneurysms there is, at least, one endothelial cell subpopulation with an apparent inhibition of programmed cell death and in a proinflammatory activation status.
Key words: Aortic aneurysm. Fas antigen. Adhesion molecules. ICAM-1. Endothelial cell. Apoptosis.
Resumen
El objetivo de este trabajo era determinar si en las células endoteliales aisladas de aneurismas abdominales aórticos existe alguna condición basal preestablecida que incremente los mecanismos efectores inmunes y, por ende, nos pueda dar alguna pista acerca de los mecanismos por los que los aneurismas se perforan. Se aislaron células endoteliales de fragmentos saculares de aneurismas aórticos abdominales perforados; la expresión de diferentes moléculas relacionadas con la respuesta inmune fue analizada por citofluorometría. Los resultados mostraron la presencia de una subpoblación de células ricas en gránulos citoplásmicos, CD105 positiva y antígeno de von Willebrand negativa que mostraba una sobreexpresión de ICAM-1 y de antígeno Fas, pero sin que hubiera cuerpos apoptóticos. Las células endoteliales control obtenidas de regiones sanas de la misma aorta abdominal no mostraron sobreexpresión. En conclusión, creemos que dentro del endotelio que recubre las regiones donde se forman aneurismas, en la aorta abdominal existe una subpoblación de células endoteliales que tienen inhibido el proceso de muerte celular programada y que además se encuentran en un estado de activación proinflamatoria.
Palabras clave: Aneurisma Aorta. Antígeno Fas. Moléculas de Adhesión. ICAM-1. Células endoteliales. Apoptosis.
Introduction
An aneurysm is defined as a pathologic dilation of a segment of a blood vessel. The most common pathologic conditions associated with an aortic aneurysm are atherosclerosis, now considered an inflammatory disease1,2 and hypertension,3 but there is controversy as to whether atherosclerosis itself actually causes an abdominal aneurysm or develops as a secondary event in the dilated aorta. Familial clustering of abdominal aortic aneurysms occur in patients with a point mutation in the gene for type III procollagen,4 suggesting a hereditary basis of the disease. An increased synthesis of collagen III has been implicated5 but other additional causes, such as infection6 or enhanced enzyme activity7 have also been considered. Recent studies of abdominal aortic aneurysms using complementary DNA expression arrays have demonstrated that the pattern of gene expression reflects chronic inflammation, extracellular matrix degradation, atherosclerosis, and smooth muscle cell depletion8 and that certain inflammatory response cytokines are associated with the rate of expansion of abdominal aneurysms.9 Seventy-five per cent of aneurysms in the abdominal aorta are associated with atherosclerosis. The most common complication of abdominal aorta aneurysm is rupture,10 which has an overall community mortality rate of 67%.11
Since the cause of aneurysm rupture has not been clearly elucidated we decided to determine whether the rupture of an aneurysm had some connection with a pre-established basal condition of the endothelial cells that might augment immune effector mechanisms. Our results showed that abdominal aortic aneurysm endothelial cells have an enhanced basal expression of ICAM-1, an adhesin responsible for leukocyte tethering, and Fas antigen, a molecule connected with CD-8 lymphocyte-induced apoptosis mechanisms.
Material and methods
Fetal calf serum, L-glutamine, CPSR-3, antibiotic mixture [100x], N-[2-hydroxy-ethylpiperazine-N'-[ethanesulfonic acid] (HEPES), fucose, bovine gelatine, Triton X-100, Tris, and porcine heparin were purchased from Sigma Chemical Co. (St. Louis, MO). M-199 medium with or without phenol red, type II collagenase, RPMI-1640, liquid trypsin-EDTA (1x) were from Gibco Laboratories (Grand Island, NY). Recombinant TNF-α and endothelial cell growth supplement were from Boehringer-Mannheim Bioquimica (Mexico, D.F.). Purified mouse anti-human Von Willebrand factor, rhodamine-labelled goat anti-mouse IgG, FITC-labeled mouse anti-human CD105, ICAM-1, and phycoeritrin-labeled mouse anti-human CD95 or CD95-L were from Serotec (Raleigh, NC).
Saccular aortic aneurysm fragments of 2-3 cm in lenght were obtained from three different individuals undergoing surgery repair for abdominal aortic aneurysm at the División de Cirugía Cardiovascular, CMN "20 de Noviembre", ISSSTE. The diagnosis of aneurysm was done clinically and through echocardiography. The fragments were kept in a special medium (M-199 medium supplemented with 10% CPSR-3, 1x antibiotics mixture, 10 mM HEPES, 0.2 mM L-glutamine) and brought within two hours of sampling, to the laboratory, where they were immediately processed.
Endothelial cells from the aneurysm and normal abdominal aorta were obtained with 0.2% type II collagenase and were serially passed to gelatine-free flasks containing phenol-red M-199 medium (Gibco Laboratories, Grand Island, NY) and 20% heat-inactivated fetal calf serum and supplemented with HEPES (10 mM), penicillin (100 ug/mL), streptomycin (100 ug/mL), L-glutamine (2 mM), porcine heparin (5 IU/mL), and endothelial cell growth supplement (40 ug/mL). Trypsin-treated cells obtained from the flasks were extensively washed with 2% heat-inactivated fetal calf serum/HEPES saline solution (HSS)(1M HEPES, 0.15 M NaCl, 2.2 g/Lt glucose, 4 mM KCl, pH 7.5) and seeded at 2x105 cells/well in 500 µL of M-199 medium containing 20% of fetal calf serum and supplemented with HEPES and L-glutamine 48 hours before experiments. Endothelial cells cultured at 37ºC in a 7% CO2 humidified atmosphere were recovered with saline solution containing 0.5% trypsin/5 mM EDTA. Before being used, cells were washed thrice with saline solution supplemented with 2% fetal calf serum, 1 M Hepes, 2.2 g/Lt glucose, and 4 mM CaCl. Evaluation of Fas and Fas-ligand reactivity was performed individually for each one of the aortic abdominal aneurysms, in non-stimulated cells. Cells identified as endothelial by their characteristic morphology and presence of von Willebrand antigen, were used within three passages. Cytofluorometric assays were performed in 1x106 endothelial cells incubated with 2 µL of the relevant monoclonal antibody in 20 µL of PBS/1% Bovine Albumin/0.02% Sodium Azide (PBSA). After a 2 h incubation period, the cells were washed thrice in PBSA before being resuspended in 100 µL of PBSA/1% paraformaldehyde and read in a FACScalibur cytofluorometer (Beckton & Dickinson) kindly lent by Dr. Ricardo Lascurain, Dept. of Biochemistry, Instituto Nacional de Enfermedades Respiratorias, México.
Results
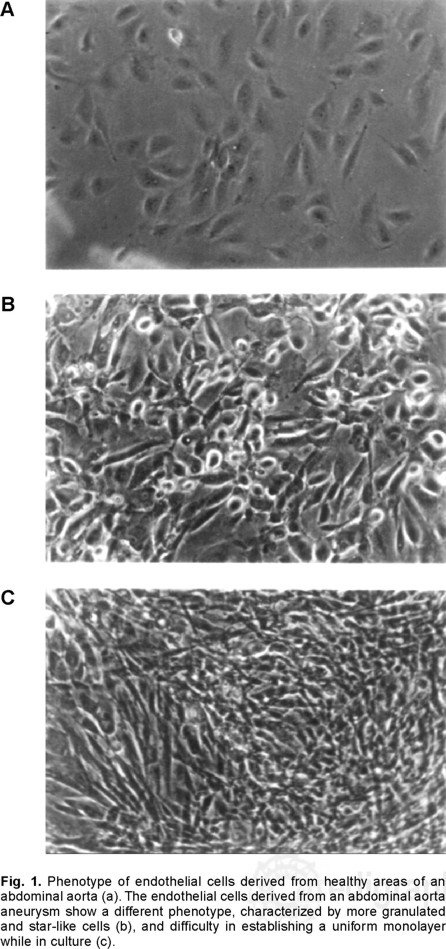
Although, most cells were CD105 and Von Willebrand antigen positive, confirming their endothelial origin, there were clear phenotypic differences between endothelial cells obtained from the healthy areas of the aneurysm fragment (NEC) versus endothelial cells isolated from the aneurysm (AEC). The typical pattern of normal endothelial cells (Fig. 1a) was replaced by more granulated, star-like and larger cells (Fig. 1b) which grew in a more deorganized pattern (Fig. 1c) and not in monolayers, as they usually do. An interesting observation was that the apparently normal endothelial cells modified their phenotype to that of cells isolated from the aneurysm after three culture passages. The cytofluorometric analysis of the endothelial cells by size and granularity allowed to recognize a subpopulation that is highly granular and large in normal (Fig. 2a) and aneurysm-derived (Fig. 2c, Fig. 3a) endothelial cells.
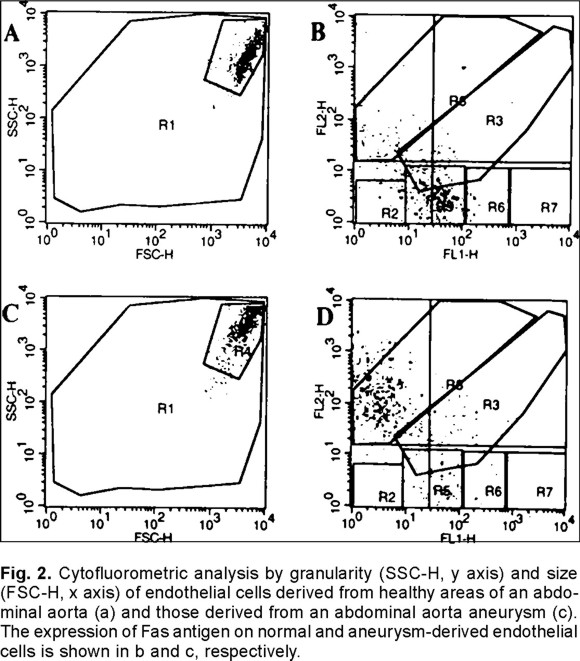
When Fas antigen was evaluated in the NEC subset only 0.8% of them were positive versus 2.8% in AEC cells, but when the big granular cell subpopulation was considered, the results showed that only 18% of this cell subpopulation expressed Fas in NEC cells (Fig. 2b) versus 72% in the similar AEC cell (Fig. 2d) subpopulation. It was also interesting to observe that the mean fluorescence intensity, which reflects number of receptors, was three-fold higher in the AEC cell subpopulation.
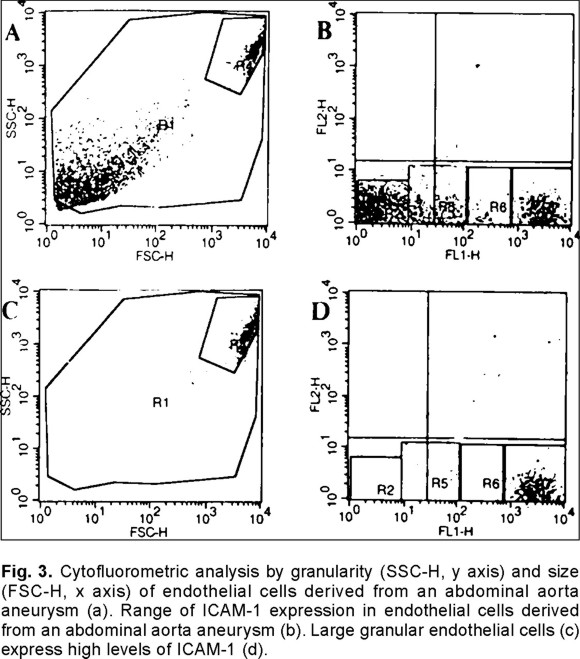
The evaluation of ICAM-1 in NEC cells showed that only 2.5% of them were positive versus 5% in AEC cells. Since there was a wide fluorescence spectrum in the AEC derived cells as shown in Fig. 3b, further cytofluorometric analysis of the cell subpopulation that embraces more than 95% of the big granular cells (Fig. 3c) showed that 64% of this cell subpopulation express high levels of ICAM-1 in NEC cells versus 71% in the similar AEC cell (Fig. 3d) subpopulation. The mean fluorescence intensity was almost identical in the NEC and AEC granular cell subpopulations (768 versus 726, respectively). It is worth noticing that 98% of the big granular cells in the AEC cell subpopulation was CD105 positive but von Willebrand antigen negative, the remaining 2% was positive for both markers.
Discussion
The knowledge of the mechanisms involved in the interaction between endothelium and immune system cells have evolved greatly,1 but it is clear that many enigmas, including the so called genetic factors, need to be addressed. Similarly to atherosclerosis,12 abdominal aorta aneurysms have an hereditary trend4 and an inflammatory background.8 The use of adhesion receptor-deficient mice has demonstrated that leukocyte-endothelium adhesion receptors play a significant role in promoting monocyte recruitment and, consequently, lesion growth.13 Resting endothelium does not express ICAM-1 and carries low levels of ICAM-2, probably used by circulating monocytes to navigate out of the vessels and into their tissue site. The interaction of the endothelium with macrophage cytokines, especially TNF-α, induces the expression of ICAM-1 in endothelial cells, which then bind leukocytes through a chemokine-driven conformational change of LFA-1 and Mac-1, heterodimeric proteins of the integrin family in leukocytes.14
Our initial observation showed that there was a dissimilarity in the phenotype of the abdominal aorta aneurysm-derived endothelial cells (E-2) compared to the control abdominal aorta endothelial cells (E-1). The most important difference was that E-2 cells have a more star-like pattern, but we also observed that E-1 cells showed a transformation to the E-2 pattern after three culture passages; thus, suggesting a switch to a less differentiated status, similar to that observed in neoplasias.15 The macroscopic observation of the cultured cells showed a small and a larger cell subpopulation, the latter contained a great quantity of granules. Based on this observation, all the remaining analyses were performed in both, total and granular, cell populations for either the E-1 or the E-2 endothelial cells.
There was an increase in the percentage of endothelial cells derived from aneurysms (E-2) that expressed ICAM-1 although the quantity of molecules expressed on the cell surface was identical as to that observed in control endothelial cells (E-1). We also found that the larger granular cell population was the main responsible of the enhanced ICAM-1 expression, but again, there were no differences in the percentage between aneurysm and control endothelial cells. Based on what is known, this enhanced level of ICAM-1 expression in aneurysm-derived endothelial cells is compatible with a "proinflammatory activation status" at least in a granule-rich endothelial cell subpopulation.
The fact that TNF-α, the main inducer of endothelial activation, is a strong inducer of apoptosis through death domain-containing molecules, such as FADD/MORT1,16 the observation that oxidized LDL activates Fas-mediated endothelial cell apoptosis17 and that ceramide arrests the endothelial cell cycle in a manner similar to TNF-α18 led us to evaluate whether aneurysm-derived endothelial cells were also more prone to enter early apoptotic death via the pro-caspase 8 pathway.19 The results demonstrated that E-2 cells expressed 3-fold more Fas antigen than E-1 cells but again this difference was due to the large granular cells. It is interesting to note that E-2 cells, having such an enhanced Fas expression, do not show the presence of apoptotic bodies nor is there a larger cell death ratio than in E-1 cells.
Taking all the results into consideration it seems that the endothelial cells lining the damaged area of the abdominal aorta aneurysm are different to those of the non-damaged abdominal aorta. The difference being in phenotype, adhesin expression, and apoptosis commitment; moreover, the actual difference was not observed when we analyzed all the tissue-derived endothelial cells but it rather seemed to be circumscribed to a larger and more granular cell subpopulation, which was more abundant in the aneurysm and showed a pattern similar to immune activated cells. Initial results demonstrated that this cell subpopulation expresses MHC-II molecules when exposed to certain bacterial proteins. We do not know what is the origin of this cell population but we do know that although it expresses CD105, a typical marker of endothelial cell, it does not express von Willebrand antigen, another molecule considered a classical marker of endothelial cells. It is possible that in the process of aneurysm formation, endothelial cells lose their capacity to be eliminated, due to inhibition of programmed cell death,20 and that this apparent "immortality" is accompanied by the loss of some differentiation markers, such as von Willebrand antigen, thus indicating a process of dedifferentiation21 or transdifferentiation,22 which would result in a switch in their phenotype. Inhibition of apoptosis has been observed in viral infectious diseases23,24 and in neoplastic cell lines where integrin β-1, another member of the cell adhesion receptors,25 occupies its ligand.26
Conclusions
We conclude that in the endothelium lining abdominal aorta aneurysms there is at least one endothelial cell subpopulation with an apparent inhibition of programmed cell death and in a pro-inflammatory activation status. It is possible that this cell subpopulation might be more susceptible to the immune-related mechanisms and the mechanical forces that have been associated with the development of an aneurysm.
References
1. ROSS R: Atherosclerosis -an inflammatory disease. New Engl J Med 1999; 340: 115-26. [ Links ]
2. SELZMAN CH, MILLER SA, HARKEN AH: Therapeutic implications of inflammation in atherosclerotic cardiovascular disease. Ann Thorac Surg 2001; 71: 2066-74. [ Links ]
3. SINGH K, BONAA KH, JACOBSEN BK, BJORK L, SOLBERG S: Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study : The Tromso Study. Am J Epidemiol 2001; 154: 236-44. [ Links ]
4. KONTUSAARI S, TROMP G, KUIVANIEMI H, ROMANIC AM, PROCKOP DJ: A mutation in the gene for type III procollagen (COL3A1) in a family with aortic aneurysms. J Clin Invest 1990; 86: 1465-73. [ Links ]
5. BODE MK, SOINI Y, MELKKO J, SATTA J, RISTELI L, RISTELI J: Increased amount of type III pN-collagen in human abdominal aortic aneurysms: evidence for impaired type III collagen fibrillogenesis. J Vasc Surg 2000; 32: 1201-7. [ Links ]
6. OHTAHARA A, SANTO Y, OGINO K: Infective abdominal aortic aneurysm. Heart 2001; 86: 126. [ Links ]
7. GOODALL S, CROWTHER M, HEMINGWAY DM, BELL PR, THOMPSON MM: Ubiquitous elevation of matrix metalloproteinase-2 expression in the vasculature of patients with abdominal aneurysms. Circulation 2001; 104: 304-9. [ Links ]
8. TUNG WS, LEE JK, THOMPSON RW: Simultaneous analysis of 1176 gene products in normal human aorta and abdominal aortic aneurysms using a membrane-based complementary DNA expression array. J Vasc Surg 2001; 34: 143-50. [ Links ]
9. JUVONEN J, SURCEL HM, SATTA J, TEPPO AM, BLOIGU A, SYRJALA H, ET AL: Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 1997; 17: 2843-7. [ Links ]
10. WOLF YG, BERNSTEIN EF: A current perspective on the natural history of abdominal aortic aneurysms. Cardiovasc Surg 1994; 2:16-22. [ Links ]
11. CASSAR K, GODDEN DJ, DUNCAN JL: Community mortality after ruptured abdominal aortic aneurysm is unrelated to the distance from the surgical centre. Br J Surg 2001; 88:1341-3, 2001. [ Links ]
12. BRESLOW JL: Genetic differences in endothelial cells may determine atherosclerosis susceptibility. Circulation 2000; 102: 5-6. [ Links ]
13. DONG ZM, WAGNER DD: Leukocyte-endothelium adhesion molecules in atherosclerosis. J Lab Clin Med 1998; 132: 369-75. [ Links ]
14. MULLER WA, RANDOLPH GJ: Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J Leukoc Biol 1999; 66: 698-704. [ Links ]
15. PRASAD KN, HOVLAND AR, NAHREINI P, COLE WC, HOVLAND P, KUMAR B, ET AL: Differentiation genes: are they primary targets for human carcinogenesis? Exp Biol Med 2001; 226: 805-13. [ Links ]
16. NAGATA S: Apoptosis by death factor. Cell 1997; 88: 355-65. [ Links ]
17. SATA M, WALSH K: Oxidized LDL activates Fas-mediated endothelial cell apoptosis. J Clin Invest 1998; 102: 1682-9. [ Links ]
18. LOPEZ-MARURE R, VENTURA JL, SANCHEZ L, MONTANO LF, ZENTELLA A: Ceramide mimics tumour necrosis factor-alpha in the induction of cell cycle arrest in endothelial cells. Induction of the tumour suppressor p53 with decrease in retinoblastoma/protein levels. Eur J Biochem 2000; 267: 4325-33. [ Links ]
19. COHEN GM: Caspases: the executioners of apoptosis. Biochem J 1997; 326: 1-16. [ Links ]
20. ZORNIG M, HUEBER A, BAUM W, EVAN G: Apoptosis regulators and their role in tumorigenesis. Biochim Biophys Acta 2001; 1551: F1-37. [ Links ]
21. YONEMASU H, TAKASHIMA M, NISHIYAMA KI, UEKI T, YAO T, TANAKA M, ET AL: Phenotypical characteristics of undifferentiated carcinoma of the pancreas: a comparison with pancreatic ductal adenocarcinoma and relevance of E-cadherin, alpha catenin and beta catenin expression. Oncol Rep 2001; 8: 745-52. [ Links ]
22. ZELIVIANSKI S, VERNI M, MOORE C, KONDRIKOV D, TAYLOR R, LIN MF: Multipathways for transdifferentiation of human prostate cancer cells into neuroendocrine-like phenotype. Biochim Biophys Acta 2001; 1539: 28-43. [ Links ]
23. TAKAMATSU M, FUJITA T, HOTTA H: Suppression of serum starvation-induced apoptosis by hepatitis C virus core protein. Kobe J Med Sci 2001; 47: 97-112. [ Links ]
24. TOLLEFSON AE, TOTH K, DORONIN K, KUPPUSWAMY M, DORONINA OA, LICHTENSTEIN DL, ET AL: Inhibition of TRAIL-induced apoptosis and forced internalization of TRAIL receptor 1 by adenovirus proteins. J Virol 2001; 175: 8875-7. [ Links ]
25. APLIN AE, HOWE A, ALAHARI SK, JULIANO RL: Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev 1998; 50: 197-263. [ Links ]
26. AOUDJIT F, VUORI K: Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene 2001; 20: 4995-5004. [ Links ]














