Introduction
Microbialites are known to have contributed significantly to "reef and "reef-like structures in the past as well as in the present (e.g. Wood, 1999, 2001; Saint-Martin, Müller, Moissette & Dulai, 2000; Olivier et al., 2004; Nose, Schmid & Leinfelder, 2006; Westphal, Heindel, Brandano & Peckmann, 2010). Disregarding the fact whether they are able or not to form ecological reefs sensu stricto (Fagerstrom, 1987) by themselves, (e.g. Neuweiler, 1993; Webb & Kamber, 2000; Neuweiler & Bernoulli, 2005; Floquet, Neuweiler & Léonide, 2012; Heindel et al., 2012; Duda et al., 2016), their role in bulding "mud mound"-like structures has been recognised since the nineties of the last century and up to date (Reitner & Neuweiler, 1995; Dupraz & Strasser, 2002; Chan et al., 2014). They may reach important percentages (more than 50%) in ancient frameworks, particularly after extinction events; most notably in the Precambrian-Cambrian transition (Zhuravlev, 1996; Turner, Narbonne & James, 2000; Shapiro, 2004; Wahlman, Orchard & Buijs, 2013), the Permian-Triassic boundary (Delecat & Reitner, 2005; Ezaki, Liu, Nagano & Adachi, 2008; Kershaw, 2017); and the Cretaceous-Paleogene boundary (Zamagni, Kosir & Mutti, 2009; Tunis et al., 2011; Astibia, López-Martínez, Elorza & Vicens., 2012). They can also dwell successfully in deep and in cryptic environments, where the influence of light is minimum, or none at all (Keupp & Arp, 1990; Reitner, 1993; Reitner, Wilmsen & Neuweiler, 1995; Webb & Jell, 1997; Guido et al., 2017).
Microbialites have often been identified in the deposits of the famous Fossillagerstätte St. Cassian Formation. They are particularly abundant in the Cipit boulders in Seelandalpe (Alpe di Specie) and Misurina (Trentino-Alto Adigio, South Tyrol, NE Italy). These olistoliths come from the Cassian platforms, and have escaped late diagenetic processes due to their swift deposition in basin areas (Fürsich & Wendt, 1977; Wendt & Fürsich, 1980; Bosellini, 1991; Russo, Neri, Mastandrea & Laghi, 1991). Microbialites may account for more than 50% of any single olistolith (Russo, Neri, Mastandrea & Baracca, 1997).
Recently, Sánchez-Beristain & Reitner (2016, 2018) have described new fossil associations either dominated by metazoans or by microbialites (up to 60% of the framework), using statistical methods. However, they did not describe any association significantly dominated by traditional metazoan "reef builders in these works. Their first work in this regard (Sánchez-Beristain & Reitner, 2016) includes five "coralline" sponge-based associations, plus one dominated by calcareous algae and hexactinellid sponges. Their second work dealing with this topic (Sánchez-Beristain & Reitner, 2018) includes four further sponge associations with a vast diversity of microencrusters. The aim of the present work is thus to determine the palaeonvironmental-palaeocological setting of the "reef" sediments forming the olistoliths, in particular those that are mainly composed by, or have an important percentage of microbialite. The detailed microfacies analysis of thin sections has allowed us to recognize three new fossil associations in the St. Cassian Formation, where microbialite accounts ca. 75%, and up to 90% of total framework, as well as a new association based on a chaetetid sponges/microencrusters and microbialites. Statistical methods proved effective in differencing similar microbialite-based associations, mainly by considering the diversity of microencrusters, which help infer palaeoenvironmental constraints. These results may be of special interest, since most Cipit boulders from the main localities where the St. Cassian Formation outcrops -namely Alpe di Specie and Misurina- are mainly dominated by a vast array ofmetazoans (Wendt, 1982; Sánchez-Beristain & Reitner, 2012, 2016).
Geological setting
The St. Cassian Formation (upper Ladinian-lower Carnian) crops out in the Dolomites (Figure 1). First designated by Münster (1841) and cartographically characterised by von Hauer (1858), it is constituted by an alternation of shales, marls and calciturbidites (Wendt & Fürsich, 1980), with maximum thickness between400 and 500 m (Bizzarini & Braga, 1978). This formation consists of two members (Upper Member and Lower Member; Figure 2). Division is performed based on ammonite biozones. The Frankites regoledanus Zone corresponds to the Ladinian Lower Member deposits, whereas the Daxatina canadensis and Trachyceras aon Zones correspond to the Lower Member deposits of the Cordevolian. In turn, the Trachyceras aonoides Zone is correlated with the Upper Member, in the Julian (Mietto et al., 2012).

Figure 1 Geographic position in northeastern Italy of the two localities (grey squares) where olistolith samples have been collected: the Alpe di Specie (Seelandalpe) and Misurina. Modified from Nützel, Joachimski & López-Correa (2010) and Sánchez-Beristain & Reitner (2016).

Figure 2 Chronostratigraphic distribution of the upper Ladinian to lower Carnian lithostratigraphic units in the Dolomites, NE Italy, including the studied "Cipit boulders" olistoliths in the St. Cassian Formation (LCD = Lower Cassian Dolomite; UCD = Upper Cassian Dolomite; Cor = Cordevolian; Jul = Julian). Modified from Müller-Wille & Reitner (1993) and Sánchez-Beristain, López-Esquivel Kranksith, García-Barrera & Reitner (2013).
Both members have the same lithological composition and are partly lateral equivalents of the Cordevolian-Julian deposits of the so-called Cassian carbonate platforms (Russo, 2005), informally known as "Lower Cassian Dolomite" and "Upper Cassian Dolomite" (Figure 2). According to Bosellini (1991), this phenomenon indicates sequences of regression and transgression. The St. Cassian Formation overlays concordantly the Wengen Formation (Ladinian) and underlies the Dürrenstein Dolomite (Carnian).
The Upper Member includes the so-called "Cipit boulders", olistoliths which constitute one of the most notable features of the formation. These olistoliths are relics of the "Cassian patch reefs", which could be related to both the Upper and Lower Cassian Dolomites (Wendt, 1982). Their preservation state has allowed an accurate taxonomical determination of their fossils and other analyses, such as the characterisation of diagenetic pathways (Russo, Neri, Mastandrea & Laghi, 1991), the determination of palaeotemperatures (Stanley & Swart, 1995; Nützel, Joachimski & López-Correa, 2010), the discovery of original organic matter (Neuweiler & Reitner 1995; Sánchez-Beristain, Schäfer, Simon & Reitner, 2011), and even the study of biogeochemical and further geochemical signatures (Tosti et al., 2014). These olistoliths mainly crop out in the region of Trentino-Alto Adige (South Tyrol), Northeastern Italy (Figure 1), at the Seelandalpe ("Alpe di Specie"; 46°36' N, 12°12' E) near the town of Carbonin (Schluderbach) and in Misurina in the Rimbianco Valley (46°35' N, 12°15' E). The samples studied here come from these two areas and were collected by the authors of this work between the mid-eighties of the twentieth century and 2008.
The reader is referred to the works of Müller-Wille & Reitner (1993), Bosellini, Gianolla & Stefani (2003), and Sánchez-Beristain & Reitner (2012) for general and specific remarks on the stratigraphy and geology of the St. Cassian Formation.
Material and methods
Material
Thirty thin sections coming from 10 "Cipitboulders" (olistoliths) were analysed. Thin section dimensions range between 7.5 x 10 cm and 10 x 15 cm.
Microfacies and chemical analyses
We carried out microfacies analysis, which included the identification and quantification of the proportion of components in the olistoliths. Two different methods were used: visual area estimation (after Bacelle & Bosellini, 1965) and area counting (Flügel, 2010). Area counting was performed by processing each thin section with aid of an AGFA SnapScan 1236 Scanner. Thin sections were analysed microscopically, and simultaneously compared to the digitalized files on-screen. This was made in order to distinguish boundaries between different components, which allowed us to establish a graphical on-screen classification. To achieve this goal, software utilities like Metamorph (licensed to the University of Göttingen) and VistaMetrix (licensed to the first author) were used.
In order to distinguish aragonite and high-Mg calcite from ferrous calcite (frequently found in secondary cements in St. Cassian samples), all thin sections were stained with Alizarine red-S following the method by Dickson (1965), to differentiate ferrous calcite from high-Mg calcite, aragonite and dolomite (compare Sánchez-Beristain & Reitner, 2012), thus allowing us to better identify different facies types. In addition, since original organic matter can be recognised in microbialites (Neuweiler & Reitner, 1995), we performed epifluorescence microscopy at some thin sections to distinguishing true organic automicrite (Reitner, 1993) from inorganic micrite.
Classification of microbialites was performed after Sánchez-Beristain & Reitner (2016), who classified microbialites into types I, II, III and IV, according to their fabric (loose peloids, densely arranged peloids, leiolite and stromatolite, respectively). The scale designed by Dupraz & Strasser (2002) was followed to quantify the abundance of all microencruster species in every thin section.
The most representative thin sections were chosen to elaborate vector graphic maps with the Corel Draw X7 software.
Microscopical observation and chemical analyses were performed at the Department of Geobiology of the GZG (Centre of Geosciences, University of Göttingen).
Statistical Analysis
The use of statistics for determining fossil associations in palaeoecology has been previously proved successful (e.g., Sepkoski, 1974; Simpson, 2007; Sánchez-Beristain & Reitner, 2016, 2018). In the present work, after quantifying all facies components in the thin sections, their corresponding values were analysed by means of statistical methods. Cluster analysis in Q-Mode was applied to all thin sections in order to group them into fossil associations. Three algorithms were used: UPGMA (Sokal & Michener, 1958), WPGMA (Sokal & Michener, 1958), and Nearest Neighbour (Legendre & Legendre, 2012). Each algorithm was coupled with the Jaccard index (Jaccard, 1912). This combination renders a presence-absence matrix of all components (organisms, microbialite, allomicrite and cements; Sánchez-Beristain & Reitner, 2016, 2018). In addition, we paired the WPGMA Algorithm with the Bray Curtis dissimilarity index. According to Bray & Curtis (1957) and Legendre & Legendre (2012), this method takes into account the individual abundance of each component in all samples.
Cluster analyses were carried out by means of the MVSP Software (1985-2009 by Kovach Computing Systems, licensed to FSB). Subsequently, the method of Sánchez-Beristain & Reitner (2016, 2018) was followed, in order to obtain the corresponding phenograms. The reason to perform a vast array of statistical algorithms was to compare their results, since both WPGMA and UPGMA emphasize in the use -or lack of use- of the arithmetic mean of all values (Sokal & Michener, 1958), while Nearest Neighbour bases on grouping clusters in an agglomerative fashion, at each step combining two clusters that contain the closest pair of elements not yet belonging to the same cluster as each other (Legendre & Legendre, 2012). Jaccard and Bray Curtis index were selected on the basis that the former takes into account similarity, while the latter rather considers dissimilarity.
Results
Microfacies Analysis
No significant difference existed between visual area estimation and area counting. In addition, five thin sections were analysed by point counting using a 1 mm-wide grid, in order to corroborate the exactness of area counting and visual estimation (Flügel, 2010). Since we did not find any difference between the results obtained by this method and the previous ones, we proceeded to interpret our results based on area counting, which is a method that combines both precision and swiftness (Bacelle & Bosellini, 1965).
The following components were identified in thin sections: fossils, microbialite, allochthonous components (detritus/ allomicrite), microsparite, and cements (Tables I and II). These components have since long been identified for St. Cassian Formation samples from olistoliths, though in different percentages (e.g. Rech, 1998; Russo, 2005; Sánchez-Beristain & Reitner, 2016, 2018). Flügel (2010) included within the term Biomorpha all bioclasts with a high degree of preservation or articulation. Usually, microbialites are not included usually within biomorpha (Flügel, 2010). However, we decided to group them along with fossils into this category, with the aim of generating a robust palaeoecological scope in the determination of fossil reef associations, considering that both metazoans and microbialites contribute to the formation of "reef" and "reef-like structures (Fagerstrom, 1987). Proportions (%) of all components at each thin section were input in a Microsoft Excel file (Table I). The content for each component from the microfacies analysis of all samples can be accessed through Table I, whereas their corresponding code can be seen in Table II. Selected Corel Draw schemes (Figures 3, 4) depict the most distinctive thin section of each association.
Table I Data of all samples analysed. See text for further details.
| MIC | Microbialite |
| Dart | Dendronella articulata |
| Prc | Precorynella sp |
| Mes | Mesophyllum sp |
| Cass | Cassianothalamia zardinii |
| Epol | Eudea polymorpha |
| B-ch | Branched ceratoporellid chaetetid |
| Sph-C | Sphaerulitic chaetetid |
| Uv | Uvanella-like thalamides |
| Teth | Tehtysocarnia cautica |
| Plv | Planiinvoluta sp |
| Mcom | Microtubus communis |
| Apfr | Alpinophragmium perforatum |
| Ter | Terebella cf T. lapilloides |
| Tub | Tubiphytes cf. T. obscurus |
| Pseu | Pseudorothpletzella- like crusts |
| Ks | Koskinobullina socialis |
| Alloc | Allochthonous components |
| UDTD | Undetermined framework builders |
| MESP | Microsparite |
| CEM | Cements |
Table II Key to all variables (cases) displayed in Table I columns. See text for further details.
| ASSOCIATION | Clustering Code | Dominating biofacies | Palaeoecological remarks |
| Microbialite - microencruster Association | M-m | Microbialite framework with diverse microencrusters. Metazoans insignificant. Algae rare. | Deep, low energy "mud mound" setting / Cryptic, shallow environment with low-energy levels / mid- to deep ramp low-energy microbialite- reef, oxygen-poor setting. |
| Dual-type Microbialite Association | 2t-M | Microbialite with uniform peloid size. Metazoans rare ("chaetetid" sponges). Very seldom microencrusters. No algae. Stromatactis with several generations of cements. | Two scenarios: a) Cryptic-deep setting with a sponge-based framework and subsequent colonization by stromatolites; or 2) Initially cryptic-deep sponge-based framework with further basinward falling and recolonization by stromatolites |
| Chaetetid - microencruster Association | C-m | Metazoan framework with branched chaetetids and subrogate corals. Microencrusters abundant and diverse. Microbialite constituting also a major framework building fraction | Quiet, semi-cryptic/deep marine setting, probably below 50 m, similar to modern Caribbean cryptic enviroments. |
| Microbialite - Terebella Association | M-T | Framework rich in microbialite. No metazoans. Some algal patches. Deep-water microencrusters. Stromatactis with several generations of cements. | Deep "mud mound", oxygen-poor setting. |

Figure 3 Microfacies maps of samples a) MI - 6 and b) MX - 5, containing the Microbialite-microencruster (M-m) association, and the Dual-type Microbialite (2tM) Association, respectively. Scale bar in a = 2.5 cm. Scale bar in b = 2 cm.
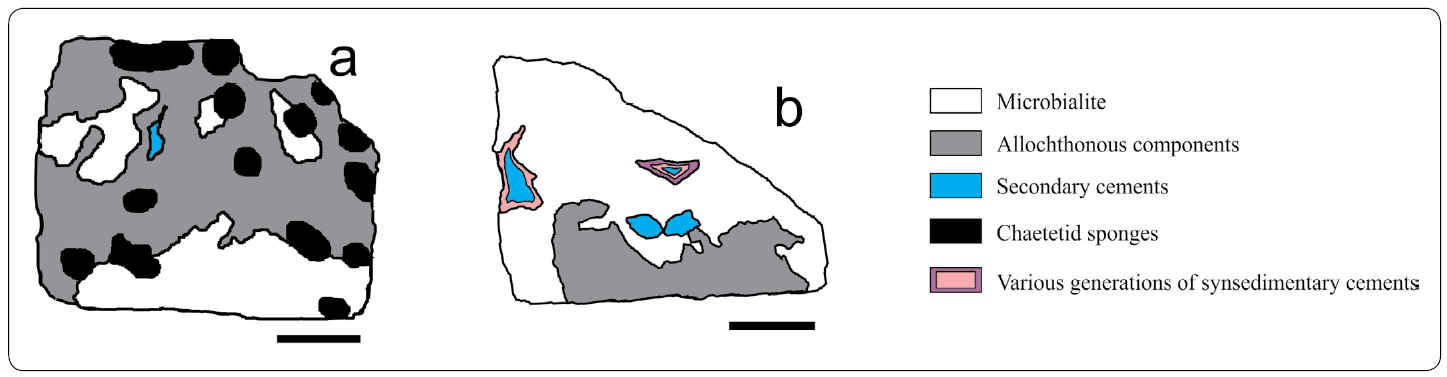
Figure 4 Microfacies maps of samples a) FSSA XXV - 3L, and b) M XL - 3, containing the Chaetetid-microencruster (C-m) Association, and the Microbialite-Terebella (M-T) Association, respectively. Scale bar in a = 2.5 cm. Scale bar in b = 2 cm.
All associations were named after their most representative attributes and are summarized in Table III.
Table III Summary of all associations described in this manuscript, with the most important remarks and constraints on their palaeoecology. See text for further details.
| Sample | Sample code | MIC | Dart | Prc | Mes | Cass | Epol | B-ch | Sph-C | Uv | Teth | Plv | Mcom | Apfr | Ter | Tub | Pseu | Ks | Alloc | UDTD | MESP | CEM | Total |
| MI - 6 | M-m 1 | 80 | 5 | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0.033 | 0.033 | 0.033 | 0.033 | 0.033 | 0 | 0.033 | 6 | 0 | 0 | 1 | 100.198 |
| MI - 10/1 | M-m 2 | 66 | 9 | 5 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0.033 | 0.033 | 0 | 0.033 | 0 | 0.033 | 14 | 1 | 0 | 1 | 98.132 |
| JR III - 106 | M-m 3 | 70 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.033 | 0.033 | 0.033 | 0.033 | 0 | 0 | 11 | 4 | 0 | 0 | 94.132 |
| JR III - 32 | M-m 4 | 72 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.033 | 0.033 | 0 | 0.033 | 0.033 | 0 | 0.033 | 13 | 3 | 0 | 0 | 95.165 |
| MI - 7 | M-m 5 | 70 | 10 | 1 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0.033 | 0 | 0.033 | 0.033 | 0 | 0 | 5 | 5 | 0 | 1 | 97.099 |
| MI - 9 | M-m 6 | 54 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.033 | 0 | 0.033 | 0 | 0 | 0.033 | 0.033 | 0 | 0.033 | 12 | 29 | 0 | 3 | 98.165 |
| M X - 5 | 2tM 1 | 85 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 5 | 94 |
| M X - 8 | 2tM 2 | 76 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 2 | 0 | 10 | 93 |
| M X - 2L | 2tM 3 | 74 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 1 | 0 | 5 | 86 |
| M X - 2R | 2tM 4 | 74 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 8 | 87 |
| MX-5 | 2tM 5 | 69 | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 15 | 100 |
| FSSA XXV 1 | C-m 1 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 42 | 0.03 | 0.033 | 0 | 0 | 0.033 | 0.033 | 0 | 0.066 | 0.066 | 29 | 1 | 0 | 0 | 96.261 |
| FSSA XXV - 7 | C-m 2 | 26 | 0 | 0 | 0 | 0 | 0 | 0 | 29 | 0.03 | 0.033 | 0 | 0 | 0 | 0.033 | 0 | 0.066 | 0.066 | 39 | 1 | 0 | 0 | 95.228 |
| FSSA XXV - 3L | C-m 3 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 37 | 0.03 | 0.033 | 0 | 0 | 0 | 0.033 | 0 | 0.066 | 0.066 | 30 | 1 | 0 | 0 | 98.228 |
| FSSA XXV - 2L | C-m 4 | 19 | 0 | 0 | 0 | 0 | 0 | 0 | 18 | 0 | 0.033 | 0 | 0 | 0 | 0.033 | 0 | 0.066 | 0.066 | 57 | 4 | 0 | 0 | 98.198 |
| FSSA XXV -5L | C-m 5 | 35 | 0 | 0 | 0 | 0 | 0 | 0 | 29 | 0 | 0.033 | 0 | 0 | 0.033 | 0 | 0 | 0.1 | 0.066 | 26 | 0 | 0 | 0 | 90.232 |
| FSSA XXV - 6R | C-m 6 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 41 | 0 | 0.033 | 0 | 0 | 0 | 0.033 | 0 | 0.066 | 0.066 | 15 | 8 | 0 | 0 | 97.198 |
| FSSA XXV 6L | C-m 7 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 36 | 0 | 0.033 | 0 | 0 | 0.033 | 0.033 | 0 | 0.033 | 0.066 | 30 | 4 | 0 | 0 | 92.198 |
| FSSA XXV -8 | C-m 8 | 35 | 0 | 0 | 0 | 0 | 0 | 0 | 34 | 0 | 0.066 | 0 | 0 | 0 | 0.033 | 0 | 0.066 | 0.066 | 22 | 2 | 0 | 0 | 93.231 |
| FSSA XXV - 2R | C-m 9 | 37 | 0 | 0 | 0 | 0 | 0 | 0 | 30 | 0 | 0.066 | 0 | 0 | 0 | 0 | 0 | 0.066 | 0.066 | 30 | 0 | 0 | 0 | 97.198 |
| JR Cas 27 | C-m 10 | 26 | 0 | 0 | 0 | 0 | 0 | 0 | 35 | 0 | 0.066 | 0 | 0 | 0 | 0.033 | 0 | 0.066 | 0.033 | 27 | 6 | 0 | 0 | 94.198 |
| M XL - 1 | M-T 1 | 84 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 2 | 3 | 1 | 11 | 101.01 |
| M XL - 2 | M-T 2 | 90 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 1 | 0 | 0.33 | 9 | 100.34 |
| M XL - 3 | M-T 3 | 70 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 1 | 2 | 1 | 23 | 97.01 |
| M XL - 4 | M-T 4 | 72 | 0 | 0 | 2 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 1 | 1 | 0.66 | 19 | 99.67 |
| M XVII - 5R | M-T 5 | 90 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 1 | 0 | 1 | 6 | 100.01 |
| M XXI - 1 | M-T 6 | 92 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.066 | 0 | 0 | 0 | 0 | 0 | 0.66 | 5 | 97.726 |
| M XXI - 2 | M-T 7 | 89 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0.66 | 5 | 97.67 |
| M XXI - 3 | M-T 8 | 80 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.03 | 0 | 0 | 0 | 0 | 10 | 1.66 | 7 | 98.69 |
| M XXI - 4 | M-T 9 | 76 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.03 | 0 | 0 | 0 | 0 | 13 | 1 | 4 | 94.03 |
Statistical Analysis
By means of both UPGMA-Jaccard and WPGMA-Jaccard analyses (UPGMA-J and WPGMA-J on the following), four different associations were found using data from Table I. These associations are: "Chatetetid" sponge-microencruster Association (C-m), Microbialite-microencruster Association (T-M), Dual-type Microbialite Association (2tM), and Microbialite-Terebella Association (M-T). Associations referred were easily identifiable by their relatively medium to high J coefficient values. In UPGMA-J (Figure. 5), J values are 0.48 for M-m; 0.557 for 2tM; 0.577 for M-T and 0.684 for C-m. In the WPGMA-Algorithm (Figure 6), J values are 0.467 for M-m; 0.557 for M-T; 0.577 for 2tM, and 0.684 for C-m. Nearest Neighbour-J (NN-J) and WPGMA-Bray Curtis analyses rendered different results (Figures. 7, 8). In the case of the NN-J method (Figure 7), both the C-m and T-M phena remained robust (J = 0.77 and J = 0.556, respectively); however, all 2tM and T-M samples are grouped within a single phenon with a J = 0.6, which furthermore has three subordinate nodes at J= 0.717, J = 0.75 and J = 0.8 (Figure 7). In the case of the WPGMA-Bray Curtis analysis, the only phenon which remains intact, is C-m (Figure 8), with a BC value of 0.355. At BC = 0.345, a gross phenon groups the three remaining associations obtained by means of both UPGMA-J and WPGMA-J. A single branch containing sample M-m 6 emerges from here. A node can be found at BC = 0.23, which encompasses two further phena: one encompassing all samples from both 2tM and T-M, and a robust phenon, which groups the remaining samples from the M-m association (M-m 1 to M-m 5).

Figure 5 UPGMA-Jaccard phenogram. Note the arrangement of the samples into strong associations for the Chaetetid-microencruster (C-m) Association (J = 0.684). Two phena, with J = 0.557 and 0.577 contain the Dual-type Microbialite (2tM) and Microbialite-Terebella (M-T) associations, respectively. The Microbialite-microencruster (M-m) Association is grouped with a not so robust phenon, at J = 0.48. See text for further details.

Figure 6 WPGMA-Jaccard phenogram. Note the arrangement of the samples into a strong association for the Chaetetid-microencruster (C-m) Association (J = 0.684). J values for Dual-type Microbialite (2tM) Association and Microbialite-Terebella (M-T) Association are inverted in comparison with the UPGMA-Jaccard phenogram (Fig. 5). M-m value is similar to Figure 5. See text for further details.

Figure 7 Nearest-Neighbour-Jaccard phenogram. Phenogram is slightly different to UPGMA-J and WPGMA-J clustering. Both Chaetetid-microencruster (C-m) and Microbialite-microencruster (M-m) phena are robust (J = 0.77 and J = 0.556, respectively). Dual-type Microbialite (2tM) and Microbialite-Terebella (T-M) samples are grouped within a single phenon with a J = 0.6. Note the three subordinate nodes at J= 0.717, J = 0.75 and J = 0.8. See text for further details.

Figure 8 WPGMA-Bray Curtis phenogram. This phenogram is relatively different to the previous ones (Figs. 5-7). Chaetetid-microencruster (C-m) Association has a strong BC value (0.355). Nonetheless, a robust phenon arising at BC = 0.345 groups the three remaining associations obtained by means of both UPGMA-J and WPGMA-J, perhaps due to its lower content of microbialite. See text for further details.
Considering that two of the procedures performed practically led to the same results (UPGMA-J and WPGMA-J), and that these two analyses show a classification based rather on the diversity of features (taxa) than in the amount of microbialite, we therefore decided to consider the associations obtained from them for the discussion and interpretation. These two types of phenograms have been successfully used in previous studies (Sánchez-Beristain & Reitner, 2016, 2018).
Description of fossil associations
Microbialite-microencruster Association (M-m; Figures. 3a, 9; 10)
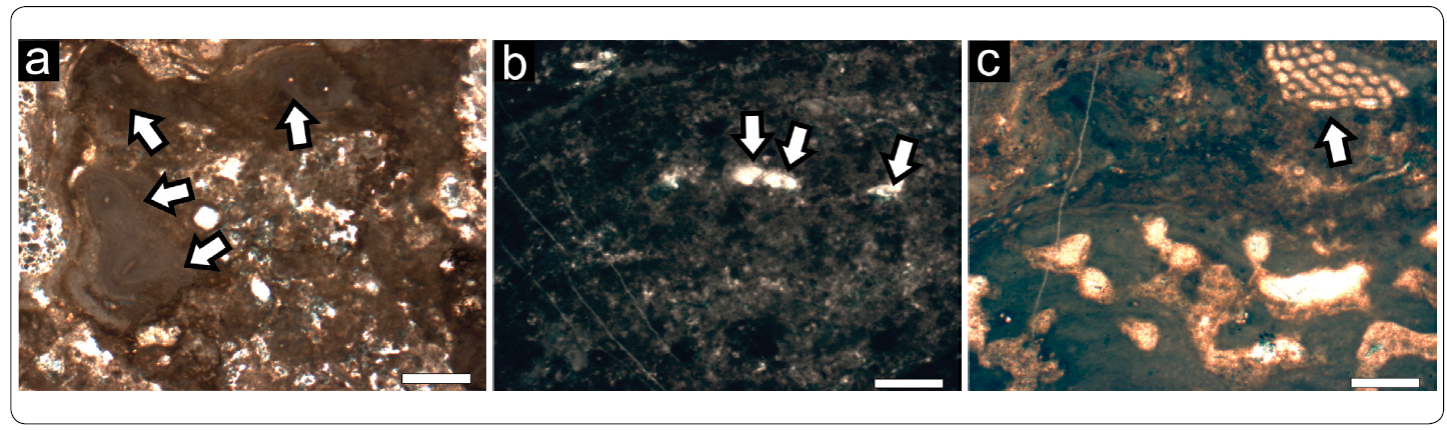
Figure 9 Microbialite - microenruster Association . a) Numerous "chimneys" of Tubiphytes cf. T. obscurus (arrows). b) Planiinvoluta sp. (arrows) in peloidal Type II matrix. c) Alpinophragmiumperforatum encrustation (arrow) on automicritic substrate. Scale bar = 500 mm.

Figure 10 Microbialite - microenruster Association. a) Microtubus communis (arrows) associated with Type III leiolites. Scale bar = 500 mm. b) Pyrite framboids (arrows), which can be found abundantly within microbialites. Scale bar = 100 mm. c) Botryoid-like cement (arrow), possibly of aragonitic composition. Scale bar = 500 mm.
Biomorpha
Dominant component is microbialite, averaging 71%. Type II (densely clotted peloids) is the most frequent. Peloids range from 20 to 100 mm in size (average = 50 mm). Occasionally Tubiphytes and Planiinvoluta-like foraminifera (Figures. 9a, 9b), sparse nubeculariids and Terebella cf. T. lapilloides can be seen. Microbialite Type IV (stromatolite) rarely appears. While Alpinophragmium (Figure 9c) and Koskinobullina socialis (compare Sánchez-Beristain, López-Esquivel Kranksith, García-Barrera & Reitner, 2013) encrustations can only be seen associated with microbialite Type II, Type III (leiolitic fabrics) appear only associated with frequent Microtubus borings (Figure 10a). Pyrite framboids, between 20 and 150 mm in size, are very common (Figure 10b).
Apart from the mentioned microproblematic fossils and foraminifera, fossils are rare. They comprise only 15% of the analysed thin sections and are represented only by the coralline sponge Precorynella as well as by few fragments of the alga Dendronella articulata. It is, however, not clear if these fragments grew in situ or rather were allochthonously incorporated in the framework, since they appear relatively infrequently.
Associated components
Microbialites present fibrous and botryoid-like cements, presumably of aragonitic origin, which could be primary vug fillings (Figure 10c). However, the most frequent type is blocky spar cement. Nevertheless, content ofcements is altogether < 1%.
Allochthonous material consists almost entirely of micrite, peloids and peloidal aggregates. In general, it proved difficult to distinguish allochthonous detritus from microbialite. However, epifluorescence analyses, which were conducted to corroborate the presence of organics, were negative in allochthonous material. Altogether, this component type accounted for no more than 13%.
Dual-type Microbialite Association (2tM; Figures 3b, 11)

Figure 11 Dual-type Microbialite Association. a) Branch of a ceratoporellid chaetetid (upper border, center - right), one of the only sponges found as a part of this association. Scale bar = 5 mm. b) Type IV stromatolitic facies located in a different growth direction (white arrow) in comparison to a ceratoporellid sponge (black arrow) and the overlying thrombolitic crust (grey arrow). t. Scale bar = 5 mm. c) Cement succession in a stromatactis cavity. Note the four generations, which include a synsedimentary cement (black arrow) and three secondary cements (grey arrows). Scale bar = 3 mm.
Biomorpha
Skeletal fossils can only be assessed by seldom ceratoporellid chaetetids, and account for no more than 5% (Figure 11a). Microencrusters are almost absent, whereas algae lack completely. The predominant component of this association is microbialite (in average 75%), most of which is assessed to Type II, yet Type IV can also be observed. Type II can be found growing as crusts on top of chaetetid sponges and consisting of peloids with a size of ~ 30 mm, whereas Type IV has frequently associated stromatactis-type cavities and very uniform, bigger peloids, mostly ranging from 50 to 70 mm in diameter. Notable however is that stromatolite-like microbial crusts may be located growing with different directions in comparison to the chaetetids and the encrusting thrombolites (Figure 11b).
Associated components
Allochthonous sediments, which conform mudstone-wackestone fabrics, include peloids and constitute less than 5%, so they do not play an important role in the composition of this association. These sediments are found in primary vugs within the thrombolitic fabric, yet they can occasionally also be found in secondary (boring) cavities within these microbialites. Sometimes sediments include some Tubiphytes cf. T. obscurus and gastropod fragments.
Cements can mostly be found in stromatactis cavities. These include bladed calcite crystals, which are interpreted as primary based on a pink-red staining, and up to several generations of sparry calcite (Figure 11c). Overall, cements account for up to 15% of the total estimated volume of the boulders of this association, thus being one of the samples with a higher cement fraction. Other types are elsewise sparry granular calcites, which fill in stromatactis as the last generation of cements.
Chaetetid-microencruster Association (C-m; Figures 4a, 12, 13)

Figure 12 Chaetetid - microencruster (C-m) Association. a) Cross section of a branch of a chaetetid sponge. Note encrustations containing Tethysocarnia cantica (arrows) in Type III Microbialite. Scale bar = 1 mm. b) A group of microencrusters (above, center) on the surface of a chaetetid sponge. Scale bar = 1 mm. Note Terebella cf. T. lapilloides (arrow) encrusted in thrombolite. c) Enlargement of b), displaying Koskinobullina socialis (arrow) and Tethysocarnia cantica (right) 500 mm. d) Pseudorothpletzella - like crust associated to Type II microbialite. Scale bar = 200 mm.

Figure 13 Chaetetid - microencruster (C-m) Association. a) Alpinophragmium perforatum (center) and Terebella cf. T. lapilloides (arrows). Scale bar = 1 mm. b) Terebella cf. T. lapilloides tubes (arrows) immerse in Type II microbialite. Scale bar = 500 mm. c) Tethysocarnia cautica (arrows) encrusted in Type III microbialite Scale bar = 500 mm.
Biomorpha
The most noteworthy component of this association is represented by chaetetids with a sphaerulitic skeletal microstructure. They perform a baffling function (Figures 12a-b). It is also noticeable that almost no other metazoan can be found in thin sections containing this association, considering that boulders studied in this work -and especially those which contain the C-m association- reached up to 40 cm in diameter. Anyhow, these chaetetid colonies comprise up to 40% of area counting measuring.
Most chaetetids are covered by Type II to Type III microbialitic crusts. Altogether, however, microbialite does not reach more than 34% of area countings. Microencrusters in this association do not differ much from other associations, and include Koskinobulina socialis (Figure 12c), Pseudorothpletzella (Figure 12d), Alpinophragmium perforatum (Figure 13a), Terebella cf. T. lapilloides (Figures 13a, b) and Tethysocarnia cautica (Figure 13c). Yet here a pattern of competence for substrate is somehow clear. While on the surface of some branches only a Pseudorothpletzella-K. socialis association can be found, on others only individuals of Tethysocarnia can be seen. In addition, microbialitic fabric surrounding these tend to have a Type III fabric, whereas microbialites associated to K. socialis and Pseudorothpletzella are rather of Type II.
Associated components
Allochthonous micrite and associated detritus can be found within primary vugs formed by microbialitic growth. They account for no more than 25% of the analysed sections and can mainly be classified as mudstones. Detritus include gastropod and bivalve shells, as well as echinoid spines which can even be seen from the outer surface of some boulders. Cements have barely any importance, not even reaching 2 % of point counting in any analysed thin section.
Microbialite-Terebella Association (M-T; Figures 4b, 14)

Figure 14 Microbialite -Terebella (M-T) Association. II. Terebella cf. lapilloides (arrows) dwelling in microbialitic substrate. Scale bar = 500 mm.
Biomorpha
The main feature of this association is its complete lack of metazoan framework builders in the seven thin sections obtained from two boulders. The only identified non-microbialitic organisms are coralline algae patches, possibly belonging to Mesophyllum. Otherwise all thin sections in average contain more than 85% of microbialite, which is abundantly inhabited by Terebella cf. T. lapilloides (Figure 14).
Associated components
Allochthonous micrite is rather scarce (no more than 1% in average), while cements constitute more than 10%. They tend to form stromatactis-type cavities within microbialites. Microsparite accounts for no more than 1%.
Discussion
Statistical Analysis
Variations of UPGMA-J and WPGMA-J algorithms may be due to the weighting of arithmetical mean, which takes place in WPGMA, having more effect in microbialite-based associations. Withal, phenograms obtained by these two methods are very similar (Figures 5, 6). This would imply that both assemblages obtained by means of UPGMA - J and WPGMA - J (2tM and M-T) could be grouped under the same fossil association, which is possibly because of the high content (percentage) of microbialite. Similar results could be observed in the Nearest Neighbour phenogram. The fact that all 2tM and M-T samples are grouped in a single branch could be attributed to their richness in microbialite.
For the Bray-Curtis phenogram, since a BC value of 0.345 is relatively low (McCune, Grace & Urban, 2002) the C-m Association is the most dissimilar of all four obtained by both UPGMA and WPGMA, perhaps due to its lower absolute content of microbialite, and to its higher metazoan / microencruster diversity. M-m Association (except for sample M-m 6) can also be found in a robust branch. All remaining samples (M-m 6, all 2tM and all M-T) are grouped in a single branch without nodes. As in the case for Nearest Neighbour, this could be attributed to their richness in microbialite, and furthermore to their scarceness in other components.
It must be stated that cluster analyses have rendered significant results while interpreting fossil "reef and "reef-like associations, especially from allochthonous or para-autochthonous samples (olistoliths), where there are not sufficient clear lateral relationships to their original depositional setting (Sánchez-Beristain & Reitner, 2016, 2018). By quantitatively determining to which extent an olistolith is composed of different components, plus using the information provided by additional features (allochthonous components and cements), it is possible to assess with certainty the percentage of each component and thus to correlate with certainty samples to a depositional environment within a "reef or "reef-like setting. Without performing cluster analyses, which consider both diversity and abundance of all components, it would have been easy to assume that samples with high microbialite percentages (over 75%) and even some microencrusters, would have constitute a single association. Furthermore, while comparing UPGMA-J and WPGMA-J (Figures 5, 6) with both NN-J and WPGMA-BC algorithms (Figures 7, 8), we clearly found four different associations, despite the similar microbialite percentage in three of them. This is the reason for which UPGMA-J and WPGMA-J were preferred over NN-J and WPGMA-BC, since the former two ponder diversity to a bigger extent than the latter two in samples dominated by a single component (Sokal & Michener, 1958; Legendre & Legendre, 2012).
Results presented statistically in this work are similar with those presented by Russo, Mastandrea & Baracca (1997), who reported microbialite-rich deposits in the St. Cassian outcrops in Punta Grohmann, NE Italy, and differ with the traditional metazoan-based frameworks from this Fossillagerstätte. According to Russo, Neri, Mastandrea & Baracca, 1997; Russo, 2005), this would represent the presence of lateral variations within the Cassian platform, which is the source for Cipit boulders.
Fossil associations
Microbialite-microencruster Association (M-m)
Since no considerable skeletal framework could be assessed, algae could seldom be found and microbialite is the most conspicuous component, M-m association could have originated at a deep, low energy «mud-mound» setting; probably at a subtidal level (Pratt, 1995 ; Reitner & Neuweiler, 1995). Pyrite framboids, which suggest a poorly oxygenated setting with bacterial activity (Wilkin & Barnes, 1997; Spadafora, Perri, McKenzie & Vasconcelos, 2010 ; Kershaw et al., 2012), further support this statement. However, stromatactis/stromatactoid cavities, which are often characteristic of these settings, are completely absent. Neuweiler & Reitner (1995) described mud mounds from the Basque Country in northern Spain that are poor in stromatactis or completely devoid of them, arguing that geopetal cavities resulting from internal sediment could be their equivalents. Nevertheless, geopetal cavities are also absent in these samples. Neuweiler (1993) described a reef setting from the Albian in northern Spain, where microbialites acted as main builders, making baffling and binding subordinate. Therefore, this possibility also remains plausible for the origin of the M-m association, especially considering the presence of aragonite cements, which, though scarce, could have a synsedimentary origin, and could thus also be associated with to the filling of primary vugs (Neuweiler & Reitner, 1995).
Similar associations were described byAurell & Bádenas (2004) for the Kimmeridgian of NE Spain, which were interpreted as shallow ramp reef settings. Nonetheless, they noticed a high abundance of Tubiphytes and Terebella (microbial-dominated reef) during stages of relative sea-level rise. The same was noted for Terebella by Rodríguez-Martínez, Reitner & Mas (2010) for the Viséan of the Guadiato Valley, Córdoba, SW Spain.
Nicol (1987) found shallow coral-dominated reef communities containing Alpinophragmium strongly associated to Tubiphytes and abundant calcareous algae in the Norian of the Northern Calcareous Alps. These results are similar to those by Reijmer & Everaars (1991) from the same region, as well as for the mid- to late Jurassic of SE Sicily (Buccheri Formation; Basilone, 2018).
Regarding Alpinophragmium, it should however not be considered as an encruster which is diagnostic for depth conditions, since it appears to have had a broad palaeoecological distribution in reef facies (Senowbari-Daryan, Schäfer & Abate, 1983), though it is often associated to particular coral genera (Stanton & Flügel, 1987). Nevertheless, the presence and abundance of these encrusters associated to the few Koskinobullina socialis and, on the other side the scarcity of algae, suggest that the boulders containing the M-m association would have originated at a mid- to deep ramp, microbialite-reef subtidal setting, under low-energy conditions and perhaps poor in oxygen. However, another possibility would imply that this association originated at a cryptic, yet shallow setting, similar to those that occur at Jamaican Caribbean in the Recent (Hartman & Goreau, 1970 ; Jackson, Goreau & Hartman, 1971; Goldberg, 2013).
Dual-type Microbialite Association (2tM)
Since microbialite is the dominating component, it can be concluded that neither macroinvertebrates nor microencrusters have an important role in the palaeoenvironmental implications of this association. Microencrusters can only seldom be found on the surface of the chaetetid skeletons, or in their associated microbialitic crusts so that a deep, subtidal setting can be inferred for these chaetetids.
Moreover, it is significant that the orientation of some overlying stromatolite-like microbial growing is completely different from that of the chaetetids. This is supported by the presence of geopetal cavities with their respective infilling, which underlie this kind of microbialite. These microbialites are not colonized by any microencrusters.
Two possibilities exist for the palaeoecological interpretation of this association. On the one hand, chaetetids and associated thrombolites could have settled first in a deep/cryptic setting, followed by the establishment of a microbial community with a different growth direction, which precipitated carbonate as a layered, stromatolitic fabric.
On the other hand, it could have been possible that these stromatolites formed even at greater depths than the precursor chaetetids, especially since no associated alga could be found. In addition, stromatolites are devoid of microencrusters. This possibly indicates an oxygen-poor setting, which may indicate depths greater than 50 m (Schmid, 1996). Furthermore, deep water stromatolites are known to exist from the Mesoproterozoic (Bartley, Kah, Frank & Lyons, 2015) and have notable representatives from the Devonian (George, 1999), Mississippian (Shen & Qing, 2008) and until the Holocene (Brachert, 1999). Considering these criteria, along with the relative abundance of stromatactis, it is highly probable that the last depositional setting for this association had been related to a deep water microbialite-rich, mud mound-like environment. Though both scenarios are possible, we believe the second is more plausible. An association based on chaetetids and thombolitic microbialites could have undergone a further encrustation of stromatolites, after some boulders containing the chaetetid-thrombolite association fell from the carbonate platform front and were deposited in a deeper setting of the slope.
Chaetetid-microencruster Association (C-m)
The most remarkable feature of this association is its overall lack of calcareous algae, as well as its distinctive microencruster settling patterns. It cannot be assessed whether competence for substrate existed between Tethysocarnia and Koskinobullina.
However, it is known from present studies that substrate competence can occur between different foraminifer species (Páez, Zuniga, Valdés & Ortlieb, 2001).
The C-m association is not very diverse; nonetheless it provides sufficient criteria to make a palaeoecological preliminar assessment. It is well known that a branching growth form generally indicates low-energy conditions for bryozoans, corals and sponges (Flügel, 2010). This phenomenon has already been recorded for other Triassic localities, particularly branching corals and solenoporaceans at the Rötelwand and Adnet complexes in northern-central Austria (Schäfer, 1979), for sponges at Feichtenstein and the Gruber Reef, near Salzburg, Austria (Senowbari-Daryan, 1980), for recent sponges at the Caribbean (Wulff, 2006; James & Jones, 2015) as well as for Upper Jurassic microbialites from the Chay Peninsula in Western France (Olivier et al., 2003). This type of growth in chaetetid sponges, the relative scarcity of microbialite, the lack of high-energy dwelling microencrusters, the fabric of allochthonous components, and the lack of algae may help to infer a quiet, semi-cryptic/deep (subtidal) marine setting, probably below 50 m (compare Keupp, Reitner & Salomon, 1989) that could therefore somehow be related to the Ceratoporella-Tubiphytes association from Sánchez-Beristain & Reitner (2018). Unfortunately, no further assessment is possible.
Microbialite-Terebella association (M-T)
Since microbialite is the dominant facies in all samples and framework builders are completely absent, a deep "mud mound" subtidal setting, poor in oxygen and sustained only by microbialites is the most probable interpretation. Abundant terebellids furthermore support this assumption (Wendt, Xichun & Reinhardt, 1989), since they can dwell successfully in low-oxygen conditions (Peybernes, Chablais & Martini, 2015), sometimes even in symbiosis with reducing bacteria (Guido et al., 2014). It is noteworthy that no stromatolite is present, in contrast to the 2tM Association. In addition, Corallinaceae are not sufficient to give evidence of a very profound depth (Riding, 1975; Round, 1981; Pratt, 1995). Nevertheless, no further palaeoenvironmental assessment can be made. Further geochemical and sedimentological studies would be needed to approach this issue.
Conclusions
Fossil associations can be effectively obtained by means of cluster analyses. Statistical analyses results indicate that most samples described in this work are strongly dominated by microbialite. These results are similar to those presented by a previous work in another St. Cassian outcrop.
Data from microencrusters provide help to infer palaeoecological constraints which allow further separation in different associations. In the case of the Cipit boulders from the St. Cassian Formation (upper Ladinian - lower Carnian, Dolomites, NE Italy), the discovery of these associations represent a solid approach in attempting to reconstruct the palaeosynecological settings of this Fossillagerstätte.
Four fossil associations were obtained by clustering: Microbialite-microencruster Association, which may have either originated in a deep "mud mound" subtidal setting, or in a cryptic environment poor in oxygen; Dual-type Microbialite Association, originating initially in a cryptic-deep (subtidal) sponge-based framework with further basin-ward falling and recolonization by layered microbialites; Chaetetid-microencruster Association, coming from a quiet, semi-cryptic or deep subtidal marine setting, which would seem to modern Caribbean cryptic environments; and, Microbialite-Terebella, representing a deep, subtidal oxygen-poor typical microbialite-based mound? environment. Significant light influence in all associations was discarded, due to the absence of algae and to the different growth direction of some microbialitic crusts.
Being minimum at most associations, macrofossils play no significant role in framework building, leaving this niche entirely to microbialite. Microencrusters help define energy constraints for the palaeoenvironment where these associations come from.
Results from this study partially disagree with the widely accepted model for the proposed palaeoenvironment for the Cipit boulders from the St. Cassian Formation. According to this model, these boulders would have come from the Cassian platforms, which correspond to shallow "reef" and "reef"-like frameworks based on metazoans (Fürsich & Wendt, 1977; Wendt & Fürsich, 1980; Wendt, 1982; Russo, Neri, Mastandrea & Laghi, 1991). Despite coming from the most studies localities for this formation, the boulders analysed in this work present apparently little lateral variations in facies and in biotic composition in comparison with other St. Cassian microbialite-rich outcrops (Russo, Neri, Mastandrea & Baracca, 1997), and a significant one with the most known localities (Wendt, 1982). This paper thus represents the first work dealing exclusively with cryptic to deep settings inferred from fossil associations within the Cipit boulders from the Seelandalpe and Misurina.











 nueva página del texto (beta)
nueva página del texto (beta)


