Introduction
Proteusmirabilis is a mobile dimorphic Gram-negative #1 bacterium member of the Enterobacteriaceae family (Coker, Poore, Li & Mobley, 2000). This microorganism produces urease and varies from a simple vegetative bacillus with few flagella (swimmer cell) to a lengthened cell with multiple flagella (swarmer cell) (Coker, Poore, Li & Mobley, 2000; O'Hara, Branner & Miller, 2000). It is frequently isolated from the gastrointestinal tracts of humans and animals (Hoeninger, 1964). P. mirabilis causes complicated urinary tract infections (UTI), mainly in patients having urinary catheters for prolonged periods or in those with structural or functional abnormalities of the urinary tract (Mobley & Belas, 1995). Some of the most frequent urinary pathologies caused by P. mirabilis are: acute pyelonephritis, chronic inflammation, periurethral abscesses, bacteremia, urolithiasis and prostatitis (Allison, Coleman, Jones & Hughes, 1992). UTI caused by P. mirabilis involves the expression of virulence factors, as adherence, motility and evasion of the host immune response, which promote bladder and kidney colonization during infection (Coker, Poore, Li & Mobley, 2000; Armbruster & Mobley, 2012).
In the last two decades, new virulence proteins known as autotransporters have been reported mainly in Gram-negative bacteria (Alamuri & Mobley, 2008). These proteins use the type V secretion system to export bacterial components from cytosol to the extracellular milieu (Henderson, Navarro-Garcia, Desvaux, Fernandez & Ala'Aldeen, 2004). Plasmid-encoded toxin (Pet) was first described in enteroaggregative E. coli 042 (EAEC) strain (Eslava et al., 1998). It is a secretory protein (104 kDa) that damages the intestinal mucosa causing: I) exfoliation of epithelial cells (enterocytes), II) increase of mucous production and III) abscess development in the cell crypts (Navarro-García et al., 1998). Pet mediated proteolysis breaks the scaffolding protein α-fodrin, inducing contraction of the cytoskeleton, loss of actin stress fibers and loss of the focal contact of cells, which leads to morphological changes like cell rounding and detachment (Canizalez-Roman & Navarro-García, 2003; Navarro-Garcia, Sonnested & Tetter, 2010; Capello et al, 2011). Pet in P. mirabilis had not been described until Gutierrez et al. (2012), showed its presence in a clinical isolate denominated as Proteus mirabilis RTX339. Pet was found in the culture supernatant and showed high homology with Pet of EAEC 042 (Gutiérrez-Lucas et al., 2012).
The morphological changes of intestinal cells due to Pet from EAEC have been widely studied in several cell lines and in the ligated loops of intestine model (Navarro-García et al., 1998; Navarro-García, Sears, Eslava, Cravioto & Nataro,1999; Navarro-García, Sonnested & Tetter, 2010; Canizalez-Roman & Navarro-García, 2003; Capello et al., 2011; Henderson et al., 1999; Villaseca et al. 2000; Betancourt-Sánchez & Navarro-García, 2009; Sainz et al., 2002; Sui, Dutta & Nataro, 2003; Rocha-Ramírez et al., 2016). However, there are not studies on the effect of Pet produced by P. mirabilis (Pet+) during UTI. Therefore, the aim of this study was to develop an UTI in a mouse model using the P. mirabilis (Pet+) strain and to analyze the cellular and morphological changes in relevant urinary organs.
Materials and Methods
Mouse strain
Female BALB/c mice, 6 weeks old weighing 17-20 g, were obtained from the Unidad de Experimentación y Producción de Animales de Laboratorio (UPEAL), at the Universidad Autónoma Metropolitana-Xochimilco. All animals were kept at 21-24°C in a 12h light/dark cycle. They were fed with Purina® and pathogen-free purified water ad libitum. Mice were handled in accordance with the international and Mexican standards for animal protection (DOF, 2002).
Bacterial strains
The P. mirabilis RTX339 strain was isolated from a patient and was characterized for secreting Pet (Gutiérrez-Lucas et al., 2012). P. mirabilis RTX267 and the EAEC 042 were also clinical isolates, the first was a non-secreting Pet isolate, and the last was an enteroagreggative E. coli isolate, characterized for secreting Pet (Nataro et al., 1995). All the strains were cultivated in Luria broth, at 37°C overnight.
Detection of pet by PCR analysis
DNA from P. mirabilis RTX339, EAEC 042 and P. mirabilis RTX267 strains was extracted using the wizard DNA purification kit (Promega) following the manufacturer's instructions. PCR was carried out using a PCR QIAGEN kit (QIAGEN). The PCR for pet detection was performed as previously described using the primers U250F (forward) 5'-TGACTCTGCATGGATTGAGC-3' and U250R (reverse) 5'-GACGCATCACTCAGTACAGT-3' (Gutiérrez et al., 2012).
Pet detection by Western blot assay
Bacterial secreted proteins were recovered from Luria broth supernatants, which were inoculated with each of the strains at 37°C overnight. Cell cultures were centrifuged at 9,000 χ g for 15 minutes. The supernatant was precipitated with ammonium sulfate at 60% of saturation and kept overnight at 4°C. Then, samples were centrifuged at 12000 x g for 45 minutes, re-suspended in PBS and dialyzed using a nitrocellulose membrane (Spectra/Por® molecular porous membrane tubing MWCO: 12,000-14,000). Finally, the dialyzed proteins were centrifuged at 24,600 χ g for 15 min and the supernatants stored at -20°C until use (Gutiérrez et al, 2012). A concentration of 100 mg from each of the protein samples were subjected to SDS-PAGE electrophoresis (12% gel) as described by Laemmli (1970). Protein bands were stained with Coomassie blue R-250 (BioRad). Proteins were transferred to PVDF membranes (Millipore) for Western blot analysis as described by Towbin et al. (1979) (Towbin, Staehelin & Gordon, 1979). One membrane was incubated with a rabbit anti-P mirabilis-Pet antibody using a 1:256 dilution, while the other was incubated with a rabbit anti-EAEC 042-Pet antibody according to Gutiérrrez et al. (2012). Latter, a goat anti-rabbit IgG coupled to horseradish peroxidase (HRP) (Thermo Scientific), was used at a 1:5,000 dilution. Reaction was detected with a DAB kit (Dako®).
Urinary tract infection
Five mice were employed in each group viz., P. mirabilis (Pet+), EAEC 042 (Pet+), P. mirabilis (Pet-) and a control group remained uninfected (inoculated only with PBS). Mice were anesthetized with ketamine [60mg/Kg] and xilazine [10mg/Kg], and transurethral inoculated with 50 μL of the corresponding bacterial suspension containing approximately 5X107 CFU (Reis et al., 2011; Hung, Dodson & Hultgren, 2009).
Bacterial CFU determination during infection
Urine and urinary organs were collected under sterile conditions at 2, 4, 7 and 10 days post-infection. The urinary organs were homogenized with 1mL of sterile PBS. Subsequently, 100 |iL of serial dilutions of organ-homogenates and urine were spread on MacConkey agar and incubated at 37 ° C for 24 hours. The colony count was recorded and the CFU/mL for the urine samples and CFU/g tissue for the urinary organs were determined. Graphs and statistical analyses (Student's t-test) were performed using the GraphPad Prism 6 software.
Histopathological evaluation of urinary tissues
Histological sections were prepared as follows: bladder and kidney of each experimental group were recovered in sterile conditions at day 7 post-infection; organs were fixed with 10% paraformaldehyde and embedded in paraffin. Serial sections of approximately 5 μηι were sliced and placed on glass slides. Preparations were stained with hematoxylin and eosin according to the procedures of the Biological Stain Commission manual (Clark, 1981). Cellular structures and morphological alterations were observed at 100X, 400X and 1000X magnifications in an optical microscope (Carl Zeiss).
Detection of Pet in urinary organs by confocal microscopy
Histological sections of each group were de-paraffinized and then permeabilized for 10 minutes with 0.2% triton X-100 at room temperature. Actin was stained with rhodamine-phalloidin (100 ng/slide) (Santa Cruz Biotechnology, USA.) for 30 minutes. Later, the slides were blocked with 4% albumin for 30 minutes. Pet was labeled with rabbit anti-Pet antibody diluted 1:100 and left overnight at 4°C, then stained with a fluorescein isothiocyanate (FITC) labeled goat anti-rabbit immunoglobulin G (IgG) (Santa Cruz Biotechnology) at 1:300 dilution for 2 hours at room temperature. Slides were mounted withvectashield® and observed in a LSM 510 Carl Zeiss confocal microscope at 63X magnifications and analyzed by LSM5 Pascal Zeiss software.
Results
Pet detection in P. mirabilis (Pet+)
The presence of Pet in P. mirabilisRTX339 was ascertained by genotypic and phenotypic approaches. The PCR results showed an amplicon of 250 pb as expected for P. mirabilis RTX339 (Pet+) and for EAEC 042, no amplification was detected for P. mirabilis RTX267 strain (Figure 1a). At the level of protein detection, the results showed the presence of a protein of approximately 104 kDa in both P. mirabilis RTX339 and EAEC 042 samples (Figure 1b), and P. mirabilis RTX267 did not expressed any protein of this molecular weight. Beside, Pet identity was confirmed by Western blot using rabbit anti-Pet antibodies raised with P. mirabilis RTX339 (Pet+) (Figure 1c) or with EAEC 042 (Figure 1d).
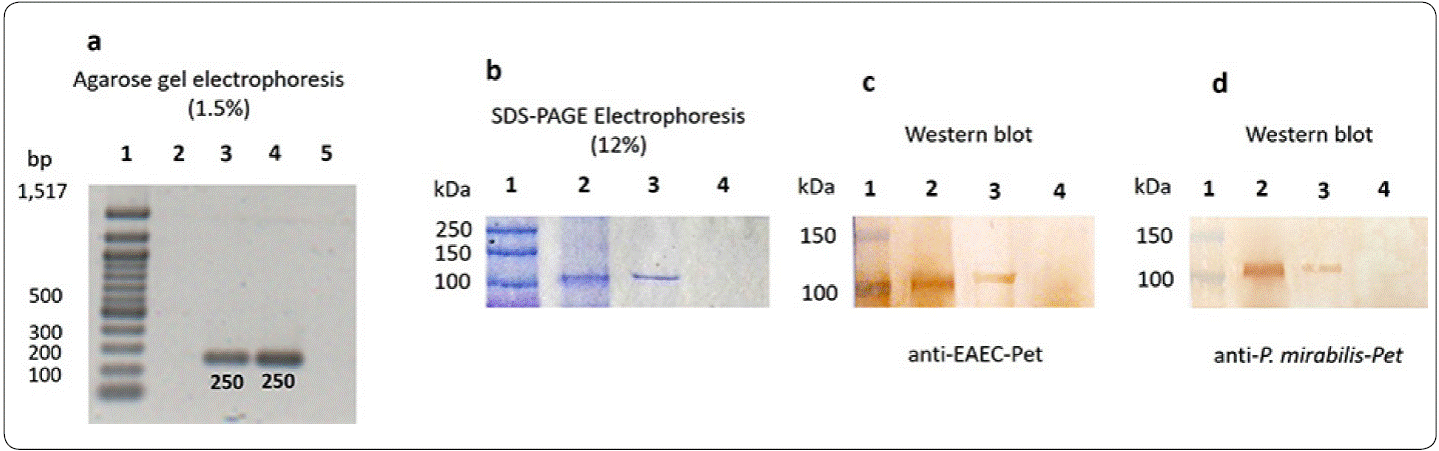
Figure 1 Pet detection in P mirabilis (Pet+). (a) DNA of each microorganism was analyzed by PCR using specific primers for Pet, the agarose electrophoresis presents a 250 pb amplicon for P. mirabilis RTX339 and EAEC 042. Line 1, pb-marker; line 2, negative control (water + all PCR components); line 3, P. mirabilis RTX339 DNA; line 4, EAEC 042 DNA; line 5, P. mirabilis RTX267 DNA. (b) SDS-PAGE-Coomassie blue stained shows a protein band of approximately 104 kDa in both P. mirabilis RTX339 (line 2) and EAEC 042 (line 3) samples; no band of 104 kDa was detected for P. mirabilis RTX267 (line 4). (c) Western Blot assay using anti-Pet antibody from EAEC 042; line 1, molecular weight marker (MWM); line 2, P. mirabilis RTX339; line 3, EAEC 042; lane 4, P. mirabilis RTX267 and (d) Western Blot assay using anti-Pet antibody from P. mirabilis RTX339.
Colony forming units (CFU) in urine and urinary organs during infection
Colony-forming unit counts in urine and urinary organs of mice infected with P. mirabilis RTX339, EAEC 042 and P. mirabilis RTX267 were carried out at 2, 4, 7 and 10 days post-infection, to determine urinary infection establishment and progress (Figure 2).
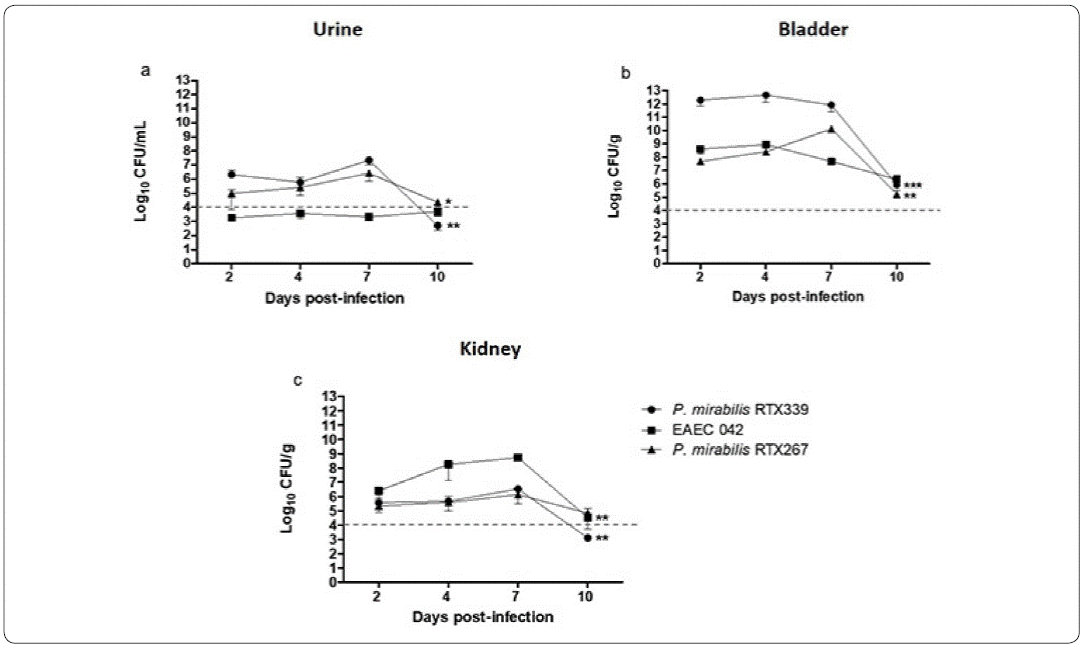
Figure 2 Colony forming units (CFU) in urine and urinary organs during infection. Colony-forming unit counts in urine (a), bladder (b) and kidney (c) of mice infected with P mirabilis RTX339, P. mirabilis RTX267 and EAEC 042. Evaluation at 2, 4, 7 and 10 days post-infection. p< 0.05: *p=0.01, **p=0.001, ***p=0.0001.
The three strains were able to establish a urinary infection after two days post-infection with CFUs higher than 104 /mL or /g per tissue, both in the urine samples and in the urinary organs, respectively. Only in the case of EAEC 042 urine samples (Figure 2a), the bacterial counts were lower than 104 CFU/ml, but in contrast, bacterial counts in bladder and kidney were above 104 CFU/ml, indicating an active bacterial infection (Figures 2b and 2c). In general, the highest bacterial counts were recovered from bladder; being particularly higher in P mirabilis RTX339 infection (Figure 2b). All three bacterial infections reached kidneys with EAEC 042 presenting the highest bacterial numbers (Figure 2c). By day 10 post-infection a significant decrease of the CFU in each of the samples was observed (Figure 2), but a complete bacterial elimination was not accomplished.
Histopathological evaluation of bladder and kidney of mice infected with different bacterial strains
Histological analysis of tissue samples from mouse urinary organs infected with each strain, showed loss of cellular integrity, exfoliation, necrosis and hemorrhage compared to urinary organs inoculated only with vehicle.
Healthy mouse bladders (Figure 3a), showed cellular structures without morphological alterations. The transitional epithelium (TE), ofthe mucosa comprising 4 to 6 cuboid cellular layers, was found broadly attached to each other. In contrast, the bladders of mice infected with P. mirabilis RTX339 (Pet +) showed the presence of exfoliated cells belonging to transitional epithelium that were rounded (ERC) and detached: in addition, elongated cells were also observed on this layer. In the lamina propria (LP), cellular and extracellular matrix damage was seen (Figure 3b). Bladders of mice infected with EAEC 042 presented similar morphological alterations to P. mirabilisRTX339 although with greater intensity (Figure 3c). The transitional epithelium of the bladders of mice infected with P. mirabilis RTX267 (Figure 3d) showed changes in the form of the cellular structures with ruffling shapes, but without cellular loss in the layer.
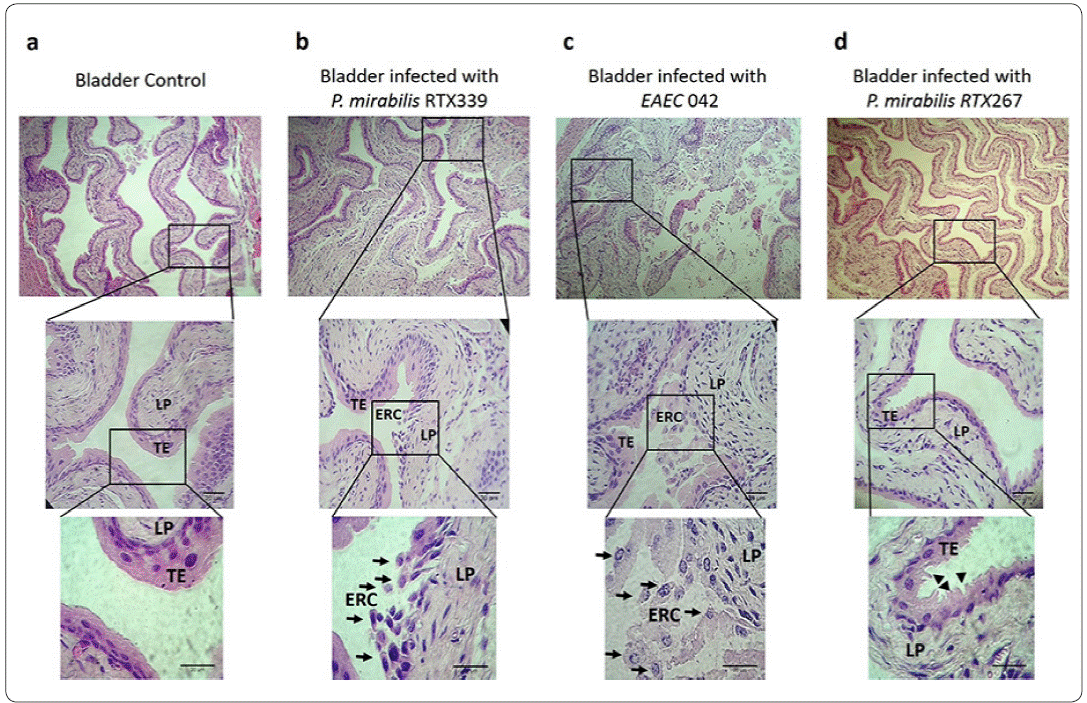
Figure 3 Histopathological evaluation of bladder tissues infected with different bacterial strains. a) Bladder tissue of mice inoculated with PBS. b) Bladder tissue of mice infected with P mirabilis RTX339 (Pet+). c) Bladder tissue of mice infected with EAEC 042 (Pet+). d) Bladder tissue of mice infected with P. mirabilis (Pet-). Histological sections were stained with hematoxylin and eosin. Arrows show the presence of exfoliated rounded cells from transitional epithelium. Arrowheads show the ruffling membrane of transitional epithelium. Bar= 20um. TE: Transitional epithelium, ERC: Exfoliated rounded cells and LP: Lamina propria.
Regarding to the analysis of kidney tissues of healthy mice, showed characteristic cellular structures like : I) glomerular cells surrounded by the Bowman's capsule and II) proximal tubules displaying uniform cuboidal cells structures firmly connected to each other (Figure 4a). In contrast, kidney tissues of mice infected withP mirabilis RTX339 (Pet+) (Figure 4b) and EAEC 042 (Pet+) (Figure 4c), showed similar histological alterations as: I) presence of exfoliated rounded cells around arteries, II) loss of proximal tubules close to glomeruli, III) loss of cuboidal cellular structures of tubules, IV) loss of crest structure and bigger size of the glomeruli and V) presences of erythrocytes between the tubules. However, kidney's tissue of mice infected with EAEC 042 presented more severe morphological changes than those infected with P. mirabilis RTX339 (Pet+). On the other hand, tissues of mice infected with P. mirabilis RTX267 (Pet-) presented: I) dilatation of proximal convolute tubules with loss of their epithelial lining, II) loss of crest structure and bigger size in glomeruli and III) absence of exfoliated cells around arteries (Figure 4d).
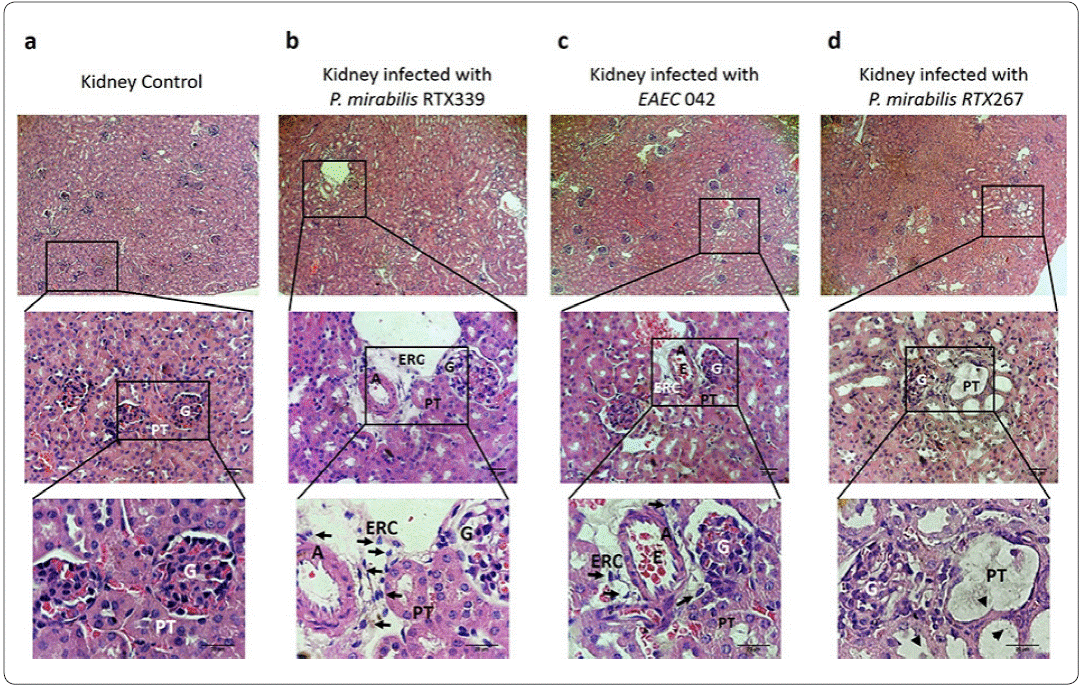
Figure 4 Histopathological evaluation of kidney tissues of mice infected with different bacterial strains. a) Kidney tissue of mice inoculated with PBS, b) kidney tissue of mice infected with P. mirabilis RTX339 (Pet+), c) kidney tissue of mice infected with EAEC 042 (Pet+), d) kidney tissue of mice infected with P. mirabilis RTX267 (Pet-). Histological sections were stained with hematoxylin and eosin. Arrows show the presence of exfoliated rounded cells around the arteries. Arrowheads show dilatation of the proximal convolute tubules. Bar= 20um. G: Glomerulus, PT: Proximal tubule, ERC: Exfoliated rounded cells, A: Artery and E: Erythrocytes.
In situ Pet detection in urinary infected tissues
An immunofluorescence assay was carried out in urinary tissues to detect in situ the presence of Pet during P. mirabilis (Pet+) infection (Figures 5 and 6). No exfoliated rounded cells, nor the presence of Pet signal were observed in bladder tissues of healthy mice (Figure 5a). The analysis of bladder tissues of mice infected with P. mirabilis (Pet+) (Figure 5b) and EAEC 042 (Pet+) (Figure 5c) showed the presence of Pet inside exfoliated rounded cells; at the same time, these rounded cells showed actin reorganization. No exfoliated rounded cells, nor the presence of Pet signal were observed in bladder tissues of mice infected with P. mirabilis (Pet-) (Figure 5d).
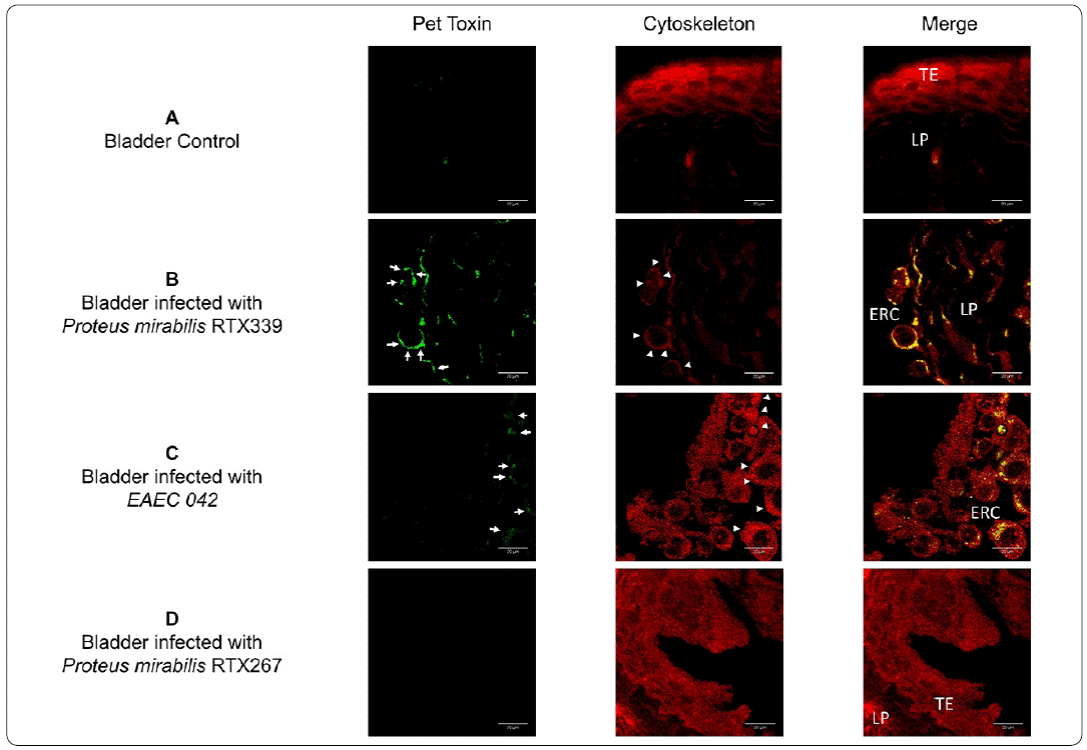
Figure 5 Detection of Pet in bladder tissue infected with different bacterial strains. a) Bladder tissue of mice inoculated with PBS, b) bladder tissue of mice infected with P. mirabilis RXT339 (Pet+), c) bladder tissue of mice infected with EAEC 042 (Pet+), d) bladder tissue of mice infected with P mirabilis RTX267 (Pet-). Arrows show the presence of Pet. Arrowheads show the presence of actin aggregates. Bar= 20um. TE: Transitional epithelium, ERC: Exfoliated rounded cells and LP: Lamina propria.
Pet was absent in the histological samples of healthy mice (Figure 6a). Regarding the analysis of kidney tissues, Pet was lesser extent in the P. mirabilisRTX339 infected kidney tissues (Figure 6b) and highly expressed in kidneys of mice infected with EAEC 042 (Figure 6c). In the EAEC 042 infected tissue Pet was observed in many structures like proximal tubules, arteries and glomeruli. In the glomeruli Pet was observed in the endothelial cells. All these structures presented beside Pet, morphological changes and cytoskeleton reorganization. Tissues from kidneys infected with P. mirabilis RTX339 had similar alterations but in lower extent. Pet was absent in samples of mice infected with P. mirabilis RTX267 (Pet-) (Figure 6d).
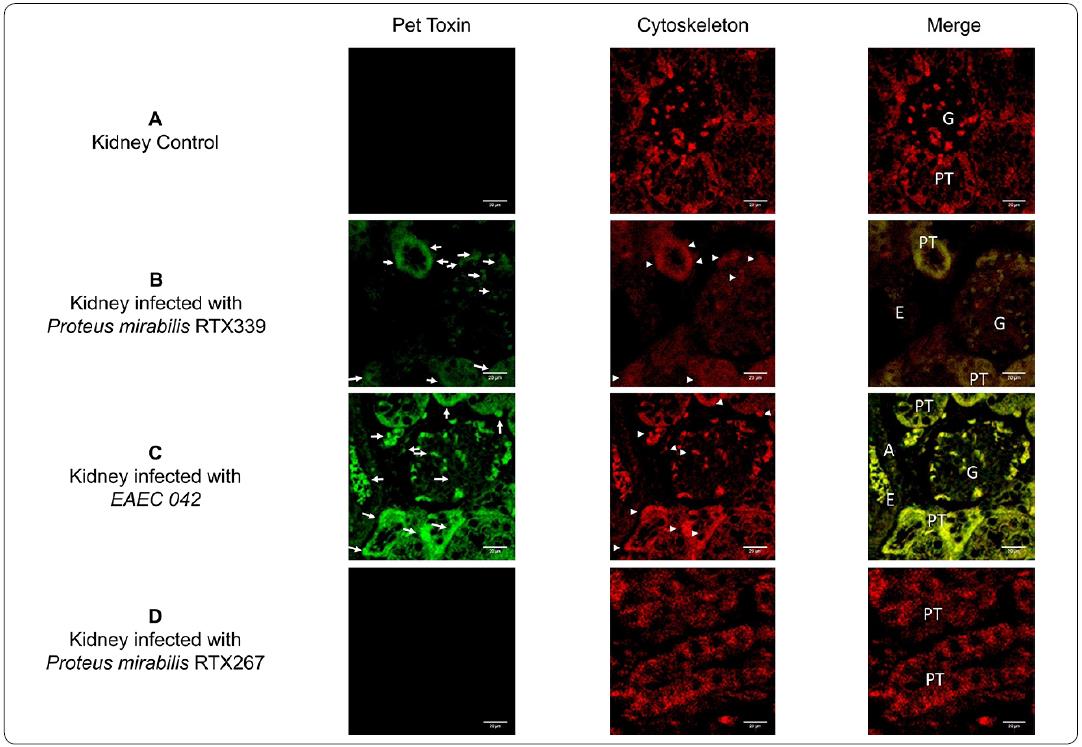
Figure 6 Detection of Pet in kidney tissue of mice infected with different bacterial strains. a) Kidney tissue of mice inoculated with PBS, b) kidney tissue of mice infected with P. mirabilis RTX339 (Pet+), c) kidney tissue of mice infected with EAEC 042 (Pet+), d) kidney tissue of mice infected with P mirabilis RTX267 (Pet-). Arrows show the presence of Pet. Arrowheads show the presence of actin aggregates. Bar= 20μιη. G: Glomerulus, PT: Proximal tubule, A: Artery and E: Erythrocytes.
Discussion
Urinary tract infection (UTI) caused by P. mirabilis is the result of a complex interaction between the pathogen and the host. During infection, the pathogen expresses numerous virulence factors, which appear to be essential for the development and during the course of infection (Coker, Poore, Li & Mobley, 2000). Pet is a member of autotransporter class of secreted proteins (Eslava et al., 1998), which belongs to the serine protease autotransporters of Enterobacteriaceae (SPATE), many SPATE have been characterized as important virulence factors for the Enterobacteriaceae family (Henderson, Navarro-Garcia, Desvaux, Fernandez & Ala'Aldeen, 2004). In this study, we describe the presence of Pet and the morphological changes in murine urinary tissues during the course of an infection with P. mirabilis (Pet+).
Pet was described and studied for the first time as a cytotoxic protein produced by EAEC 042 (Eslava et al., 1998). A recent study of an atypical enteropathogenic E. coli clinical isolate also showed the expression of Pet (Ruiz et al., 2014). In this study, we corroborate the presence of Pet in P. mirabilis RTX339 (Pet+) strain through genotypic and phenotypic assays, as previously demonstrated by Gutiérrez et al, (2012). Pet is not normally present in P. mirabilis, so, it is possible that pet was acquired by P. mirabilis RTX339 by horizontal gene transfer from an EAEC (Pet+) strain. Pet is encoded in the virulence plasmid (PAA) in EAEC and is a mobile unit that favors horizontal gene transfer processes. The process of how P mirabilis (Pet+) acquired pet and how it was incorporated into the bacterial chromosome, are issues that have to be elucidated.
To observe the characteristics of the host - P. mirabilis (Pet+) interaction, in this study we first developed an experimental murine UTI with P. mirabilis Pet+ clinical isolates. Both strains of P. mirabilis (Pet+ and Pet-) used in the study were isolated from human urine from several UTI occurring simultaneously in a hospital facility; one of them was reported to causes a severe UTI (Gutiérrez-Lucas et al., 2012). EAEC 042 strain in our study was employed because this strain is characterized for secreting Pet. Several reports have demonstrated that EAEC strains may develop UTI (Olesen et al., 2012; Herzog et al., 2014). Also, previous studies showed that in order to establish an UTI (bladder colonization) in murine models, over than 104 CFU are required (Hannan, Mysorekar, Hung, Isaacson-Schmid & Hultgren, 2010). In this study could establish a bladder colonization from the second day of infection with the three strains under study, with CFU loads higher than 104, in our model, by the tenth day post-infection a significant decrease of the CFU in urine, bladder or kidney each was observed, without a complete bacterial eradication (Figure 2).
Many in vitro an in vivo assays have been performed to understand the mechanism of action of Pet from EAEC 042. Pet induces cytotoxic and cytopathic effects on different cell lines, in rat ileal loop and in the intestine of mice infected with EAEC 042, modifying the morphological conformation of enterocytes from cuboidal to a rounded shape, this modification is due to the proteolysis of α-fodrin scaffolding protein of the cytoskeleton, resulting in reorganization of actin filaments (Navarro-García et al., 1998; Navarro-García, Sears, Eslava, Cravioto & Nataro, 1999; Navarro-García, Sonnested & Tetter, 2010); Canizalez-Roman & Navarro-García, 2003; Capello et al., 2011; Henderson et al., 1999; Villaseca et al., 2000; Betancourt-Sánchez & Navarro-García, 2009; Sainz et al., 2002; Sui, Dutta & Nataro, 2003 ; Rocha-Ramírez et al., 2016). Previous histological analysis of the ileal loop and the intestine of mice inoculated with purified Pet and EAEC 042 revealed the hypersecretion of mucus and the presence of abundant exfoliated rounded cells belonging to the intestinal mucosa (Navarro-García et al., 1998; Sainz et al., 2002; Rocha-Ramírez et al., 2016). In our study, the histological analysis of urinary tissues of mice infected with P. mirabilis (Pet+) and EAEC 042 (Pet+), showed the presence of exfoliated rounded cells, which are in accordance to those exfoliated rounded cells found in the intestinal infection by EAEC 042. Furthermore, we also observed greater degree of damage in urinary tissues of mice infected with EAEC 042 compared to urinary tissues of mice infected with P. mirabilis(Pet+). Furthermore, the presences of Pet in those sites with histological alterations, was confirmed by immunofluorescence (Figures 5 and 6). The presence of Pet also correlated to zones with actin reorganization. These results are also in agreement with morphological changes observed in cell lines treated with purified Pet or infected with EAEC 042 (Canizalez-Roman & Navarro-García, 2003; Capello et al., 2011; Navarro-García, Sears, Eslava, Cravioto & Nataro, 1999; Sui, Dutta & Nataro, 2003; Betancourt-Sánchez & Navarro-García, 2009). Furthermore, we observed a greater production of Pet in urinary tissues of mice infected with EAEC 042 in comparison with urinary tissues of mice infected with P. mirabilis (Pet+); these differences could be related to: I) that in case of EAEC 042 pet is harbor in a multicopy plasmid (Eslava et al., 1998), in comparison to P. mirabilis (Pet+), which harbors a single copy plasmid in the bacterial genome (Gutiérrez-Lucas et al., 2012); and II) differences in adhesion of the pathogen to the urinary epithelium, this feature is important for Pet delivery; in case of EAEC 042 the aggregative adhesion fimbria (AFF) and Pet are harbored in the plasmid PAA, which as was mentioned earlier is present as a multicopy plasmid in EAEC 042 (Eslava et al., 1998), AFF has been correlated with the adhesion to urinary cells in an in vitro model of UTI (Boll, Struve, Olesen, Stahlhut & Krogfelt, 2013).
Even though in the present study we could demonstrate morphological alterations and the presences of Pet in urinary tissues infected with P. mirabilis (Pet+), additional studies are needed to confirm the mechanism of action of Pet produced by P. mirabilis (Pet+) during urinary tract infection and how adherence factors as mannose resistant Proteus like fimbriae, mannose resistanKlebsiella fimbriae, Non agglutinating fimbriae also named urothelial cell adhesion, Proteus mirabilis fimbriae and ambient temperature fimbriae are correlated to delivery of Pet in host cells.
Conclusions
This study shows the first evidences of pathological alterations induced in an experimental UTI caused P. mirabilis (Pet+), suggesting that this toxin plays an important role in the pathogenesis of P. mirabilis (Pet+).
Conflict of interest: The authors declare that they have no conflict of interest.
Ethical approval: All applicable national, and/or institutional guidelines for the care and use of animal were followed.











 nueva página del texto (beta)
nueva página del texto (beta)


