Introduction
Since ancient times, mankind has used plants for medicinal purposes to treat or prevent diseases. With time, their use and commercialization have increased due to various socio-economic factors. Plants of the Chrysobalanaceae family have been used in traditional medicine in some countries; notably, Mexico and Brazil, where it is used to treat various diseases, such as leucorrhoea, bleeding, hypoglycemic, diabetes, and antiangiogenic (Alves, Satiro & Perrelli, 2012; Araújo-Filho et al., 2016; Carnevale, Pilon, Da Silva & Castro-Gamboa, 2013). C. icaco stands out for its nutritional and ethnopharmacological qualities because within its composition of both the leaves and the fruit are significant amounts of phytochemicals such as pomolic acid, which induces apoptosis in HL-60 leukemia cells (Fernandes, Weinlich, Oliveira, Amarante-Mendes & Rocha, 2007), sesquiterpenes and diterpenes with analgesic and antiinflammatory activity (Araújo-Filho et al., 2016), cytotoxic anthocyanins in HT-29 colon cancer cells, anti-inflammatory and antioxidant (Venancio et al., 2016), rutin and myricetin with antifungal activity (Silva et al., 2017). Although C. icaco is a source of other phytochemicals like terpenes, most often flavonoids like flavonols and anthocyanins are most studied in fruits. These molecules stand out for their hypoglycemic, antiobesogenic, hypolipidemic, and antioxidant activity, these in vitro and in vivo models, so could become a profitable and sustainable alternative, in the treatment of these conditions (White et al., 2016a; White et al., 2016b; Portela-de-Sá et al., 2020; Nayak et al., 2011; Ferreira-Machado et al., 2014; de Oliveira et al., 2013), in addition to the fact that no acute toxicity has been observed (Ribeiro et al., 2020).
Non-communicable diseases like diabetes, overweight, obesity, and some factors related to metabolic syndrome (MetS), such as hypertension, are the main cause of death in the world. Non-communicable diseases represent a serious public health problem since they are an economic burden to health care systems. Moreover, current pharmacological treatments cause various side effects that can affect the patient's lifestyle, which is why they are sometimes discontinued, contributing to pathological complications. The factors of MetS have also been recognized as a preponderant risk factor for the development of complications derived from respiratory diseases (Costa et al., 2020). This research aimed to compile specific literature on the effect of C. icaco in metabolic syndrome, its phytochemistry, and ethnopharmacological studies.
Methodology
The scientific literature search was carried out during August-December 2020, using the scientific name (Chrysobalanus icaco L.) as the search term. The selected databases were PubMed, Web of Science, Science Direct, Springer, Taylor and Francis, ACS, Google Scholar, and Scopus. Only those studies were selected where chemical aspects, in vivo/in vitro evaluations, structural elucidations of secondary metabolites, and pharmacological activities of fruits, leaves, roots, or other parts of C. icaco were studied. Articles in English and Spanish were considered.
Public health and chronic degenerative diseases in México
According to the National Institute of Public Health of México (INSP, 2020), the main causes of death in the Mexican population are heart diseases (20.1%), diabetes (15.2%), malignant tumors (8.12%), liver diseases (5.5%) and accidents (5.2%). During 2020, diabetes moved to third place, only surpassed by SARS-CoV-2 (INEGI, 2021). It is estimated that by 2030, México will be the sixth country with the most cases of diabetes, with 16.8 million diagnosed adults, while by 2045, the cases will increase to 22.3 million (Saeedi et al., 2019). These numbers are alarming for México's public health, so the next investigations related to the search for bioactive compounds should be oriented towards the treatment of these pathologies, in addition to implementing by the Ministry of Health robust programs for the prevention of MetS factors and diabetes.
Metabolic Syndrome (MetS) in México
According to the National Heart, Lung, and Blood Institute (NIH, 2020), MetS is defined as a group of different risk factors that can increase the risk of coronary heart disease, diabetes, or stroke. Five risk factors have been proposed to diagnose it: abdominal obesity, excess fat in the stomach; an elevated level of triglycerides; low HDL cholesterol levels; high blood pressure, and elevated basal blood sugar. Insulin resistance (abnormal functioning of the use of insulin) increases the risk of suffering from MetS; it is also more common to present it if you suffer from obesity or overweight, both together with sedentary lifestyles are the main cause of its appearance (NIH, 2020; IDF, 2020; Lanktree & Hegele, 2018).
Recent research on the prevalence of MetS in Mexican cancer patients determined that the mean age of the studied population was 55 years, mostly female (81%). The most frequent type of cancer was breast (42%), with 42% of the patients showing overweight. Besides, 42% showed hypertension and 50% suffered from diabetes with mean clinical values of 121.5 mg/dL of glucose. Some authors attribute the high prevalence of MetS to the high incidence of cancer in México (Flores et al., 2019).
Romero-Velarde et al. (2015) conducted a study with 102 obese Mexican children and adolescents. It was found that MetS prevailed from 37.5 to 54.5%, mainly associated with suffering from insulin resistance and high birth weight. Magaña, Moreno-Mascareño, Angulo & de la Peña (2020), mention that the prevalence of MetS in school children from Sinaloa, México (8-11 years, n = 155), is 10.3%, associated with the prevalence of obesity with low levels of high molecular weight adiponectin (4.5 μg/mL). A longitudinal study, carried out over 6 years in 1,046 Mexican adults, mentioned that the predictive factor for developing MetS is the increase in the body mass index (Perez-Rodriguez, Talavera & Salmeron, 2021).
An analytical cross-sectional nationally representative study carried out on Mexican adults (n = 4595), mentioned that the percentage of people with MetS who required health services was 51.3%, with high triglycerides 55.5%, abdominal obesity 50.5%, hypertension 50.6%, high cholesterol 65.1%, as important factors in the diagnosis of MetS (Ortiz-Rodriguez, Aldaz-Rodríguez, González-Robledo, Villa, Bouzas, Pastor & Tur, 2021).
Gutiérrez-Esparza, Infante, Vallejo & Hernández-Torruco (2020), used new bioinformatics tools such as machine learning algorithms to determine the most appropriate health parameters for the diagnosis of MetS in patients from the Ignacio Chavez National Institute of Cardiology in México City. Their results showed that the preponderant parameters for a correct diagnosis are fasting plasma glucose, triglycerides, Waist-to-Height-Ratio, HDL Cholesterol, Body Mass Index, diastolic blood pressure, and Waist Circumference.
A recent study points out that the minor allele G of the ADIPQ11377 C<G polymorphism constitutes a risk factor for the development of MetS since this gene is related to obesity and its development. The study was carried out in Fresnillo, Zacatecas, México (García et al., 2021). Likewise, the prevalence of diabetes and its relationship with MetS has been investigated in indigenous communities of Baja California, México. The prevalence of MetS is 53.1%, mostly in women than in men (Pacheco et al., 2018). In other Mexican ethnic groups, obesity and visceral fat are the main factors that manifest themselves, mainly in women (55.6%), while low HDL-cholesterol levels (75.8%) occur in both sexes. This is associated with the lack of education, so it is important to reinforced prevention efforts in vulnerable populations such as ethnic groups and rural areas of the country (Mendoza-Caamal et al., 2020).
Health professionals under 29 years of age from Guadalajara, Jalisco, México revealed that at least one or more components of the MetS, highlighting abdominal obesity (27.2%), followed by hypocholesterolemia (6.6%), were associated with a BMI greater than 25 kg/m2 and negatively associated with physical activity (Betancourt-Núñez, Márquez-Sandoval, Babio & Vizmanos, 2018).
Obesity and diabetes in México
The problem of obesity in children and adolescents in México is worrying due to the high prevalence in children aged 5-11 years (35.6%) and adolescents 12-19 years (38.4%), representing that 1 of each 3 people are overweight or obese. As a whole, the prevalence of overweight and obesity in men and women over 20 years of age is 72.5% and 9.4%. According to INEGI (2020), a significant degree of obesity is observed in all states of México, highlighting those of the North (Baja California, Sonora, Chihuahua, Coahuila, Nuevo León, Tamaulipas) and the coastal and southern zone (Veracruz, Tabasco, Campeche, and Yucatán), closely correlating with more cases of hypertension, particularly in Baja California, Sonora, and Chihuahua.
Diabetes occurs uniformly throughout the country, highlighting the states of Sonora, Tamaulipas, and Nuevo León. Overall, obesity, hypertension, and diabetes estimates rank first in Sonora. According to the National Health and Nutrition Survey (ENSANUT), in 2018, more than 8.6 million Mexicans over the age of 20 suffered from diabetes, 15.2 million had hypertension, 19% had high cholesterol and triglyceride levels, with women being the most affected in the three diseases. Besides, 29.0% mentioned doing less than 2.5 hours per week of physical activity, in addition to the fact that 85.8% said they consume sweetened non-dairy beverages daily (a food that is not nutritionally recommended), so that 75.2% of the Mexican population in 2018, were overweight and obese (39.1% men and 36.1% women) (INSP/INEGI, 2018).
Relationship of inflammation with MetS
Obesity is considered a chronic state of inflammation because adipose tissue activates metabolic pathways involved in inflammation such as TNF-α, c-JunN-terminal kinases, secretes adipokines such as interleukin IL-6, leptin, adiponectin, and resistin. Also, by increasing the size of the adipocytes, oxidative stress and reactive oxygen species (ROS) is generated, produced by excess lipolysis and hypertrophy of adipocytes, which in turn produces inflammatory cytokines (IL-6, IL-8, IL-18, TNF-α) and chemoattractants of macrophages altering the functionality of adipose tissue, the insulin resistance of damaged tissue, for example, pancreatic islets and liver, and initiates a local inflammatory process modifying the metabolism of biomolecules, increasing basal lipolysis and increasing the escape of free fatty acids. The pathogenesis of MetS remains uncertain; however, the mechanisms of inflammation present in obesity are associated with the progression of the factors involved in it (Fernández-Travieso, 2016; Salazar-Gómez et al., 2020).
Canto-Osorio, Denova-Gutiérrez, Sánchez-Romero, Salmeron & Barrientos-Gutiérrez (2020), conducted a cohort study with 399 Mexican participants, where they concluded that a highly inflammatory diet (high consumption of red and processed meats, high-fat dairy products, soft drinks, and refined cereals), is closely related to MetS, mainly with hypertension, obesity abdominal, and hypercholesterolemia. It is worth mentioning that the diet of Mexicans is characterized by high consumption of corn by-products and high consumption of "modern diets" such as carbonated beverages, fast food, and processed foods, which provides an ideal setting for the development of MetS.
Conventional and alternative treatments for MetS
Due to the medical implications of the metabolic syndrome, where at least three metabolic disorders are involved, its treatment constitutes a great challenge, since the drugs must act alone or in combination to treat obesity, diabetes, hypertension, and dyslipidemias (Serván, 2013; Lim & Eckel, 2014). In this sense, Table I shows the different treatments applied to MetS. A recent special article published in Nutrition Reviews, Aceves-Martins, López-Cruz, García-Botello, Gutiérrez-Gómez & Moreno-García (2021), compiles original intervention research to treat obesity in Mexican children and adolescents, which included 2,302 participants from 11 states of México. It highlighted that most of the interventions focused on reducing dietary calories, increasing physical activity, and modification of habits such as sedentary lifestyle. Also, it emphasized about the psychological and pharmacological support. Efforts should be focused to control and/or prevent obesity due to potential major comorbidities such as diabetes and hypertension.
Table I Current treatments and recommendations for factors related to MetS. Modified from Fernández-Travieso (2016); Lim & Eckel (2014); Heymsfield & Wadden (2017); Wagh & Stone (2004) and May, Schindler & Engeli (2020).
| Pathologies linked to MetS |
Recommendations | Pharmacotherapy |
|---|---|---|
| Obesity | Modify routines, eat a balanced and healthy diet, do physical exercises for at least 30 min a day, avoid a sedentary lifestyle, foods high in carbohydrates and saturated fats, avoid smoking, and consume toxic substances (alcohol and drugs). Behavioral therapy, counseling. |
Sibutramina, rimonabat, orlistat, liraglutide, bupropion/naltrexone, cathin, lorcaserin, phentarmine/topiramate. |
| Insulin resistance | A balanced diet, low in simple carbohydrates, specialized nutritional counseling, daily physical exercise. |
Metformine, glitazones. |
| Atherogenic dyslipidemia |
Low-calorie diets, consume fiber, moderate products high in lipids and carbohydrates. Consume specific functional foods, moderate consumption of alcohol and refined sugars. |
Statins, nicotinic acid, fibrates, ezetimibe. |
| Hypertension arterial |
Low sodium diet, avoid foods high in lipids and carbohydrates. Physical exercise daily. |
ACE inhibitors*, calcium channel blockers, angiotensin II receptor blocker. |
| Diabetes mellitus | Avoid foods with simple carbohydrates, sweets, sugary drinks. Exercise daily for at least 30 minutes. |
Insulin, meglitinides, sulfonylureas, α-amylase, and α-glucosidase inhibitors |
*Angiotensin Converting Enzyme.
On the other hand, specifically in the treatment of diabetes, Mexican patients, once they are diagnosed with the disease, turn to home remedies and medicinal plants, based on their ethnopharmacological knowledge, even discontinuing their pharmacological treatment (Andrade-Cetto & Heinrich, 2005; Escandón-Rivera, Mata & Andrade-Cetto, 2020). In the same way, the use of medicinal plants to control blood glucose levels or aid in the treatment of diabetes has been examined worldwide (Zekry, Badawy, Ezzelarab & Abdellatif, 2021; Ota & Ulrih, 2017; Sakulnarmrat & Konczak, 2012). Therefore, the investigation of new sources with hypoglycemic potential should be a priority in the public health area, due to the great natural resources present in México and other parts of the world such as South America, Australia and Africa.
In México, there are a large number of medicinal plants with activity hypoglycemic, anti-obesogenic, and hypocholesterolemic, for example, the "tronadora" (Tecoma stans), where two hypoglycemic alkaloids, tecomanin, and tecostatin, have been identified (Ibarra, Cantú, Verde & Oranday, 2009). Interviews with residents of Malpasito, Huimanguillo, Tabasco, mention that the plants mostly used to treat diabetes in their locality is the nopal (Nopalea cochenillifera), "maguey morado" (Tradescantia spathacea), "cundeamor" (Momordica charantia), "chicozapote" (Manilkara zapota) and "vicaria" (Catharanthus roseus) (Villareal-Ibarra et al., 2015). In Oaxaca, highlighting the traditional use of "maguey del pasmo" (Agave potatorum), "palo mulato" (Bursea simaruba), "caunashana" (Calea ternifolia) and "coral" (Hamelia patents), to treat diabetes, however, chemical and biological studies are lacking to elucidate its bioactivity (Castro, Villa, Ramírez & Mosso, 2014).
On the other hand, some plants and/or fruits have presented antiobesogenic and thermogenic effects, due to the presence of flavonoids, phenolic acids, alkaloids, dietary fiber, and proteolytic enzymes, such as those from guarana, yerba mate, green tea, green coffee, artichoke, papaya, pineapple, plantago and Garcinia cambogia (Espinosa, 2016). Other tropical fruits such as Juçara (Euterpe edulis), presents anthocyanins which showed their efficacy in treating obesity and hypercholesterolemia in clinical studies (Jamar et al., 2020). The pomegranate (Punica granatum), due to its content in phenolic acids such as ferulic, caffeic, and gallic, presents in vitro inhibitory capacity of enzymes related to hyperglycemia and obesity (Chukwuma, Mashele & Amaka, 2020).
Traditional plants of Chimaltenango, Guatemala, Hamelia patens, Neurolaena lobata, Solanum americanum, and Croton guatemalensis, showed hypoglycemic activity due to stimulation in insulin production in a murine model (Andrade-Cetto, Carola, Cabello-Hernández & Cárdenas-Vázquez, 2019). A meta-analysis, based on controlled clinical studies, indicate that C. sinensis, P.vulgaris, G. cambogia, N. sativa, I. gabonensis, and C. fimbriata, are effective for the treatment of obesity, inflammation, hypercholesterolemia, and other factors related to MetS, again the major compounds identified have been catechins and flavonoids (Payab et al., 2019).
Andrade-Cetto & Herinrich (2005) collected information on 306 plant species with hypoglycemic activity used in México, highlighting for their effectiveness in vivo and in vitro studies the "Guarumbo" (Cecropia obtusifolia), "Cola de caballo" (Equisetum myriochaetum), and "Guayacán" (Acosmium panamense), to name a few. The compounds responsible for this activity are mainly flavonoids (rutin, quercetin, kaempferol, isorhamnetin, kaempferitrin, catechin), terpenoids, and aromatic compounds. The flavonoids have presented a greater number of compounds identified in Mexican plants with hypoglycemic activity. Effects have occurred with acute and chronic administration and are related to inhibition of a-glucosidases, increase insulin levels, and inhibit glucose 6-phosphatase activity (Escandón-Rivera et al., 2020).
Likewise, Ficus carica fruits have presented the antioxidant and inhibitory potential of enzymes related to MetS, inhibiting the radical DPPH with an IC50 of 134.44 ± 18.43 μg/mL, inhibition of α-glucosidase, α-amylase, and pancreatic lipase with an IC50 of 320.9 ± 51.389, 315.89 ± 3.83, and 230.475 ± 9.65 μg/mL, respectively, this is due to the content of total phenols and flavonoids, 104.65 ± 5.51 mg/g GAE and 81.67 ± 4.0 mg/g QE respectively, in addition to acids saturated organics (Mopuri, Ganjayi, Meriga, Koorbanally & Islam, 2018). Ramírez, Zavala, Pérez & Zamilpa (2012), evaluated the inhibitory potential of α-glucosidase and lipase, in 23 medicinal plants used in traditional Mexican medicine, denoting "clavillo" (Ludwigia octovalvis), "zacapal" (Iostephane heterophylla), "ajenjo" (Artemisia absinthium), "higuerilla" (Ricinus communis) and the "prodigiosa" (Calea ternifolia), as potent inhibitors of a-glucosidase, while "clavillo", "tronadora" (Tecoma stans), "huizache" (Acacia farnesiana), and "ajenjo" highlighted due to their inhibitory properties of pancreatic lipase, the phytochemicals present in the extracts are flavonols, flavones, and caffeoyl derivates.
Some of the aforementioned plants are only used for ethnopharmacological purposes, so their collection is limited to specific areas. By not growing them for food and/or industrial purposes, knowledge is only transmitted from generation to generation, making the research and dissemination process difficult. On the other hand, C. icaco is a fruit for food purposes mainly (México, Central America, South America, and Africa), so the disclosure of its phytochemical composition and biological activities can help to encourage its use and consumption, helping to preserve the food safety of the regions where it is produced, in addition, its cultivation can subsequently be intensified for commercial and industrial purposes to obtain nutraceuticals (with previous clinical studies) representing a profitable and sustainable source. Furthermore, C. icaco has high plasticity, acidic or alkaline soil requirements, resistance to drought, high tolerance to salinity and wind, and its nutritional content is low, so its adaptation to temperate and/or dry zones could be carried out (Brown & Frank, 2018).
Chrysobalanus icaco
C. icaco is used around the world, highlighting its medicinal and food uses and is known as cocoplum, gbafilo, fat-pork, icaco, cacco, abajeru, grageru, zicaque, icacillo, and caramio, to name a few (Araujo-Filho et al., 2016; Prance, 1972; Davies & Zibokere, 2011). Its distribution is quite extensive, due to its wide plasticity to establish itself in various plant associations and soils with high concentrations of salt. It is mainly found indigenously in tropical areas, low forests, beachvegetation, and mangroves with sandy soils. It appears as a small tree that can measure from 1.5 to 7 m in height and approximately 5 to 20 cm wide; its leaves are alternate, simple, and leathery, with an orbicular shape, with a bright green color, in size, they present high variation. The fruit is a drupe, with a size of 2 to 5 cm long; the pulp is white and cottony, attached to the seed, has a soft texture, and is edible, presenting a sweet flavor; it exposes 1 or rarely 2 seeds with flat-convex cotyledons (Espinosa-Osornio, Vargas-Simón & Engleman, 2002). It is normally available from spring to summer, and both immature and ripe fruits can be found in the same bunch; the immature fruits have a green color, as they ripen, the color turns to pink, purple and white tones (Brown & Frank, 2018).
It is found in the United States of America, particularly in the area of Florida. In México, the southern states stand out: Chiapas, Yucatán, Oaxaca, Quintana Roo, Guerrero and Tabasco; however, it is also found in Tamaulipas. Central America and the Caribbean locate it in the Bahamas, Jamaica, Costa Rica, Panamá, and some Antilles islands. In South America, it is found in Venezuela, Colombia, Brazil, Ecuador, Suriname, Guyana, and French Guyana. It is also present in Africa, highlighting Senegal, Nigeria, Ghana, and Congo. In Asia, it is located in India, Vietnam, Singapore, and in Pacific islands such as Seychelles and Fiji (Davies & Zibokere, 2011; Prance, 1989; Prance & Sothers, 2003; Espinosa-Osornio, Vargas-Simón & Engleman, 2002; Vargas-Simón, Soto-Hernández, Rodríguez-González & Escalante-Estrada, 2000).
Ethnobotanic use of C. icaco
C. icaco is a low domestication fruit, and it is semi-cultivated mainly in home gardens as an ornamental plant, shade tree, or as living fences. In some localities, it is usually consumed fresh, in jams, or as a drink. In South America, its consumption has been popularized by preparing artisan sweets; however, its industrialization has yet to be exploited. In Colombia and Venezuela, it is cooked to prepare syrups (Prance, 1972). The seed is roasted and is consumed like peanuts; the seed has high oil content, which is why it has been used to make candles. It has great economic, nutritional, and medicinal value in Africa, as it is used to treat hypertension, fever, gastrointestinal diseases, and malaria. In Nigeria, a soup is prepared from the seed (Davies & Zibokere, 2011; Hernández, Martínez-Moreno & Castañón-Nájera, 2018; De Oliveira et al., 2013). The leaves, stems, and roots are used as infusions by natives of Arraial do Cabo, Ilha do Cardoso, Marudá, Pará State, Recife, Pernambuco, Rio de Janeiro, and other localities of Brazil. C. icaco is even sold in traditional markets for medicinal purposes, mainly to treat diabetes and high cholesterol. It is also used as a craft and a fishing bait (Coelho-Ferreira, 2009; de Albuquerque, Monteiro, Alves & Cavalcanti, 2007; Fonseca-Kruel, Rodrigues, Dunn & Prance, 2020).
Other uses given in Brazil are to treat chronic diarrhea, bleeding, abdominal pain, dysentery, and inflammations; for this, an infusion of the fruits, roots, stem, or leaves is prepared (Araújo-Filho et al., 2016; Alves et al., 2012). In El Salvador and Trinidad, infusions of fruits, leaves, roots, and stems are used to treat stomach ailments and bleeding (Castilho & Kaplan, 2011).
In México, its food, medicinal and ornamental uses stand out. It is consumed fresh, or injellies and jams, likewise, the oil of the seed is used for cooking other foods (Jiménez, Méndez, Lesher, Molina & Hernández, 2011). It is important to disseminate the medicinal properties of these fruits since research could be carried out to improve the quality of life of those who consume them and exploit the transformation industry, promoting greater income for the region.
Phytochemicals from C. icaco
Different secondary metabolites have been isolated and identified from fruits, leaves, roots, seeds, and stems of C. icaco (Table II). Most studies have focused on phenolic compounds mainly flavonoids such as anthocyanins and flavonols, one of the most abundant flavonoids is myricetin and its glycosylated forms, which are considered chemotaxonomic markers of C. icaco (Paracampo, Prance, Poppi & Da Silva, 2017) (Figure 1).
Table II Phytochemicals present in C. icaco.
| Part of C. icaco |
Chemical class |
Chemical constituents | References |
|---|---|---|---|
| Leaves | Flavonoids, phenolics acids |
Myricetin, Myricetrin 3-O-glucuronide, Myricetin 3-O-glucuronide, Myricetin 3-O-glucuronide, O-glycosylated myricetin-3-O- glucuronide, Myricetin 3-O-pentoside, Quercetin-pentosyl-hexoside, Myricetin-rhamnosyl-pentoside, Myricetin-rhamnosyl-pentoside- sulphate, Quercitrin, Quercetin, Myricitrin, Myricetin-3-O- rhamnoside, Myricetin 3-O-rutinoside, Quercetin 3-O-rhamnoside, Rutin, 7-O-methyl-kaempferol, Gallic acid, Ellagic acid, Sulfate- hydroxymethoxy-flavone. |
Bastos et al., 2017; Da Silva et al., 2013; Barbosa et al., 2006; White et al., 2016a; Ribeiro et al., 2020; Paracampo et al., 2017; Castilho & Kaplan, 2011; Venancio et al., 2016. |
| Triterpenes | Lupeol, Lupenone, Pomolic acid, Triterpene-dihexoside, Triterpene- rhamnosyl-hexoside (isomer I), Triterpene-hexosylpentoside, Triterpene-dipentoside (isomer I), Trihydroxy-cinnamoyl-Triterpene, Triterpene-pentosylhexoside (isomer I), Trihydroxy-oxotriterpene- dipentoside, Triterpene-rhamnosyl-glucuronide, Triterpene-aglycone. |
Vargas, Mendes, Azevedo, Pessoa & Uller, 2010; Fernandes et al., 2003; Castilho & Kaplan, 2011; Ribeiro et al., 2020. |
|
| Sterols | Stigmasterol, Campesterol, Sitosterol, Stigmast-4-en-3-one, Stigmast-5,23-dien-3b-ol, Stigmast-4,22-dienone. |
Castilho & Kaplan, 2011; Vargas et al., 2010. |
|
| Miscellaneous | Fenantrene-1,4-dione, 2-tridecen-1-ol, 4,8,12,16-tetramethyl- heptadecan-4-olide, 6,10,14-trimethyl-2-pentadecanone, 9,17-octadecadienal, Dotriacontane, Ethyl octadecanoate, Ethyl palmitate, Hentetracontanol, Hentriacontane, Heptacosane, Manoyl oxide, Nonacosane, Neofitadiene, Nerolidol E, Z, Octacosane, Squalene, Triacontanal, Triacontane. |
Vargas et al., 2010. | |
| Roots | Diterpenes | 15-oxo-ent-kaur-16-en-19-oic acid, 15-oxo-ent-kaur-16-en-19- oic acid methyl ester, 11b-hydroxy-15-oxo-ent-kaur-16-en-19-oic acid methyl ester, 3a,16b,17-trihydroxy-ent-kauran-2-one, 16a,17- dihydroxy-ent-kauran-3-one, 16b,17-dihydroxy-ent-kauran-3-one. |
Gustafson et al., 1991. |
| Fruits | Anthocyanins | Delphinidin-3-glucoside, Cyanidin 3-glucoside, Petunidin 3-glucoside, Peonidin 3-glucoside, Delphinidin 3-(6”-oxaloyl) arabinoside, Delphinidin 3-(6-acetoyl) galactoside, Petunidin 3-(6-acetoyl) galactoside, Petunidin 3-(6”-oxaloyl) arabinoside, Peonidin 3-(6-acetoyl) glucoside, Peonidin 3-(6-oxaloyl) arabinoside, Petunidin 3-(6′′-succinyl)rhamnoside, Petunidin 3-acetylglucoside, Petunidin 3-(6′′-acetoyl)galactoside, Quercetin 3-arabinoside, Apigenin-7-O-glucoside, Delphinidin 3-(6′′succinyl)rhamnoside, Peonidin 3-(6′′-succinyl) rhamnoside, Delphinidin 3-5 diglucoside, Cyanidin 3-arabinoside. |
Venancio et al., 2017; Venancio et al., 2016; de Brito et al., 2007; Vargas- Simón, Soto-Hernández, Rodríguez-González & Escalante-Estrada, 2000; Vargas-Simón et al., 2002. |
| Phenolic acid and flavonoids |
Ellagic acid derivative, Myricetin pentoside, Quercetin derivative. | Venancio et al., 2016. | |
| Carotenoids | All-trans-lutein, 9-cis-neoxanthin,All-trans-β-carotene, 9-cis- violaxanthin, All-trans-violaxanthin. |
Venancio et al., 2016. | |
| Seeds | Fatty acids | Palmitic acid, Stearic acid, Oleic acid, Linoleic acid, Arachidic acid, α-eleostearic acid, α-parinaric acid, α-licanic acid, Eleostearic acid, Parinaric acid, Licanic acid, 4-oxoparinaric acid, Myristic acid, Margaric acid, Linolenic acid, Eicosanoic acid, Trans-linoleic acid. |
Gunstone & Subbarao, 1967; Medeiros et al., 2017 |
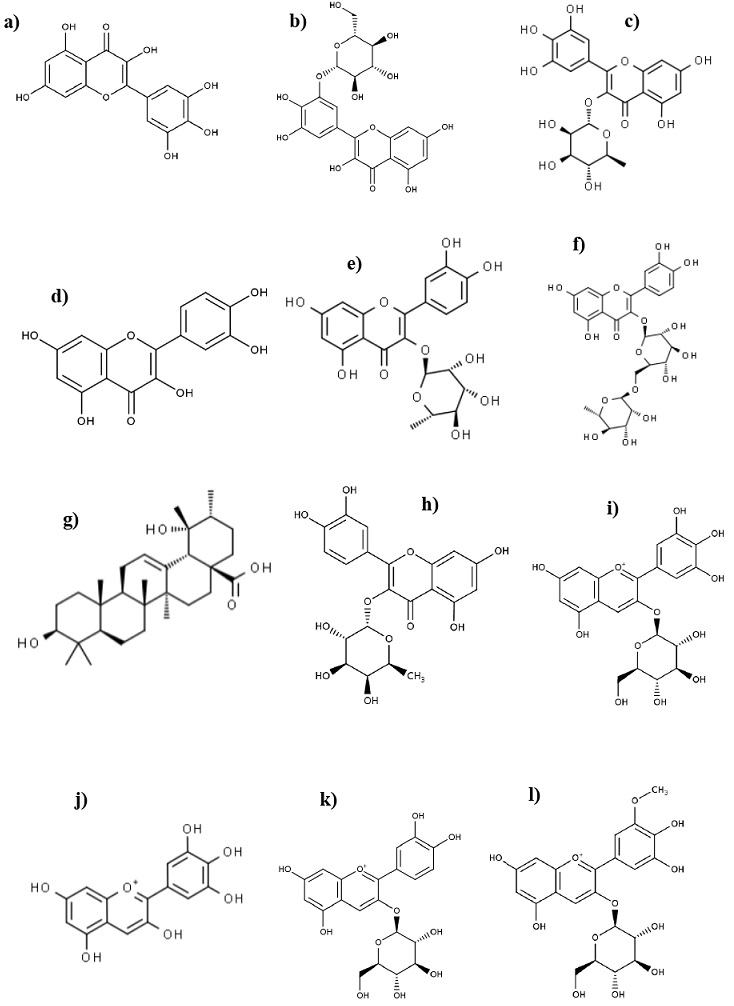
Figure 1 Main compounds isolated from C. icaco: a) Myricetin; b) Myricetin 3-0-glucuronide; c) Myricitrin; d) Quercetin; e) Quercitrin; f) Rutin; g) Pomolic acid; h) Quercetin 3-0-rhamnoside; i) Delphinidin-3-O-glucoside; j) Delphinidin; k) Cyanidin 3-glucoside; l) Petunidin-3-glucoside (Kim et al., 2021; Neveu et al., 2010).
Da Silva, Campos, Teixeira & Prado (2013), mentioned that C. icaco leaves have a content of total phenolic compounds of 51.30 ± 2.71 mg GAE/g and total flavonoids 6.64 ± 1.08 mg CE/g, gallic acid 0.45 mg/g, myricetin 0.78 mg/g, and quercetin 0.14 mg/g. Which stands out in comparison with some aromatic and medicinal plants commonly used in world gastronomy and ethnopharmacology (Skerget, Kotnik, Hadolin, Rizner, Simonic & Knez, 2005; Katalinic, Milos, Kulisic & Jukic, 2006). De Brito et al. (2007), mentions that the majority of anthocyanins in C. icaco fruits are derived from delphinidin, petunidin, and peonidin with a content of 100 mg/100 g DW, 367 mg/100 g DW, and 25 mg/100 g DW, respectively. Venancio et al. (2017), identified glycosylated and acylated anthocyanins from C. icaco fruits, highlighting the delphinidin 3-(6"-acetoyl) galactoside or delphinidin 3-(6"-oxaloyl) arabinoside (1,396 μg/mL), delphinidin-3-glucoside (1,162 μg/mL), peonidin 3-(6"-acetoyl) glucoside or peonidin 3-(6"-oxaloyl) arabinoside (689 μg/mL), petunidin 3-(6"-acetoyl) galactoside or petunidin3-(6"-oxaloyl) arabinoside (611 μg/mL), cyanidin 3-glucoside (382 μg/mL), and petunidin 3-glucoside, peonidin 3-glucoside (345 μg/mL).
Barbosa, Peres, Gallori & Vincieri (2006), identified in leaves of C. icaco, through HPLC/MS glycosylated flavonols, in particular derivatives of myricetin and rutin such as myricetin 3-O-glucoronide, myricitrin, myricetin 3-O-rutinoside, and quercitrin, these compounds have shown antioxidant, antiobesogenic, and hypoglycemic activity (White et al., 2016a). Castilho & Kaplan (2011), isolated 7-O-methylkaempferol of the leaves of C. icaco, which was active against S. aureus and S. pyogenes.
Biological activities of C. icaco
It has been observed that particularly flavonoids such as flavonols (myricetin) and anthocyanins, decrease biochemical parameters related to MetS, such as hypercholesterolemia, obesity, inflammation, and diabetes, this in clinical studies, in addition to delaying the pathogenesis of comorbidities such as Non-alcoholic fatty liver disease (NAFLD), and inhibit inflammatory cytokines, helping to reduce cardiovascular events, this due to the antioxidant activity of flavonoids, thanks to the hydroxyl groups present; therefore these compounds are of high bioactive importance for human health due to their safety and efficacy (Zhang, Xu, Zhang, Liu & Chen, 2021; Ginwala, Bhavsar, Chigbu, Jain & Khan, 2019).
According to some studies, different parts of C. icaco, like fruits and leaves, have been shown to exert important physiological activities for humans. Most studies have reported its hypoglycemic, hypocholesterolemic, and antiobesogenic characteristics, which are closely correlated in the manifestation of MetS. The seed also has flavonoids, which exert antioxidant and antibacterial activity (Villagra et al., 2020). Table III summarizes the observations obtained from in vitro and in vivo tests on the action of C. icaco extracts on these pathologies.
Table III Anti-diabetic, antiobesogenic and anti-inflammatory effects of C. icaco extracts.
| Part of C. icaco |
Extract and doses | Biological activity | Observation analyzed | References |
|---|---|---|---|---|
| Leaves | Aqueous/100 mg/kg | Anti-diabetic (In vivo) | A similar effect to metformin was demonstrated in rats with chronic diabetes. |
De Oliveira et al., 2013 |
| Aqueous/200 mg/kg | Anti-diabetic (In vivo) | Fasting blood glucose and insulin levels were reduced; weight gain and fat storage in the liver were inhibited. |
White et al., 2016b | |
| Aqueous/0.7 mg/mL | Effects in triglycerides serum concentrations (In vivo) |
Induced a significant reduction in rates of triglycerides in the blood of diabetic rats. |
Ferreira- Machado et al., 2014 | |
| Aqueous/200 mg/kg | Effects in lipogenesis and adiposity (In vivo) |
The extract improved glucose tolerance, decreased adipose mass gain, reduced leptin and gene expression of acetyl-CoA-carboxylase |
Portela-de-Sá et al., 2020 | |
| Aqueous/0.35 mg/mL | Effects in adiposity and glycemic homeostasis (In vivo) |
The extract decreased adipose tissue gain, triglyceride levels, and lipid excretion in feces, in addition to increasing locomotor activity and normalizing insulin sensitivity and glucose tolerance. |
White et al., 2016b | |
| Stem bark | Aqueous/200, 400 mg/kg | Anti-inflammatory (In vivo) |
Anti-inflammatory activity is similar to indomethacin. |
Bezerra et al., 2014 |
| Fruits | Hydromethanolic (anthocyanins) / 1.0 to 20.0 μg/mL gallic acid equivalents |
Anti-inflammatory (In vitro) |
Decreased TNF-α, IL-1β, IL-6, and NF- κB1 protein expressions. |
Venancio et al., 2017 |
| Lyophilized/400 mg/kg/day | Anti-inflammatory (In vivo) |
Decreased IL-1 β and Tnf-α. | Venancio, Almeida & Antunes, 2018 | |
| Aqueous /2 mL/kg | Anti-diabetic (In vivo) | The extract had a significant effect on normalizing blood glucose levels and decreased body mass. |
Nayak et al., 2011 |
Anti-diabetic activity and effects in lipogenesis and adiposity
The anti-diabetic potential of aqueous extracts of C. icaco leaves was investigated.A similar effect to metformin was demonstrated in rats with chronic diabetes. The administration of 100 mg/kg reduced blood glucose levels to 205.1 ± 12.8 mg/dL, similar to the administration of metformin at doses of 500 mg/kg, which obtained 201.4 ± 14.2 mg/dL. Compared with the latter treatment, a weight gain of only 14.8 ± 1.4% was observed, while with metformin, 51.9 ± 2.8%. This plant's anti-diabetic potential may be due to flavonoids, triterpenoids, steroids, and saponins since they have antioxidant activity, minimizing free radicals contributing to the generation of diabetes (De Oliveira et al., 2013).
In another study, the anti-diabetic activity and the inhibition of weight gain were analyzed by administering 200 mg/kg of aqueous extract of C. icaco leaves in mice fed a high-fat diet for 10 weeks. They observed that after 4 weeks of administration of the extract, the levels of insulin and glucose are taken in the fasting state decreased (132.3 ± 6.4 mg/dL), coupled with the inhibition of weight gain, gaining only 26.9 ± 1.3 g, while that in the group with a high-fat diet, without administration of the extract, 31.0 ± 2.2 g were obtained. Fat storage in the liver was decreased, despite the high-fat diet administered, obtaining a 72.60% lower lipid infiltration than the group administered with a high-fat diet without extract, which obtained 90.25%. It was observed in biochemical parameters that the group with a diet high in fat and extract obtained 178.3 mg/kg of total cholesterol, being even lower than the control group 236.8 mg/kg. Anti-diabetic activity is associated with the content of chemical compounds such as rutin, quercetin, and quercitrin (White et al., 2016b).
Aqueous extracts (2 mL/kg body weight, twice a day) of C. icaco fruits have shown hypoglycemic activity in rats with streptozotocin-induced diabetes. A normalization of fasting blood sugar is likely on day 18, with a 19% decrease. The fruit is rich in anthocyanins that have shown anti-diabetic effects through the regulation of glucose transporter-4 and prevention of insulin resistance and pancreatic apoptosis. The inhibitory action of the a-glucosidase was due to natural acylated anthocyanins. There was a decrease in body mass, attributing it to the regulatory effect of the adipocytokine gene, which improves the function of adipocytes, which are related to obesity and diabetes (Nayak et al., 2011).
The aqueous extract of C. icaco leaves exerts effects on adiposity and the action mechanism of acetyl-CoA-carboxylase (ACC) gene and protein expression, which plays a key role in lipogenesis. Male Wistar mice, fed a high-fat diet and 200 mg/kg/day of the extract for 4 weeks, were used for the experiment. It was concluded that the extract improved glucose tolerance, decreased fat mass gain, decreased leptin, and the expression of the ACC gene (p < 0.05), so the extract canbe used to treat obesity, thanks to the flavonoids it contains (Portela de Sá et al., 2020). In a different experiment, the activity of the aqueous extract of C. icaco leaves in adipocytes, and glycemic homeostasis was corroborated, 0.7 mg/ mL and 0.35 mg /mL were administered to groups of mice subjected to a high-lipid diet. The extract did not affect food intake or weight gain; however, it decreased adipose tissue gain, triglyceride levels, and excretion of lipids in feces, in addition to increasing locomotor activity and normalizing sensitivity insulin and glucose tolerance (White et al., 2016a).
Ferreira-Machado et al. (2014), report that the administration for 35 days of 0.7 mg/mL of aqueous extracts of C. icaco leaves shows a significant reduction in triglyceride levels was observed in diabetic rats, the values being the same as those of the control group (80 mg/dL).
Conclusions
Chrysobalanus icaco is a rich source of phytochemicals with potential against metabolic syndrome. The most commonly identified phytochemicals with potential against MetS in this species are anthocyanins, phenolic compounds, and flavonols such as myricetin and quercetin. In this review, we showed that C. icaco leaves and fruits have potential hypoglycemic, hypocholesterolemic, anti-inflammatory, and anti-obesogenic activity. Considering the promising results of C. icaco, we suggest further research in methods to yield higher phytochemical content, bioavailability, and toxicological studies. The sensory, nutritional, and bioactive characteristics of C. icaco make it an attractive fruit for consumers, so it can potentially be valued as a super-fruit. It is important to disseminate scientific information to promote its production and commercialization. In addition, its agronomic conditions indicate that it is an exploitable crop with adaptability in Mexican territory.











 nova página do texto(beta)
nova página do texto(beta)


