Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Archivos de cardiología de México
versão On-line ISSN 1665-1731versão impressa ISSN 1405-9940
Arch. Cardiol. Méx. vol.71 no.2 Ciudad de México Abr./Jun. 2001
Comunicaciones breves
Unusual giant patent ductus arteriosus associated with ventricular septal defect and discrete aortic coarctation. A case report
Conducto arterioso persistente gigante asociado con comunicación interventricular y coartación aórtica leve. Informe de un caso
Marcelo Basave,* Alberto Rangel,* Héctor Albarrán,* Óscar Nandayapa**
Hospital de Especialidades. CMN La Raza. IMSS, México, D.F. México.
* Departamento de Hemodinamia.
** Departamento de Registros Gráficos.
Correspondence
Alberto Rangel.
Departamento de Hemodinamia. Hospital de Especialidades. CMN La Raza.
Seris y Zaachila s/n. Col. La Raza. México, D.F. México. C.P. 02990.
Tel: 57821088, Ext. 1025.
E-mail: rangel_albertomx@yahoo.com.mx
Aceptado: 17 de octubre de 2000
Abstract
The authors present the case of a 26 years old female, 56 kg weight and 154 cm height, with a giant patent ductus arteriosus (2.4 cm of internal diameter), ventricular septal defect, discrete preductal narrowing of the aortic arch and pulmonary artery hypertension that did not diminished after 100% oxygen breathing. The authors speculate about the origin of the giant ductus here presented, based on hemodynamic and embryological data.
Key words: Giant ductus arteriosus. Ventricular septal defect. Aortic coarctation.
Resumen
Presentamos el caso de una mujer de 26 años de edad, con 56 kg de peso y 154 cm de estatura; persistencia de conducto arterioso gigante (2.4 cm de diámetro interno), comunicación interventricular, discreto estrechamiento preductal del arco aórtico e hipertensión arterial pulmonar que no disminuyó después de la inhalación de oxígeno al 100%. Con base en los datos hemodinámicos y embriológicos, especulamos, sobre el probable origen de la dilatación ductal.
Palabras clave: Conducto arterioso gigante. Comunicación interventricular. Coartación aórtica.
Introduction
The giant patent ductus arteriosus (PDA) was defined as a ductal external diameter > 1.5 cm.1 Cases of patients with PDA measuring 2.5 cm or more of external diameter are rarely reported in the medical literature. Recently, the cases of two children with surgically corrected giant PDA were reported (internal diameters 2.1 cm and 2.2 cm respectively).2 We present the case of an adult with an uncommon giant PDA (internal diameter 2.4 cm) associated with ventricular septal defect (VSD), and a discrete preductal narrowing of the aortic arch. Etiology of giant PDA is not well defined but its structural, hemodynamic, and embryologic features may be related with the associated defects. In order to maintain life, some cardiac malformations, such as pulmonary atresia, hypoplastic left ventricle and interrupted aortic arch (IAA) nearly always require the patency of a wide ductus arteriosus after birth.3 Because of the unusual wide diameter of PDA present in the patient, we speculate about the likely factors (hemodynamic and genetic) which led morphogenetically to the giant PDA and its associated malformations in the patient here presented.
Case report
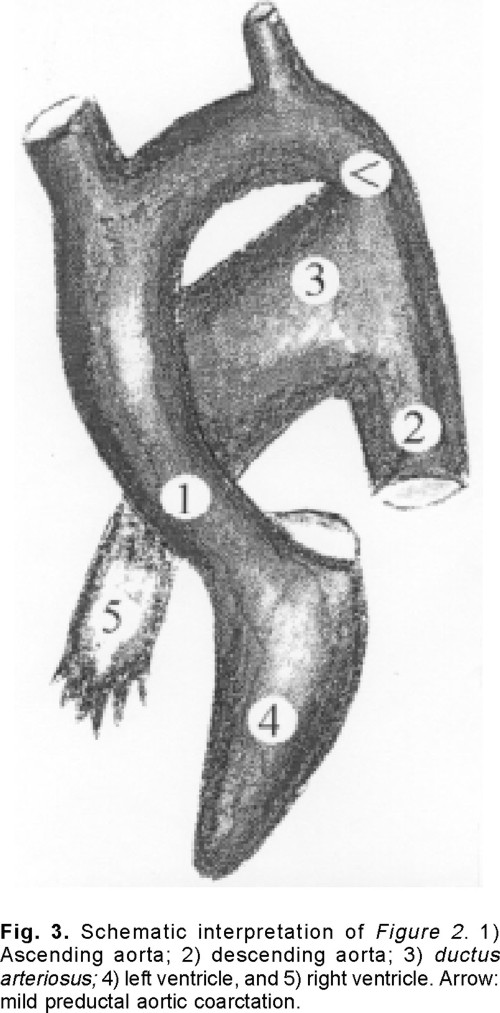
It is the case of a 26 years old woman (56 kg weight, and 154 cm height), in NYHA functional class I, with clinical, radiographic and electrocardiographic signs of PDA and VSD, associated with pulmonary artery hypertension. The transthoracic echocardiogram revealed a membranous VSD associated with almost 2 cm wide PDA and preductal aortic narrowing (Fig. 1). Intravascular catheterization (Table I) showed: a) left-to-right blood flow shunt; b) pulmonary artery hypertension and a 14 mmHg gradient between the ascending and descending aorta; c) during 100% oxygen inhalation, left-to-right blood flow shunt increased, pulmonary vascular resistance decreased and pulmonary artery pressure remained unchanged. The angiography (Fig. 2) showed the presence of a giant PDA (internal diameter 2.4 cm) associated with a discrete preductal aortic narrowing and a VSD (Fig. 2). Figure 3 shows a schematic interpretation of Figure 2.


Discussion
In 1964, Oldham et al reported 817 cases of patients with PDA, 31 (4%) presented PDA with an external diameter > 1.5 (the biggest being 2.5 cm). They definedthegiantPDAasaductalexternal diameter > 1.5 cm.1 In 1981, John et al.2 found in 131 patients with PDA, three cases with PDA outside diameter > 3.0 cm. Fisher informed about 117 adults with PDA. The biggest PDA found in this case was 2.5 cm outside diameter.3 In 1996, Chen et al. reported the case of an 18 years old girl and a 34 years old female with PDA measuring 2.1 cm and 2.2 cm of internal diameter, respectively.4 Our patient has an internal PDA diameter of 2.4 cm, documented by the angiography. Its external diameter is almost certainly comparable with the biggest reported by others.
In our patient, a wide PDA was associated with VSD and preductal aortic coarctation. Association among congenital cardiopathies can be:5 A) Fortuitous, i.e. VSD with PDA. B) Obligate, i.e. tricuspid atresia with atrial septal defect. C) Secondary to hemodynamic disturbances due to the primary cardiopathies, i.e. atrial septal defect and mitral valve prolapse.6 D) Secondary to alterations of anatomical structures, whose embryological origin is similar, i.e. aneurysm of the sinus of Valsalva with aortic insufficiency and VSD.7 No associated cardiac defects were found in most of the cases of wide PDA described in medical literature. In these cases, the origin of PDA wideness remains unexplainable.
In our patient, probably there is a fortuitous relationship between the ventricular septal defect with the PDA. However, it is possible that arterio-venous blood flow shunt through the VSD could contribute hemodynamically to enlarge the ductus arteriosus during the patient fetal life; specially when the descending aortic pressure is less than the ascending aortic pressure caused by relative aortic narrowing present in our patient.8,9 Wide PDA is associated with IAA. In our patient aortic obstruction was irrelevant. Patency of the ductus arteriosus after birth may result in cardiac failure.10 But, it may provide the only way to preserve systemic or pulmonary arterial blood flow in presence of other cardiac malformations like pulmonary artery atresia; hypoplastic left ventricle; IAA or some severe forms of aortic coarctation.11 From this point of view, it is explainable the persistence of a wide ductus arteriosus in most cases of IAA and unexplainable the wideness of PDA in absence of a complete closure of the aortic arch, as it occurred in our patient. Therefore in our case there is no obligate association between the giant ductus arteriosus and the aortic coarctation. If such association is not fortuitous, it could have an embryological origin. In our patient, aortic obstruction was preductal. There are no doubts that preductal aortic coarctation is a preceding stage of IAA. This is because preductal aortic coarctation, unperforated IAA with anatomical continuity between the pre and post-coarcted segments, and IAA with interruption between such segments are rings of the same chain. So for, our patient's preductal aortic coarctation is a frustrated case of IAA.
Aortic coarctation and hypoplasia of the aortic arch present anatomical, histological and embryological differences. Anatomically, aortic coartactation is usually a shelf-like lesion and the ligament of the ductus arteriosus almost always located between the isthmus and the descending aorta. It has been proposed that aortic coarctation may be related with an abnormal pattern of fetal blood flow in the ductus arteriosus. The shelf-like structure dorsally located in the aortic wall resembles a normal vascular bifurcation. Some blood flows proximally into the aortic arch and the remainder distally into the descending aorta.1213 Histologically, the shelflike tissue is indistinguishable from ductal tissue and is thought that closure of the ductus arteriosus after birth contributes to narrow the aortic isthmus by shrinkage of ductal tissue extended within the isthmic wall.14 On the other hand, hypoplasia of the aortic arch is a particular entity in which the portion of the aorta between the left subclavian artery and the ductus arteriosus (isthmus) remains uniformly narrowed after infancy. Its histological structure is the same as the rest of the aorta and it is frequently associated with other major cardiac malformations like VSD or PDA.
In the present case, the ductus arteriosus remained widely patent because shrinkage of the ductal elements did not occur. Formation of a shelf-like structure in the aortic wall opposite to the ductus did not occur. Pre-isthmic coarctation of the aorta was mild, in spite of the partial absorption of the left paired dorsal aorta between the sixth brachial arch and the tenth intersomite segment, which surely took place during the fetal life.
In our patient we exclude the possibility of a pseudo-coarctation of the aorta because in this entity, an elongation of a redundant aorta produces a "kicking" at the attachment of the ligamentum arteriosum. In this case, no pressure difference between, proximal and distal segments of the aorta is present.15 When the arch is interrupted, there is no evidence of arch
segment at the site of interruption, and sometimes an unperforate segment of arch is found when the arch is interrupted. Atresia of the aortic arch is an intermediate stage between severe tubular hypoplasia and aortic interruption. As it occurs in severe aortic coarctation, IAA takes place at the preductal level716 and most of the blood flow to the descending aorta takes place via a wide PDA.
Conclusions
Three cardiac malformations are associated in the case here presented. The most relevant one is the unexplainable wideness of the ductus arteriosus. Such wideness could be caused by the associated cardiopathies and explained as follow: 1) There is a fortuitous relationship between the three cardiac defects; in this case the cause of the ductal wideness is unknown. 2) Ductal wideness could be related to the hemodynamic disturbances caused by the increased arterio-venous blood flow shunt through the ventricular septal defect, promoted by the discrete aortic coarctation during the patient fetal life. 3) Incomplete or frustrated preductal IAA and PDA wideness developed simultaneously as it were genetically programmed as it occurs in the unperforated or interrupted aortic ascending and descending segments.
Acknowledgement. We are in the debt of Mrs. Maggie Brunner for their expert help in the correction of the English language of this manuscript.
References
1. Oldham H, Collins N, Pierce G, Sabiston D, Blalock A: Giant patent ductus arteriosus. J Thorac Cardiovasc Surg 1964; 47: 331-336. [ Links ]
2. John S, Muralidharan S, Jairaj P, Mani G, Sukumar I, Cherian G: The adult ductus: Review, of surgical experience with 131 patients. J Thorac Cardiovasc Surg 1981; 82: 314-319. [ Links ]
3. Fisher R, Moodie D, Streba R, Gill C: Patent ductus arteriosus in adults; long-term follow up. Nonsurgical versus surgical treatment. J Am Coll Cardiol 1986; 8: 280-284. [ Links ]
4. Chen Y, Chiu C, Lee C, Lai W, Hwang Y: Surgical correction of giant patent ductus arteriosus. J Cardiovasc Surg (Torino). 1996; 37: 309-312. [ Links ]
5. Rangel A, Pérez J, Baduí E: Cardiopatías congénitas en el adulto. Distribución de frecuencia, edad, género y presión arterial pulmonar. Arch Inst Cardiol Méx 1997; 67: 303-317. [ Links ]
6. Liberthson RR, Boucher CA, Fallont JT, Buchey MJ: Severe mitral regurgitation: a common occurrence in the aging patients with atrial septal defect. Clin Cardiol 1981; 4: 229-232. [ Links ]
7. Rangel A, Chávez E, Espinoza I: Interruption of the aortic arch in adults. Arch Inst Cardiol Méx 1999; 69: 144-148. [ Links ]
8. Shinebourne E, Elseed A: Relation between fetal flow patterns coarctation of the aorta, and pulmonary blood flow. Br Heart J 1974; 36: 492-498. [ Links ]
9. Gittenberger-Degroot A: Persistent ductus arteriosus: Most probably a primary congenital malformation. Br Heart J 1977; 39: 610-618. [ Links ]
10. Weidman W, Blount S Jr, Dushane J, Gersony W, Hayes C, Nadas A: Clinical course in ventricular septal defect natural history study. Circulation 1977; 56 (Suppl I): 156-169. [ Links ]
11. Roberts W, Morrow A, Braunwald E: Complete interruption of the aortic arch. Circulation 1962; 26: 39-59. [ Links ]
12. Hutchins G: Coarctation of the aorta explained as a branch point of the ductus arteriosus. Am J Pathol 1971; 63: 203-209. [ Links ]
13. Talner N, Berman M: Postnatal development obstruction in coarctation of the aorta: Role of the ductus arteriosus. Pediatrics 1975; 56: 562-569. [ Links ]
14. Wielenga G, Dankmeijer J: Coarctation of the aorta. J Pathol Bacteriol 1968; 95: 265-274. [ Links ]
15. Smyth P, Edwards J: Pseudocoarctation, kinking or bulcking of the aorta. Circulation 1972; 46: 1027-1032. [ Links ]
16. Bharati S, Lev M: The surgical anatomyofthe heart in tubular hypoplasia of the transverse aorta (preductal coarctation). J Thorac Cardiovasc Surg 1986; 91: 79-85. [ Links ]














