Introduction
vertebrate immune system has been classically divided in two main groups. The innate immune system able to mount an early non-specific response when an antigen is encountered in the system, and the adaptive immune system which responds slowly but is highly specific due to somatic recombination of antigen receptors (Murphy, 2014). Another hallmark of the adaptive immunity is its capacity to enhance the response to a certain antigen upon re-exposure. This enhanced response, termed immunological memory, is faster and more robust than the first one, conferring the organism with the capacity to combat stronger infections more efficiently. Until very recently, immunological memory was thought to be exclusive to the adaptive immune cells: T and B lymphocytes; however, in the past decade a growing amount of data has challenged this dogma and suggest that a memory-like mechanism can also take place in innate immune cells.
Historically, the use and development of vaccines were the first application to exploit the immunological memory of adaptive immune cells for therapeutic purposes, by taking advantage of the high specificity of T and B cells to recognize an antigen. However, from the very first time a human vaccine (smallpox vaccine) was introduced, non-specific protection (against measles, scarlet fever and syphilis) was reported. During the 1930´s, an epidemiological study suggested non-specific effects of Bacille Calmette-Guérin (BCG) vaccine improving the survival of infants (Naeslund, 1931). Moreover, a recent study supported this result showing that administration of BCG vaccine to low birth weight children diminished neonatal sepsis, infections in the respiratory tract and fever. In addition, many other observations across the globe support the fact that immunization improves survival due to non-specific effects.
Mechanistic insight explaining these observations has started to be studied in laboratory conditions in the past decade, and points towards a recently identified type of memory residing in innate immune subpopulations. In this review we will present and discuss the relevant cell types and the molecular mechanisms so far elucidated in the innate immunological memory field.
Vertebrate innate memory
The study of innate immunological memory in vertebrates started in the second half of the XX century, when J. Triboulry reported lower pulmonary schistosomula numbers after infection with Schistosoma mansoni, if mice were previously vaccinated with BCG. This experiment was performed in athymic nude mice, hence demonstrating that the observed effect was T lymphocyte independent, suggesting a phenotype totally dependent on the innate immune compartment (Tribouley, Tribouley-Duret, & Appriou, 1978). For decades, macrophages were thought to confer this protection due to its capacity to activate and secrete inflammatory cytokines. However, as innate immunity research progressed, other innate immune cell subsets with a certain kind of memory were described (Martinez-Gonzalez et al., 2016; Sun, Beilke, & Lanier, 2009). Nowadays there is a vast amount of evidence pointing out that monocytes, macrophages and natural killer cells (NKs) change their gene expression after encountering an inflammatory stimulus and some of this activated cells, persist in the organism and confer protection against a secondary stimulus, a phenomenon named trained immunity (Netea, Quintin, & Van Der Meer, 2011). Recently, this kind of recall response was described for Innate Lymphoid Cells (ILCs), non-immunological cells and even for Hematopoietic Progenitors (HSCs)(Kaufmann et al., 2018; Martinez-Gonzalez et al., 2016; Naik et al., 2017) (Figure 1). Hence, growing evidence supports the idea of a trained immunity, independent of adaptive immune cell types, which seems to be conserved among innate immune and non-immune cell types. In the following section we will focus on reviewing the current progress reported in different innate immune cell types.
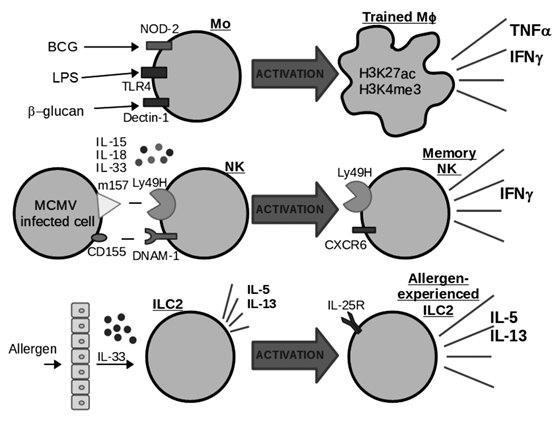
Figure 1: Mechanisms involved in the generation of innate memory. Generation of Trained macrophages (Mφ) from monocytes (Mo), Memory Natural Killer Cells (NK) and allergen-experienced type 2 innate lymphoid cells (ILC2). The different activation requirements and memory phenotypes for these innate cell types are depicted. Figure based on data obtained from (Kleinnijenhuis et al., 2012; Madera & Sun, 2015; Martinez-Gonzalez et al., 2016; Nabekura et al., 2015, 2014; Paust et al., 2010; Quintin et al., 2012; Sun et al., 2009).
Evidence of immunological memory within innate immune cell subsets
Mononuclear phagocytes
Macrophages were first described by Elia Metchnikoff as the phagocytic component of the immune system (Tauber, 2003). Since then, many other functions of these cells have been discovered (Mosser & Edwards, 2008; Murray & Wynn, 2011). Additional macrophage effector functions, besides phagocytosis of microorganisms, includes engulfment of apoptotic cells and production of reactive oxygen species and soluble cytokines, to alert other cell types that an infection is taking place. Macrophages have been classified as classical or alternatively activated, depending on the type of pathogen they encounter and more recently even regulatory macrophages have been described (Fleming & Mosser, 2011; Wang, Liang, & Zen, 2014).
For a long time, cumulative evidence suggested that macrophages could have memory properties and enhance its response upon a secondary infection. Most of these observations were based on studies were an infection conferred protection against an unrelated microorganism in a lymphocyte-independent manner (Bistoni et al., 1986; Van’t wout, Poell, & Van furth, 1992). One of these reports proves that immunization with an attenuated-virulence Candida albicans strain, protects mice against a second challenge with a virulent C. albicans strain and even against a bacterial infection (Bistoni et al., 1986). Furthermore, adoptive transfer of macrophages from an immunized donor to control mice, is sufficient to confer protection and inhibition of lymphocyte functions, using a cytostatic drug does not impair such protection (Bistoni et al., 1986, 1988).
The molecular mechanism involved in the macrophage activation by C. albicans is based on the recognition of β-glucan, a molecule present on the surface membrane of the yeast by the myeloid cell C-type lectin receptor: dectin-1. Dectin-1 ligation, induce the activation of Necrosis Factor kappa-B (Nfκ-B) in a Raf-1 dependent manner; which in turn, promotes changes in the epigenetic landscape of monocytes leading to increased Tumour Necrosis Factor α (TNF-α) and Interleukin 6 (IL-6) RNA levels. Moreover trained monocytes displayed markers of both, classical and alternatively activated macrophages as well as Dendritic Cell (DC) markers. Altogether these data suggests that monocyte activation is not skewed to a certain type of polarization (Quintin et al., 2012).
Another pioneer study, demonstrated that macrophages from previously BCG-immunized mice, were more efficient at producing H2O2 and internalized more C. albicans than control mice. Immunized animals also had higher resistance against a systemic C. albicans infection compared with non-immunized controls (Van’t wout, Poell & Van furth, 1992). BCG immunization activates monocytes to produce proinflamatory cytokines such as IFN-γ, IL-1β and TNF-α in response to a second stimulus, either from bacterial or fungal origin (Kleinnijenhuis et al., 2012). Monocyte activation depends on the NOD-2 pathway, when NOD-2 is activated by its ligand, muramyl dipeptide (MDP); it promotes the autophosphorylation of RIP2, a serine threonine kinase. RIP2 then, phosphorylates the inhibitor of kappa-B a (IκBa), which results in the NFκB activation and translocation to the nucleus. Similar to C. albicans activation, BCG induced activation promoted a global change on the epigenetic landscape of these cells (Kleinnijenhuis et al., 2012).
Interestingly, cross-activation of macrophages is not restricted to bacteria or fungal pathogens; since latent infection with two subtypes of Herpes viridae, confers protection against Listeria monocytogenes (a gram-positive intracellular bacteria) and Yersinia pestis (a gram-negative extracellular bacteria). This protection is maintained for up to three months and depends on IFN-γ in the microenvironment but seems to be completely independent on T cell activation upon re-exposure (Barton et al., 2007). However, this study fails to demonstrate macrophage-specific memory because no adoptive transfer experiment using trained macrophages was performed into naïve hosts. Importantly, not all types of viruses were able to confer protection against L. monocytogenes infection (Barton et al., 2007) which leads to a fundamental question: how specific is innate immunological memory?. At least for mononuclear phagocytes, Pattern Recognition Receptors (PRRs) seem to be required for the priming and recall responses (Bowdish, Loffredo, Mukhopadhyay, Mantovani, & Gordon, 2007). However, thorough studies comparing the primed cellular responses against different types of pathogens are still missing. It would be important to determine if different priming stimulus could be better at conferring protection against particular types of pathogens. Also, it would be interesting to know if costimulatory molecules and cytokine signalling are necessary for the development of trained immunity, as has been largely recognized for adaptive immune memory (Murphy, 2014).
Innate lymphoid cells
ILCs are the most recent group of innate cells discovered. They share a common progenitor with T and B lymphocytes and have a similar morphology; however ILCs lack RAG-dependent antigen receptors: BCR or TCR, and have no markers of myeloid or dendritic cells (Spits et al., 2013). For a long time ILCs were though to lack memory responses but growing evidence demonstrate otherwise.
Natural Killer cells (NKs) were the first cells from this group to be described (1975) and for a long time the most studied subset of ILCs. Early evidence for adaptive-like immunity in NKs came from studies in a contact hypersensitivity (CHS) model (Leary, Goodarzi, Drayton, & Andrian, 2006). This model is widely used to test specific immune memory, and T and B lymphocytes have been implicated in the elicited inflammatory responses. NKs are also able to respond to a CHS challenge in a lymphocyte-independent manner, hapten-specific and long lasting, as has been proven to be able to elicit recall responses even a month after the primary stimulus (Leary, Goodarzi, Drayton & Andrian, 2006).
Specificity of NKs has also been reported in viral infection models (O’Sullivan & Sun, 2015) where it has been found to be analogous to T cell activation. T cells need three independent signals to be fully activated and become functional effector cells: 1) antigen-receptor interaction, mediated by TCR in lymphocytes; 2) a costimulatory stimulus, CD28; and 3) a cytokine signal that skews polarization of T cells. To gain more mechanistic insight into what are the requirements for NK memory induction, particular infection models have been exploited. In this regard, Mouse Cytomegalovirus (MCMV) infection is the most studied model for memory NK formation. Once other target cells have been infected, NKs are able to recognize the MCMV encoded protein m157 via its Ly49H receptor (1st signal) and proliferate in an IL-12, IL-18, and IL-33 dependent manner (3rd signal) (Madera & Sun, 2015; Nabekura, Girard, & Lanier, 2015; Sun et al., 2012). A co-stimulatory signal (2nd signal) required for NK cell memory expansion has also been described. DNAM-1 (CD226) is constitutively expressed by NK cells and upon MCMV infection; it binds its cognate antigens CD155 and CD122, both upregulated in infected cells (Nabekura et al., 2014). After MCMV infection has been cleared, NK numbers diminish but Ly49H+ NKs frequency remains high. These subset of NK cells, persist several months in the liver on a CXCR6-dependent manner and are able to respond to a second challenge with the same virus (Paust et al., 2010). In addition, Ly49H+ NK cells are able to confer protection after been adoptively transferred to naive, lymphocyte-deficient mice (Sun, Beilke & Lanier, 2009). However, the mechanism for specific memory formation against other types of viruses remains to be elucidated (O’Sullivan & Sun, 2015). An important aspect related to the molecular identity responsible for NK memory response, is the lack of known antigen-receptor interactions. To specifically recognize different types of viruses, without a RAG-dependent receptor, different antigen-specific receptors should be expressed on the surface of NK cells. This theoretical idea is unlikely, due to the large viral diversity and may imply that virus specific NKs could be found in homeostatic conditions. Moreover, even if the specific receptor were to be expressed only in inflammatory conditions, the viral diversity would still remain as a persistent issue. In any case, this is a topic of great interest and further studies may shed light into this problem as it promise to open new therapeutic venues to treat major viral infections affecting humankind such as HIV.
More recently, memory-like NKs (MLNKs) have also been identified using soluble cytokines. In vitro stimulation or priming with IL-12, IL-15 and IL-18 yields activated NKs with enhanced IFN-γ production and cytotoxic activity. Importantly, these cells are able to respond to IL-12 stimulation in vivo, supporting the idea of its potential for therapeutic intervention. MLNKs lack specificity to antigens but have been proposed as a novel treatment to fight tumour growth (Fehniger & Cooper, 2016).
Another subset of ILCs, ILC2s has been recently reported to have memory-like properties (Martinez-Gonzalez et al., 2016). Martinez-Gonzalez and colleagues reported that upon intranasal administration of allergens, lung-resident ILC2s proliferate and secrete IL-5 and IL-13 promoting lung inflammation. After that, lung ILC2s number decrease to almost basal levels, and a subpopulation of allergen experienced ILC2s migrates to the mediastinal lymph node. Allergen experienced ILC2s were defined as IL-25 receptor (IL-25R) expressing cells, presumably able to respond more efficiently to a secondary challenge. This activation seems to be dependent on IL-33 signalling and, since ILCs have no TCR or BCR, it is antigen independent. Future studies need to be performed in order to elucidate if other ILCs subsets (ILC1s or ILC3s) can also become experienced, are long-lived upon activation or not, and if so; to identify and determine the type of stimulus driving this response.
Basophils
The study of basophils during memory response has been mainly focused on their role during the development of adaptive immune responses. During a memory response, basophils capture antigen specific IgE antibodies through the expression of the IgE receptor on their surface, allowing a rapid binding of the antigen and clearance of the pathogen (Mack et al., 2005). In a model of memory response using the protein allophycocyanin mixed with heat-killed Bordetella pertussis, upon rechallenge 6 weeks later, basophils respond to this challenge and represent an important source of IL-6 and IL-4 in the bone marrow and spleen (Denzel et al., 2008). This enhanced response completely depends on the ability of specific antigen recognition by IgE antibodies, and hence a humoral product of the adaptative immune response (Denzel et al., 2008). It has also been demonstrated that basophils play a key role in the expulsion of Nippostrongylus brasiliensis after a primary infection, even in the absence of Th2 cells (Ohnmacht & Voehringer, 2010). Surprisingly, the expulsion of this hookworm after a secondary infection, seem to be mainly dependent on basophils, and independent of mast cells and CD4 T cells (Ohnmacht & Voehringer, 2010). Given that basophils are an important source of IL-4 and IL-13 under parasitic infections (Sullivan et al., 2011), and since both cytokines are required for the enhanced worm expulsion, it is likely that the lack of both cytokines from this source, could affect the ability to resolve the infection during a reinfection challenge. In this context, it is unlikely to assume that the basophils exposed to a first encounter with a parasite, could acquire a trained phenotype; mainly because basophils have a life span of 60 hours and hence, could not persist until a secondary exposure (Schwartz & Voehringer, 2011). Alternatively, it has been recently shown that infection can induce changes in haematopoiesis during infection (Glatman Zaretsky, Engiles, & Hunter, 2014), which can lead to changes in epigenetic and transcriptomic signatures, thus conferring progenitors the ability to generate a protective innate immunity against distinct pathogens. Interestingly, this phenomenon seems to be a common feature of trained memory (Kaufmann et al., 2018; Mitroulis et al., 2018). In conclusion, even though so far there is no such experimental evidence for parasitic antigens and basophil precursors; it is possible that similar to what has been described in other models, this type of infection could induce epigenetic changes on the basophil precursors, priming newly generated basophils derived from this precursor to enhance expression of effector cytokines such as IL-4 and IL-13 after a secondary infection, all these speculations would need to be tested and experimentally demonstrated or discarded in the future.
Neutrophils
Neutrophils are short-lived cells from the innate immune compartment, whose primary function during an infection is mediated by their ability to phagocyte, produce reactive oxygen species, release neutrophil extracellular traps (NETs) and different types of proteases (Grainger & Grencis, 2014). Recently, an unexpected function of neutrophils was described, based on its ability to interact and influence the function of other cell populations. Such novel function reside on the neutrophil´s ability to induce a memory like phenotype in macrophages. During a primary infection with N. brasiliensis, neutrophils polarize to an N2 phenotype that later drives the development of a long-lived resident macrophage population in the lung, which subsequently confers protection during a secondary infection with the same parasite (Chen et al., 2014). This induction of innate memory of macrophages has also been associated with ILC2s and CD4 T cells in the lung (Bouchery et al., 2015). Even though, neutrophils have not been directly implicated with a long-standing innate memory; we could hypothesize, that similar to what we previously discussed for basophils, nematode infections could prime neutrophil progenitors to enhance and improve the immune response upon re-exposure to the same or similar pathogen.
Mechanisms for trained immunity
Different groups have been trying to understand the molecular mechanisms of trained memory induction in innate cells. Growing evidence suggests that the metabolic state and epigenetic reprogramming is essential for the acquisition of the innate memory. In fact, the accumulation of specific metabolites such as mevalonate and fumarate; modulates the epigenetic processes that impact the functional program of the cell, and therefore are able to prime an immune cell, even in the absence of a foreign antigen or pathogen.
Macrophage activation via a wide range of different stimuli including LPS, poly (I:C), type I interferon, and β-glucan, leads to a process of metabolic switch from oxidative phosphorylation to glycolysis, resulting in more lactate production, a process called the Warburg effect (Kelly & O’Neill, 2015). This metabolic reprogramming depends on the activation of the Hypoxia inducible factor 1 alpha (HIF-1α) and the mammalian target of rapamycin (mTOR). Activation of these enzymes, leads not only to the increased expression of glycolytic genes, but also to the upregulation of several other genes like beclin-1, STK11, JHDM1D or FOX4, associated with autophagy, chromatin remodelling, signal transduction and modulation of transcription, respectively; promoting the acquisition of a trained phenotype on the macrophages (Chen et al., 2014).
Importantly, not only glucose metabolism is relevant for the priming of immune cells, since glutaminolysis and cholesterol synthesis pathways are also essential for the modulation of trained immunity and epigenetic reprogramming. Stimulation with β-glucan leads to the metabolism of different aminoacids, including glutamine. Glutamine metabolism generates glutamate, α-ketoglutarate and succinate semialdehyde, giving substrates for the Krebs cycle such as fumarate and succinate. In this regard, it has been shown that monocyte stimulation with fumarate, promotes trimethylation of histone 3 at K4 (lysine) (H3K4me3) as well as acetylation at K27 (H3K27ac). Fumarates are able to inhibit KDM5 (a histone demethylase), which in turn leads to the increased H3K4me3, mainly at the promoters of inflammatory cytokines. Fumarate also inhibits hydroxylation of HIF-1α, which promotes its stabilization and transcriptional activity (Arts et al., 2016). In the case of cholesterol synthesis, mevalonate, a metabolite of this pathway, has also been reported as an inducer of trained immunity in monocytes. Specifically, exposure to mevalonate induces enrichment of H3K4me3 on the promoters of IL-6 and TNFα, a phenotype also observed in monocytes upon exposure to β-glucan (Bekkering et al., 2018).
Surprisingly in the case of fumarate and mevalonate, single exposure of the monocyte to any of these metabolites, are sufficient for the acquisition of epigenetic modifications related to trained memory, even in the absence of antigen exposure. In this same study, qualitative and quantitative differences modulated by the combined effect of such metabolites and antigen recognition was reported. More recently, additional evidence in agreement with the influence of metabolites in the induction of innate memory, describes the influence of western diet on long-lasting trained immune memory in myeloid cells (Christ et al., 2018).
Histone modifications are not the only epigenetic marks that could alter the cell responses after a first immunization. Other molecules induced after activation, that could impact on transcriptional profile expressed by any cell are microRNAs (miRNAs): stable molecules able to modify gene expression even after the initial stimulus has faded. Several microRNAs regulate innate and adaptive immune functions. miR-146 and mi-R155 have been shown to regulate the inflammatory cell response decreasing or increasing inflammation, respectively (Monticelli & Natoli, 2013). Others have been shown to inhibit the production of inflammatory cytokines, such as miR-187, which inhibits TNF-α, IL-6, and IL-12p40 after TLR4 stimulation (Rossato et al., 2012). Importantly, these molecules are short lived and seem to be transiently induced upon a primary activation; hence, its contribution to the establishment of trained immunity is unlikely, however studies addressing this subject have to be performed in order to discard or confirm this hypothesis.
Even thought there has been some advance and increasing research interest on the trained immunity area; the molecular mechanisms driving this induction remain to be elucidated. So far, it is clear that metabolism plays an important role in this process, leading to modifications at the chromatin level on genes associated with inflammation (Figure 2). However, many important questions remain unanswered including the relationship between pathogenesis and environment, and how they influence the establishment of this type of memory.

Figure 2: Molecular events leading to trained memory. During a first challenge or priming phase, the innate cell is activated and its metabolism changes towards a glycolytic pathway. The activation of glycolytic enzymes induces activation of key transcription factors such as HIF-1α (Hypoxia inducible factor 1 alpha) and mTOR (mammalian target of rapamycin). Activation of other metabolic pathways in parallel, leads to the production of metabolites including fumarate and malate. These metabolites as well as the transcription factors induce changes at the level of chromatin leading to the enrichment of H3K27 as well as H3K4me3 in the promoters and enhancers of proinflammatory genes. Upon re-exposure, the primed chromatin with its epigenetic modifications, allows for the rapid expression of the proinflammatory genes promoting a more efficient response and rapid control of the pathogen.
Microbiota and trained immunity
Cumulative evidence in recent years demonstrates the impact of the gut microbiota diversity on immune subset differentiation and immune regulation. Identification of the bacterial populations driving Th17 or Treg differentiation in homeostasis and pathogenesis (Honda & Littman, 2012), had greatly contributed to our understanding on how the interaction with commensal communities strongly impacts the nature and kinetics of the immune response. In this regard, little is known about the role of the gut microbiome and the development of trained immunity.
A pioneer study suggests a possible role for the microbiota in priming mononuclear phagocytes. For instance, trimethylation of lysine 4 on histone 3 (H3K4me3: an epigenetic mark associated with the acquisition of trained immunity), in promoters of genes associated to an inflammatory response (vgr., Il6, Ifnb1, Tnfa) in macrophages and dendritic cells, is dramatically reduced in germ free mice. Interestingly, the physiological consequence of this phenotype observed in germ free mice, leads to incorrect priming of NK cells and deficient antiviral response upon MCMV infection (Ganal et al., 2012), mostly due to a deficient production of inflammatory cytokines by phagocytes. A similar study, focusing on parasite infection susceptibility regulated by microbiota, described how the microbiome composition affects the immune response against Entoameba histolytica (Burgess & Petri, 2016). In this study, bone marrow derived dendritic cells from mice colonized with SFB (Segmented Filamentous Bacteria), produce more IL-23 and have an intrinsic tendency to migrate to lamina propria upon infection with this parasite. Whether the impact of the microbiota in shaping trained immunity is long-lasting or relies on a constant exposure to the commensals, are aspects currently under study. Antibiotic treatment of mice before MCMV infection, leads to deficient proinflammatory cytokine secretion in macrophages, which in turn impairs activation of NK cells. This suggest that in the context of a viral infection, the absence of the microbiota leads to an impaired immune function (Abt et al., 2012). A similar phenotype was observed in the acquisition of innate immune memory in Anopheles gambiae. In this model, acquisition of resistance to Plasmodium infection in mosquitos is achieved by its pre-exposure to ookinetes of the malaria parasite. In this context, once there is a Plasmodium infection, an enhanced clearance of the pathogen is observed in pre-exposed mosquitos. Interestingly, this enhanced clearance was decreased when the gut microbiota is eliminated. So this protective response is regulated by the presence of microbiota (Rodrigues, Brayner, Alves, Dixit & Barillas-mury, 2010).
Regarding the impact of the microbiota on regulating priming of immune precursors, increasing amount of evidence indicates that the gut microbiome could impact on stem cell derived myeloid cell development, which in turn affects systemic immune function (Josefsdottir, Baldridge, Kadmon & King, 2017; Khosravi et al., 2014). It has also been shown that vaccination with BCG induce global epigenetic reprogramming in hematopoietic stems cells, promoting protection against reinfection and the acquisition of a trained phenotype (Kaufmann et al., 2018). Importantly, it has been recently shown that dysfunction of the microbiota impairs vaccination, particularly deficiencies on antibody production (Lynn & Pulendran, 2017). Hence it is possible that the microbiota promotes not only the development of a correct humoral response, but could also promote an epigenetic reprogramming on hematopoietic stem cell precursors.
As for the inducer signals derived from the microbiota that could promote trained immunity, some hints point to some bacterial metabolic products including short-chain fatty acids (Burgess, Gilchrist, Lynn & Petri, 2017) or “tonic” signals, that activate TLRs in mononuclear phagocytes and regulate the balance between tolerance and inflammation. However, future research has to be done in order to identify and elucidate the molecular mechanisms in which microbiota could shape and promote trained immunity.
Immunological memory in other cells
For decades, it was believed that the adaptive immune memory had a unique capacity to be able to rapidly respond to previously encountered pathogens, and that this memory was conserved through evolution from jawless vertebrates to humans (Boehm, 2012). Adaptive memory depends on the ability to generate cells with specific receptors competent to recognize a wide range of different antigens. The generation of these receptors in B and T cells, BCR and TCR respectively, depends on the process of recombination of specific regions of the DNA and somatic hypermutation, ensuring diversity and promoting affinity maturation for foreign antigen recognition (Arya & Bassing, 2017). For compiled evidence related to the mechanisms involved in memory acquisition in different immune subsets see Table I.
Table I: Comparative features of the immunological memory mechanisms in different immune cell subsets. The immunological memory observed in different innate cell subsets: Monocytes (Mo), Natural Killer Cells (NK) or Type 2 Innate Lymphoid Cells (ILC2) differs from that provided by adaptive immune cells: lymphocytes. Major differences include the duration of the immunological memory, the cytokines needed and the cellular mechanism for memory formation. Pattern Recognition Receptors: PRR; T-cell receptor: TCR; B-cell receptor: BCR; Interferon gamma: IFN-γ, Interleukin: IL; Unknown: ?. Table based on data from Bowdish, Loffredo, Mukhopadhyay, Mantovani & Gordon, 2007; Grainger & Grencis, 2014; Kaufmann et al., 2018; Kleinnijenhuis et al., 2012; Quintin et al., 2012, Madera & Sun, 2015; Nabekura, Girard, & Lanier, 2015; Sun et al., 2012; Sun, Beilke, & Lanier, 2009, Martinez-Gonzalez et al., 2016 and Murphy, 2014.
| Feature | Mo | NK | ILC2 | Lymphocyte |
| Specific memory | No | Yes | No | Yes |
| Duration | Months | Months | Months | Years |
| Antigen receptor | PRR | Virus specific | No | TCR/BCR |
| Costimulation | ? | Stimulatory | ? | Stimulatory/inhibitory |
| Activating cytokines | IFN-γ | IL-12, IL-18, IL-33 | IL-33 | IL-7, IL-15 |
| Memory mechanism | Epigenetic modifications | Epigenetic modifications | Epigenetic modifications | Somatic recombination and hypermutation |
| Precursor reprogramming | Yes | ? | ? | No |
Notably, recent observations reported that an equivalent mechanism based on DNA editing takes place in bacteria and archaea. These modifications promoted a memory-like acquired immunity to phages through the incorporation of sections of foreign DNA into the bacterial genome. Acquisition of adaptive immunity depends on the incorporation of exogenous DNA coming from plasmids and viruses, into Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) within the bacterial genome. Those insertions are later transcribed into small interfering RNA (CRISPR-RNA or crRNA) that hybridize with a trans-activating RNA (tracrRNA) to guide specific endonucleases Cas for specific cleavage of complementary repeats in the viral or phage genome (Barrangou & Marraffini, 2014). This system confers immunity against viral infection because when the virus reinfects the bacteria, the Cas endonuclease with the guide RNA, specific to the viral DNA previously inserted into the bacterial genome, is now able to bind to the invading phage DNA and rapidly degrade it. This system has not only shed some light into the mechanisms of bacterial defense against virus but is also provided a powerful tool that is greatly impacting genome editing techniques (Glass, Lee, Li, & Xu, 2018).
Similar to what has been recently described for bacterial immune defense, recent advances in the study of the molecular mechanisms involved in pathogenesis, survival and evasion, has led to the discovery of similar mechanisms of defense in several organisms including virus. Viruses, particularly giant viruses from the Mimiviridae family are target of infection with different virophages. This type of phage uses the viral factory of the host to propagate at the expense of its viral host (La Scola et al., 2008). It was shown that such mimivirus were resistant for infection with different strains of virophages, and that the acquisition of resistance was dependent on a CRISPR-Cas-like system, including a cluster of genes called MIMIVIRE or mimivirus virophage resistance element. The genes from this cluster encode for a helicase-nuclease R350 and nuclease R354, which confer enzymatic activities involved in the cleavage of foreign nucleic acid. Similar to the mechanism of defense described above for bacteria and archaea, foreign repeated DNA sequences where identified in the MIMIVIRE cluster of these viruses, suggesting a similar adaptive defense mechanism for protection upon reinfections (Levasseur et al., 2016).
As mentioned by Levasseur et al, CRISPR-Cas and MIMIVIRE systems confer a complete immunity against reinfection. These systems confer: “1) Prevention of viral absorption and genome injection, 2) Cleavage of the invading genome based on the self/non self discrimination principle and 3) Blockage of phage replication”. All together, these new encouraging data, indicates that the development of immunological memory is necessary for the survival and adaptation of any organism, even for those entities previously unrecognized such as bacteria and viruses.
Concluding remarks
Growing evidence suggests that the acquisition of a memory-like defense response, is not restricted to the adaptive immune cellular subsets in vertebrates and higher organisms, but is also present in the innate compartment and even in primitive forms of life such as bacteria.
Albeit both arms of the immune system (innate and adaptive) can induce memory cell subsets after an immunization, certain differences between them exist. One of the most crucial is the permanence of protection: memory on innate cells does not last as long as the one in adaptive cells. This difference is mostly due to the nature of genomic changes; while T and B cells undergo permanent gene changes by recombination and somatic hypermutation to produce highly specific receptors¸ innate cell adaptations rely on epigenetic modifications which are labile and reversible by cellular mechanisms such as cell division. In addition, even though both adaptive and innate memory cells will have an enhanced response at a secondary challenge, adaptive immune memory cells will only be activated if exactly the same antigen is encountered. In contrast, innate memory cells are able to respond to diverse antigens once they have been primed. Additional differences between innate and adaptive memory are summarized in Table I.
The molecular mechanisms controlling this memory feature involve either genome editing or alternative mechanisms to control gene expression such as epigenetic changes in the genomic landscape. Given that only a subpopulation of the innate effector cells persists as memory-like trained cells, it would be interesting to determine if different cell types or tissue resident subsets have different responses to a certain pathogen (vgr, airway cell populations responding more efficiently to worm infections), in order to develop novel immunizing therapies targeting specifically those cellular subsets involved in the acquisition of an enhanced response.
Although some progress have been made aimed to elucidate the formation of memory subsets in different immune populations, functional interactions between memory and effector cells, or even between memory populations of different cells types remains to be determined.
As fascinating as trained immunity can be, further research is required to understand the molecular mechanisms that control this enhanced response, and careful consideration needs to be taken into account before clinical application, including possible negative side-effects that could come in parallel such as immune overactivation that could lead to tissue damage or autoimmune disease development. Recent evidence has shown that certain neurological diseases can be shaped by trained microglia. In mouse models of Alzheimer’s disease and focal brain ischemia, LPS-trained microglia increased brain inflammation compared to control animals with non-trained microglia (Wendeln et al., 2018). LPS systemic treatment increased the total amyloid β levels and the IL-1β levels which indicate a worse disease prognosis in Alzheimer and ischemia respectively. On the other hand, growing evidence supports a role for trained immunity in the efficiency of vaccines. A recent study demonstrates that BCG vaccination increases the production of IL-1β which confers protection against yellow fever virus infection in humans (Arts et al., 2018). Future studies must be aimed to evaluate the duration of this effect and to carefully study the possible adverse effects of these therapies.
Importantly, all these recent evidence highlights how far we are from completely understanding the biology of the immune system, and how our initial classification of innate and adaptive response seems now simplified and misconceived.
Current research is now focused to fully understand the regulatory pathways that control trained immunity as well as elucidating the efficacy of priming innate cells to confer protection against viral infection and cancer. Future research on innate function of T cells (Kawabe et al., 2017) and adaptive function of innate cells, will be imperative to comprehend the complex interactions and mechanisms operating during a functional immune response and to employ this knowledge in more efficient and effective new ways to prevent, treat and cure diseases.











 nueva página del texto (beta)
nueva página del texto (beta)


