Introduction
Milking parlors produce several types of waste as the sewage with solid waste (faeces, food remains and mud) and liquids (water, urine, milk remains and cleaning solutions for milking equipment), which are a consequence of milking (Charlón, 2009). The wastewater from the milking parlor is rich in nutrients, such as protein, fat and lactose, originating from wasted milk, which contributes to organic matter, phosphorous and nitrogen present in liquid waste, bringing about contamination problems due to the high values of biochemical oxygen demand (BOD) or chemical oxygen demand (COD), in addition to representing a source of harmful bacteria, such as the coliforms (Kern, Idler & Carlow, 2000; Shah & Patel, 2013; Wu & Zhu, 2015).
To assist in the treatment and disposal of sludge and wastewater, different technologies have been developed, one of which it was proposes the use of effective microorganisms (EM) in several number of applications, including agriculture, livestock, gardening and landscaping, composting, bioremediation, septic tank cleaning, algae control, and others (Shalaby, 2011; Shah & Patel, 2013). Among the species that make up the EM mixtures are yeasts such as Saccharomyces cerevisiae (SC) and lactic acid bacteria (LAB) (Hoyos et al., 2008; Shalaby, 2011; Shah & Patel, 2013). On the other hand, bacterial species belonging to the Bacillus genus are known to help in the mineralization and reduction of the accumulation of organic matter in the water (Anh, Huong & Dung, 2010).
Various studies have reported that the use of mixtures of microorganisms containing LAB and SC in the treatment of wastewater achieved the reduction of COD, the bioabsorption of metal ions, the reduction of alkalinity and the content of total dissolved solids, among others benefits (Rodríguez, Miranda, Olivas & Sosa, 2008; Namsivayam, Narendrakumar & Arvindkumar, 2011; Herrera & Corpas, 2013; Matute-Almeida, Galindo & Delahais, 2017). Likewise, species of the genus Bacillus have been used in water treatment plants, managing to reduce the BOD by 71.93% (Safriti, Priadie, Miranti & Astuti, 2015), degrading the organic matter present in wastewater from food industries (Huertas, 2010), among other benefits. The main objective of this research was to assess the use of microbial mixtures on the treatment of liquid waste from a milking parlor. In this regard, the specific purposes of this work were: to evaluate the growth kinetic both Bacillus´s species mixture and mixture of LAB under “in vitro conditions” as well as, to study the effect of the Bacillus´s mixture and LAB´s mixture on the quality of liquid wasted (“in vivo conditions”). There is not works related with the specific use of these microorganisms on the treatment of liquid waste from a milking parlor. Finally, the principal contribution of this paper was to compare both the “in vitro” conditions of each mixture of microorganism and their applications “in vivo”.
Materials and methods
Site of investigation
The research was conducted at the Agroindustrial Biotechnology Laboratory of the Institute of Chemistry and Technology and at the Milking Room of the Bovine Section of the Institute of Animal Production. Both institutes are located in the Faculty of Agronomy of the Central University of Venezuela, Campus Maracay.
Biotechnological process
Dairy wastewater
The effluents were collected from the milking, washing and disinfection process in the Milking Parlor of the Bovine Section of the Animal Production Institute. The following analyzes were carried out on the samples of liquid wastewater: pH (Olmos, 1987); electrical conductivity (EC) expressed in μS (Andem, Okorafor, Eyo & Ekpo, 2013); total dissolved solids or TDS, expressed in ppm (Jimenez-Noda, Cova, Trías, Vega & Manganiello, 2017) and total coliforms bacteria count (TC) by the plate counting method (Covenin, 1984). The quantification of coliform bacteria on a solid medium involves plating with violet red bile agar (VRBA) and counting dark red colonies.
Microorganisms
Two (2) microbial mixtures were used: the first mixture (MM1) included an isolate of lactic acid bacteria (LAB) identified as q11 collected from artisanal white cheese, and belonging to the brand name of the Agroindustrial Biotechnology Laboratory and commercial Saccharomyces cereviseae, and the second one it was the microbial mixture (MM2) composed of four (4) Bacillus species: Bacillus subtilis, Bacillus licheniformis, Bacillus amyloliquefaciens and Bacillus megaterium have been obtained from soil samples and belonging to the culture collection of the Agroindustrial Biotechnology Laboratory. The strains were routinely stored at 4 °C in agar slants tubes of brain-heart infusion (BHI) (Bacillus species) and manrogosa-sharpe (MRS) (LAB q11). The Bacillus species were transferred every 24 h in BHI broth at 37 °C and a shaking frequency of 300 rpm until a steady state of growth was reached. As long as, the isolate q11 was cultivate in MRS broth at 37 °C in static flask. Initial population in both mixtures was about 109 CFU/mL, the quantification was performed by plating treated cultures on MRS agar (q11) and BHI agar plates and counting colonies.
Study of the growth kinetics of the microorganisms under study in the liquid waste
Microorganisms growth was studied by pouring 100 mL of the residues in Erlenmeyer flasks with a capacity of 500 mL, separately inoculating each microbial mixture and incubating at 37 °C in static flask. Sampling was carried out during 0, 24, 48 and 72 hours and the pH was measured (Olmos, 1987); the EC in μS (Andem et al., 2013); TDS expressed in ppm (Jimenez-Noda et al., 2017) and TC by the plate counting method (Covenin, 1984). Likewise, it was carried out the plate count of the microorganisms under study, the yeasts (Covenin, 1990), the LBA isolate (Savadogo et al., 2004) and the Bacillus bacteria (Lara-Mantilla & Burgos-Portacio, 2012).
Effect of the microbial mixture on the quality of the waste
Once the growth of the MM in the milking parlor wastewater was evaluated, the in situ effect “in vivo” of the application of the MM was studied, which presented the best results in the kinetics of the residues. Liquid waste samples were collected during weeks 0, 1, 2, 3 and 4, from the effluent stream by slowly submerging the container with minimal surface disturbance and allowing the sample stream to flow smoothly into the container with minimal splashing. This operation was repeated three times until reaching a gallon of sample. Immediately, the pH was measured (Olmos, 1987); the EC in μS (Andem et al., 2013); TDS expressed in ppm (Jimenez-Noda et al., 2017) and TC by the plate counting method (Covenin, 1984). Likewise, the microorganisms of the microbial mixture were counted using standard plate count method (Lara-Mantilla & Burgos-Portacio, 2012).
Statistical analysis and experimental design
The test was carried out under a completely randomized design, with three replicates for each analysis and microorganism. The experimental unit was represented by the residues and the treatments were the two microbial mixtures: MM1 (isolated LAB q11 and SC) and MM2 (Bacillus species). An analysis of variance (ANOVA, α = 0.05) was performed with the collected data to determine if there is an effect on the quality of the liquid residues the milking parlor and the means were separated by the least significant differences (LSD) procedure among the treatments to determine which of the mixtures was more efficient. Subsequently, an analysis of variance (ANOVA, α = 0.05) was applied in order to determine if there was an effect on the quality of the liquid residues of the milking parlor applied in situ “in vivo” and the mean comparison test was applied of the least significant difference (LSD) to the samples collected through weeks 0, 1, 2, 3 and 4 (Gutiérrez & de la Vara, 2012), to determine if the quality of the residues was improved.
Results and discussion
Study of the growth kinetics of the microorganisms under study in the wastewater of the milking parlor
The analysis of variance (ANOVA) applied to the collected data, showed that there are highly significant differences (p ˂ 0.01) between the treatments for all the variables studied. In Figure 1, the behavior of the pH during the treatment with the mixtures can be observed.

Figure 1 Experimental comparison of pH values along the time. Were measured the changes in pH in the treatments [One of the treatments was a mixture made up of an isolate of lactic acid bacteria (LAB) identified as q11 and commercial Saccharomyces cereviseae; the other one was a microbial mixture of four (4) Bacillus species]. It was taking readings every 24 hours during the process time (from 0 to 72 hours). Three (3) repetitions were made per reading. Each point in the graph represents the mean of the repetitions. Fisher’s test of Least Significant Differences was applied to determine differences between treatments from each other. Different letters indicate significant differences (p < 0.05).
In the treatments studied, the pH increased reaching values of 8.65 for the mixture of isolate q11 and SC (MM1) and of 8.75 for MM2 (p < 0.05) at 72 hours, which, according to Chae-Woo, Yun-Seok & Kye-Heon (2009) and Nemutanzhela, Roets, Gardiner & Lallo (2014) can be attributed to the generation of bacteriocins, which release ammonium ions increasing the pH.
It is important to highlight that in the water purification processes, the pH is chemically stabilized by incorporating lime, bringing it to values of pH 8, in this way the putrefaction of the organic matter contained in the treated water is avoided while it is biodegraded and being promoting the precipitation of elements such as phosphorous in the form of phosphates and metals in the form of hydroxides or insoluble salts (CENTA, 2008).
Regarding the total dissolved solids and the electrical conductivity in the studied treatments, it seen to be that the values in both parameters decreased as the kinetics was in progress (Figures 2 and 3, respectively). In accordance with Cirelli (2012), this is because of these variables are closely related, since the salts dissolved in the water were part of the total solids and the first influences directly on the electrical conductivity of the waters. Figure 4 shows a positive relationship between these parameters, with an average correlation of 0.76 [R2 = 0.7763 (MM1) and R2 = 0.7378 (MM2)]. It has been pointed out that electrical conductivity is a useful indicator of total dissolved solids (TDS) because the conduct of current in an electrolytic solution depends mainly on the concentration of ionic species, since conductivity is the capacity of water to conduct an electric current, and the dissolved ions acting as the conductors. This relationship (TDS and electrical conductivity) is a complex matter, depending on the presence of ions, their total concentration, mobility, valence, relative concentration and the temperature at which the measurements were studied, correlations ranging from 0.5 up to 0.9 were founded (Siosemarde, Kave, Pazira, Sedghi, & Ghaderi, 2010; Shinde, Pathan, Raut & Sonawane, 2011; Alí, Mo & Kim, 2012; Pal, Samal, Roy & Roy, 2015; Choo-in, 2019).

Figure 2 Comparison total dissolved solids values against time. Were measured the changes in the total dissolved solids concentration in the treatments [One of the treatments was a mixture made up of an isolate of lactic acid bacteria (LAB) identified as q11 and commercial Saccharomyces cereviseae; the other one was a microbial mixture of four (4) Bacillus species]. It was taking readings every 24 hours during the process time (from 0 to 72 hours). Three (3) repetitions were made per reading. Each point in the graph represents the mean of the repetitions. Fisher’s test of Least Significant Differences was applied to determine differences between treatments from each other. Different letters indicate significant differences (p < 0.05).

Figure 3 Comparison electrical conductivity values against time. Were measured the changes in the electrical conductivity values in the treatments [One of the treatments was a mixture made up of an isolate of lactic acid bacteria (LAB) identified as q11 and commercial Saccharomyces cereviseae; the other one was a microbial mixture of four (4) Bacillus species]. It was taking readings every 24 hours during the process time (from 0 to 72 hours). Three (3) repetitions were made per reading. Each point in the graph represents the mean of the repetitions. Fisher’s test of Least Significant Differences was applied to determine differences between treatments from each other. Different letters indicate significant differences (p < 0.05).

Figure 4 Lineal regression curves and correlation analysis between total dissolved solids and electrical conductivity in the studied treatments. Variations in the concentration of total dissolved solids were measured and readings were taken every 24 hours during the liquid waste treatment process (from 0 to 72 hours), to determine if there is a correlation with electrical conductivity. Each point on the graph represents the mean of the Three (3) repetitions. Likewise, the equations of the trend lines drawn for each applied microbial mixture are presented. The treatments presented coefficients of determination R2> 0.7, so it can be said that there is a correlation between the content of dissolved solids and the electrical conductivity that can be attributed to the presence of electrical ions dissolved in the medium.
Likewise, a lower content of TDS and a lower value in EC were recorded in the treatment with MM2 (p < 0.05) at 72 hours. Furthermore, the EC reported in both treatments was lower than that obtained by Mostafa, Metwally, Nanis & Eltohamy (2014), who pointed an EC of 2,470 µS/cm in wastewater treated with bacterial cultures. Similarly, it was important to note that the values obtained in this paper are less compared than the maximum permissible limit of 1,000 ppm and 1,500 µS/cm of electrical conductivity (Advíncula Zeballos, García Junco, García Armas, Toribio Tamayo & Meza Contreras, 2014; Jímenez-Noda et al., 2017) at 72 hours of treatment.
Regarding the growth of the microorganisms forming part of the mixtures, it was determined that the three species used (isolated from lactic acid bacteria q11, SC and the Bacillus species), were able to grow in the effluents from the milking parlor (Figure 5). However, it was observed that the growth of the MM2 mix differed significantly (p < 0.05) from the MM1 mix. The lower growth of microorganisms in the MM1 mixture can be attributed to the absence of sugars in the medium, since an initial sugar concentration between 10 and 15% has been reported to favor SC productivity (Arifa, Madiha & Tasnim, 2010; Dhanasekeran, Lawanya, Saha, Thajuddin & Panneerselvam, 2011).

Figure 5 Microbial density from milking parlor wastewater treated with microbial mixtures. Were measured the changes in the microorganism populations applied [One of the treatments was a mixture made up of an isolate of lactic acid bacteria (LAB) identified as q11 and commercial Saccharomyces cereviseae; the other one was a microbial mixture of four (4) Bacillus species]. It was taking readings every 24 hours during the process time (from 0 to 72 hours). Three (3) repetitions were made per reading. Each point in the graph represents the mean of the repetitions. Fisher’s test of Least Significant Differences was applied to determine differences between treatments from each other. Different letters indicate significant differences (p < 0.05).
Finally, as observed in Figure 6 the behavior of TC in the treatments studied. The statistical tests applied indicate that the MM1 and MM2 mixtures affected the TC population (p < 0.01) and that 72 hours after the kinetics, no TC were not observed in the MM2 flasks (p < 0.05).
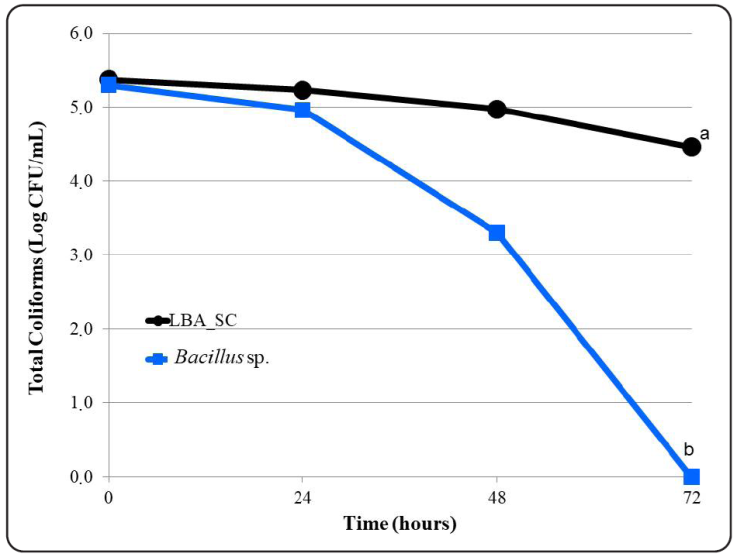
Figure 6 Total coliforms density from milking parlor wastewater treated with microbial mixtures. Were measured the changes in the coliforms populations in the treatments studied [One of the treatments was a mixture made up of an isolate of lactic acid bacteria (LAB) identified as q11 and commercial Saccharomyces cereviseae; the other one was a microbial mixture of four (4) Bacillus species]. It was taking readings every 24 hours during the process time (from 0 to 72 hours). Three (3) repetitions were made per reading. Each point in the graph represents the mean of the repetitions. Fisher’s test of Least Significant Differences was applied to determine differences between treatments from each other. Different letters indicate significant differences (p < 0.05).
Various studies have indicated that as TC are an indicator of microbial quality, the decrease in their population suggests a reduction in the growth of pathogenic microorganisms, such as: Staphylococcus aureus, Salmonella spp., Clostridium perfringens, Clostridium botulinum, Clostridium chauvonei, Campylobacter spp., Escherichia coli, Bordetella spp. and Corynebacterium spp. (Joerger, 2003; Fiorentin, 2005; Peinado, Ruiz, Echávarri & Rubio, 2012). The reduction in the coliform population probably was due to the probiotic character of Bacillus species and lactic acid bacteria, which not only inhibit the growth of coliforms but were capable of neutralizing and their possible toxins, if it were the case (Phianphak, Rengpipat, Piyatiratitivorakul & Menasveta, 1999; Lutful Kabir, 2009).
In summary, from data tabulated as shown in the Table I, the microbial mixtures used improved the quality of the residues in the milking parlor. However, statistical tests showed that this effect was greater in the treatments with the MM2 mixture (p < 0.05), meaning that this was the mixture applied to the effluents in situ.
Table I Means (±standard deviation) of milking parlor wastewater physicochemical parameters before and after use microbial mixes (72 hours).
| Parameters | Wastewater | MM1 Treatment | MM2 Treatment | Maximum limit allowed* |
|---|---|---|---|---|
| pH | 8.08±0.06c | 8.65±0.06b | 8.75±0.055a | 4.5-9 |
| Total Dissolved Solids (ppm) | 816±4.48a | 775±22.48b | 703±28.38c | 1,000 |
| Electrical Conductivity (μs/cm) | 1,784±4.48a | 973±23.71b | 753±38.35c | 1,500 |
| Total Coliforms (Log CFU/mL) | 5.34±0.048c | 4.47±0.4b | 0a | - |
According to regulation 5.021: Gaceta Oficial 5.021 (1995).
** Means within a row with Different superscript indicate significant difference at p < 0.05 according to Least Significant Difference (LSD) test statistics.
Effect of the microbial mixture of Bacillus species on the quality of liquid waste in the milking parlor
Statistical tests applied to the data collected, determined the existence of highly significant differences in all the variables under study during treatment (p < 0.01). In the Figure 7, the behavior of the pH was observed and how it increases until reaching a value of 8.76 at the fourth week of the biological treatment. Which, as previously it was pointed out, this behavior might be attributed to the generation of antimicrobial agents (probably bacteriocins), which release ammonium ions as increasing the pH (Chae-Woo et al., 2009; Nemutanzhela et al., 2014).

Figure 7 Experimental pH (●), Total Dissolved Solids (○) and Electrical Conductivity (■) values against time. The variation in the experimental parameters of the dairy liquid residues to which the Bacillus species mixture was applied was measured. It was taking readings every 7 days during the process time (from 0 to 4 weeks). Three (3) repetitions were made per reading. Each point in the graph represents the mean of the repetitions. Fisher’s test of Least Significant Differences was applied to determine variances between treatments from each other. Distinct letters indicate significant differences (p < 0.05).
Based on the TDS (Figure 7), they increased during the first week of treatment, this could be due to the process of degradation of organic matter present in the effluents, releasing ions into the medium (Chaurasia et al, 2005; Yilmaz, Soran & Beyatli, 2006; Cirelli, 2012); coupled with the milking cow population increased, since 5 new births occurred in the course of this investigation, which implied an increment in feces and therefore in the volume of effluents, considering that on average, can be recovered 0.363 kg of solid matter (DM) per cow and per day. If it was considered that an adult cow produces between 4 and 5 kg DM of manure per day and assuming that 90% of the recovered solid fraction corresponded to fecal matter (the remaining 10% corresponds to food and mud), it can be inferred that in milking parlor, between 7 and 9% of the daily total are deposited, this, without considering the residues due to colostrum, spilled milk, urine and washing water, since these are very difficult to estimate (Charlón, 2011).
Changes in EC, it was determined that it remained constant during the first week of treatment and began to decrease after 2 weeks of incorporation of the MM2 mixture (Figure 7). The decrease in EC is indicative of the reduction of ions in the residues (salinity), meaning that the microbial mixture could be used as an alternative treatment of the residues since it uses biodegradable organic matter from the wastewater, as nutrients of a bacterial population suspended in the water to be treated (suspended biomass). The treated water can be used for irrigation whether it is previously subjected to some disinfection method (chlorination, ozone, ultraviolet radiation), which corresponds to the tertiary or advanced treatment used to achieve a purer, even potable water, if desired. The objectives of advanced treatment are to eliminate the organic load remaining from a secondary treatment (biological treatment), disinfect it to eliminate pathogenic microorganisms, undesirable color, and odor, remove detergents, phosphates, and residual nitrates, which cause foam and eutrophication (Wetland, 2007).
In addition, an antagonistic effect was observed between the microorganisms that make up the MM2 mixture and the coliforms, since the presence of the latter was not detected in the plate counts carried out during the last two weeks of the study (Figure 8). The statistical tests applied showed the existence of significant differences (p < 0.05) between the populations of the microorganisms accounted during this study. This result coincides with that indicated in various investigations, about the ability of Bacillus species to reduce the population of coliforms such as Escherichia coli, Shigella spp., Klebsiella pneumoniae, Enterobacter aerogenes, among others (Bhoonobtong, Sawadsitang, Sodngam & Mongkolthanaruk, 2012; Vijayaram & Kannan, 2015; Sharif et al., 2016, Tariq, Sudha & Reyaz, 2016),

Figure 8 Microorganisms density from milking parlor wastewater treated with MM2 against time. Were measured the changes in the bacterial populations. It was taking readings every 7 days during the process time (from 0 to 4 weeks). Three (3) repetitions were made per reading. Each point in the graph represents the mean of the repetitions. Fisher’s test of Least Significant Differences was applied to determine variances between treatments from each other. Distinct letters indicate significant differences (p < 0.05).
It can be concluded that the MM2 mixture obtained from four Bacillus species (Bacillus subtilis, Bacillus licheniformis, Bacillus amyloliquefaciens and Bacillus megaterium), was able to improve the quality of the residues from the milking parlor upon reaching an electrical conductivity and total dissolved solids concentration below the allowed limit for agricultural irrigation water (1,500 μS/cm and 1,000 ppm, respectively) under the conditions in which this experiment was carried out, in addition, there was an antagonistic effect over the total coliform population.
Conclusions
Under the conditions in which this research was carried out “in vitro”, the two studied microbial mixtures demonstrated the ability to grow and improve the environmental quality of the wastewater from the milking parlor of the Animal Production Institute (Faculty of Agronomy of the Central University from Venezuela). Regarding the application on site “in vivo” of the MM2 mixture, it reduced the electrical conductivity and the total solids dissolved in the residues, as well as inhibited the growth of total coliforms, even with the daily discharges during regular activity in a milking parlor.











 nueva página del texto (beta)
nueva página del texto (beta)


