INTRODUCTION
Analysis of heavy metals in wastewater is important because of their significant role in various complex processes, such as surface soil and water loading, bioaccumulation in living organisms, cloud stability, atmospheric catalysis, and increases in the frequency of air and water-borne diseases[1,2]. The concentrations of trace metals in the atmosphere are continuously increasing due to both natural and anthropogenic emission sources, such as winds, storms, volcanic eruptions, biomass burning, evaporation, fossil fuels combustion, ore smelting, thermal power plant fly ash, and unsustainable use of natural resources. Trace metals are subject to biogeochemical cycles that determine their presence and concentrations in different compartments of the environment: soil, groundwater, surface water, air, and living organisms. Human intervention can alter the metal concentrations in these compartments and change the distribution of metals in the environment. The toxicological importance of metals is enormous because of their ubiquity, their extensive industrial and domestic use, and their environmental persistence. This persistence depends upon the characteristics of the chemical compound that the metal comprises, which determine the metal's environmental mobility and its bioavailability[3]. The metals in the hydrosphere are of environmental importance because of their interactions with solid geological materials, their influence on biological processes, and their interactions with the atmosphere by evaporation processes[4]. Biosorption is a technology that represents an alternative to conventional water treatments for heavy metal recovery. This technology allows the reuse of agricultural and industrial residues. Biosorption is a term that describes the removal of polluting agents from aqueous solutions by using biomass. The mechanism of removal by biosorption is not controlled by metabolism, but mainly by surface adsorption. In contrast, the term bioaccumulation describes an active process of metal removal that requires the metabolic activity of a live organism[5]. Heavy metal removal by biomaterials such as biosorbents offers an alternative for toxic metal removal from industrial effluents[6]. It is known that biomaterial residues such as agents seaweed, bacteria, fungi and certain aquatic flora have the ability to concentrate and accumulate metals from dilute aqueous solutions[7]. When selecting the metals of interest to examine their removal or recovery options by the application of appropriate technologies, the following considerations are mainly taken into consideration: environmental pollution problems and deterioration of several ecosystems with the accumulation of many toxic metals[8].
MATERIALS AND METHODS
Isolation of bacteria
For the growth and isolation of bacteria, a 1 mL sample of activated sludge was deposited in a tube containing 9 mL of a sterile saline solution (NaCl to 0.5%). The tube was warmed to a temperature of 80 °C over the course of 20 minutes, resulting in thermal selection of bacteria that are capable of forming spores The tube was then incubated at 36 °C for 24 hours. After treatment 100 μL from this sample was spread in duplicate onto plates with medium Luria Bertani (LB) agar and nutritious agar). Plates were then incubated at 36 °C for 24 hours. Strains were characterized by growing in LB agar and nutritious agar prepared with CdCl2, MnCl2, CrCl2 and PbCl2 at 50 and 100 mg L-1. A total of 37 strains were isolated, 16 of which grew in LB agar and 31 of which grew in nutritious agar.
Effects of metals on bacterial growth
The solutions with heavy metals were sterilized by using 0.45 μm pore-size sterile filters. The strains that could tolerante the highest heavy metal concentration were selected and identified. Two strains (C-13 and C-16) displayed growth at concentrations of 50 mg L-1 for all the metals. Growth was recorded after 1-3 days of incubation at 36 °C. The lowest concentration of metal that completely prevented growth was termed the minimal inhibitory concentration (MIC).
The metal susceptibility of each strain was determined using CdCl2, MnCl2, CrCl2 and PbCl2. Liquid cultures in LB medium were incubated in a shaker at 37 °C until the suspension reached an OD600 between 0.4 and 0.5. A volume of 100 μL of each strain was spread in LB medium diluted to 50% of normal strength. A volume of 5 μL of each metal with concentrations of 50 μg/mL to 300 μg/mL was spotted on the agar plates in triplicate and plates were incubated overnight at 37 °C. After overnight incubation, inhibitory or clear zones were recorded and MIC (the lowest concentration of metal ion which completely inhibited growth) determined.
Determination of Cd, Cr, Mn and Pb content in solutions
To determine the biosorption capacity of the Bacillus sp. (C13 and C16), each of the subject treatment samples were analyzed using a GBC double beam 932AA Atomic Absorption Spectrophotometer coupled with a System 3000 graphite furnace accessory, a GF3000 graphite power supply and a PAL3000 furnace auto-sampler controlled by an Intel personal computer. For background correction, a deuterium lamp was used. Pyrolytically coated graphite tubes and boosted discharge hollow cathode lamps (Photron Super Lamp) were used for Cd and Pb analysis at 228.8 and 217.0 nm, respectively. Hollow cathode lamps (Photron) for Cr and Mn, at 357.9 and 279.5, respectively, were used before and after sorption equilibrium established.
Isolation and molecular characterization
Microscopic characterization was carried out by means of the Wirtz's tension using 7.6% malachite green and 0.25% saffron, observing the presence of bacterial endospores[9]. Amplification of a 16s rDNA gene sequence was performed by PCR with the conserved eubacterial primers fD1 and rD1[8]. The reactions were performed in 30 μL volumes using the Platinum PCR Supermix High Fidelity system (Invitrogen). Amplification conditions using a Bio-Rad C1000 Thermal Cycler were: 94 °C (2 min), 30 cycles of 94 °C (30 s), 45 °C (40 s), and 72 °C (2 min), with a final 5 min chain elongation at 72 °C. The amplification products were purified using the DNA clean concentrator-5 kit (Zymo Research) according to the specifications of the manufacturer. The sequencing reactions were performed by LANGEBIO, Mexico. The obtained 16s rDNA sequences were aligned against nucleotide sequences from the GenBank[9] and the Ribosomal Database Project (RDP)[10] using the ClustalX2 method[11]. Sequencing reactions were performed by the Macrogen Korea Institute (Seoul, Republic of Korea) using the Sanger technique[12]. The 16s rDNA sequences were aligned and compared by BLAST[13] against nucleotide sequences from GenBank[14], the best results were recovered and the alignment of the sequences was performed with the ClustalW program[15]. Phylogenetic reconstruction was done with the MEGA6 program[16] using the following parameters: maximum likelihood[17] with 1000 bootstrap replicates, which shown a tree > 50% support.
Metal Biosorption in dead biomass
Two strains of Bacillus sp. (C13 and C16) were inoculated into 250 mL of LB medium in 250 mL Erlenmeyer flasks and incubated on a shaker at 150 rpm for 24 h at 36 °C. The cells were grown to exponential phase, harvested by centrifugation at 14000 rpm for 30 minutes at room temperature and washed three times with deionized water. Cells were dried in an oven at 80 °C for 12 hrs. Tests biosorption in batch system were performed with each strain in 250 mL Erlenmeyer flasks with 90 mL of a solution of Cd, Cr, Mn and Pb of 50 mg L-1 and adding 10 mL of 24 hour culture with a dead biomass concentration of 1 g L-1[18].
For biosorbent activate the two treatment conditions for metal were provided for the two strains:
Alkaline treatment: Before contact with the metal solution, 800 μL of 0.5 M NaOH was added to 50 mg of dead biomass for 5 minutes to remove possible ions and to activate functional groups of the microorganisms according to the description by[19-22].
Acid treatment: In this treatment, 800 μL of 0.1 MHClO4 was added to 50 mg of dead bacterial biomass for a contact time of 5 minutes in each experimental flask. The experiments were performed in 125 mL. For both treatments, samples were taken every 5 minutes to measure the concentration of metals adsorbed in a 1-hour period. This was continued for 3 days by taking samples every 12 hours.
Batch tests were carried out in 250 mL Erlenmeyer flasks to check the influence of starting metal concentration (50 mg L-1), in order to check the possible maximum removal of metal ions. A control assay accompanied each experiment.
At the end of each experiment, flasks were removed from the shaker and solutions were separated from the biomass by filtration through filter of 0.45 μm pore-size. 0.5 M solutions of NaOH and HCl were used to adjust pH of the medium to 10.
The results obtained in this study showed that the alkaline treatment is more effective in removing metal ions from an aqueous system. Therefore, alkaline pH of 10 was selected to be the optimum pH for all further studies.
RESULTS AND DISCUSSION
Effects of metals on bacterial growth
Activated sludge is a dynamic, multi-component system, whose properties are continually modified by microbial and chemical processes. Some metals are essential to microorganisms and therefore required by them, whereas others are toxic, even in small quantities. The measurement from the culture incubates for 15 hours were in agreement according to the growth resistance in solid media. However, when the strain C13 in media containing 50 mg L-1 Cd and Mn (panel a and d, Figure 1), showed a slightly longer lag phase that in absence of metals. The growth curve of the strain C13 in the media containing Cr and Pb follow the same grow pattern as the control, we did not observe inhibition for Cr and Pb at 50 mg L-1. On the other hand, when the strain C16 was grown in media containing 50 mg L-1 Cd (panel a, Figure 2), it followed the same growth pattern as the control. However, the growth in presence of media containing 50 mg L-1 Cr, Mn and Pb (panel b, c and d, Figure 2), the growth showed a slightly longer logarithmic phase that in absence of metals. In comparison to previous studies, the Bacillus sp. (C13 and C16) were able to grow at high concentrations of Cd, Cr, Mn and Pb in liquid media, which might be important to survival in presence of metals under natural conditions[23].
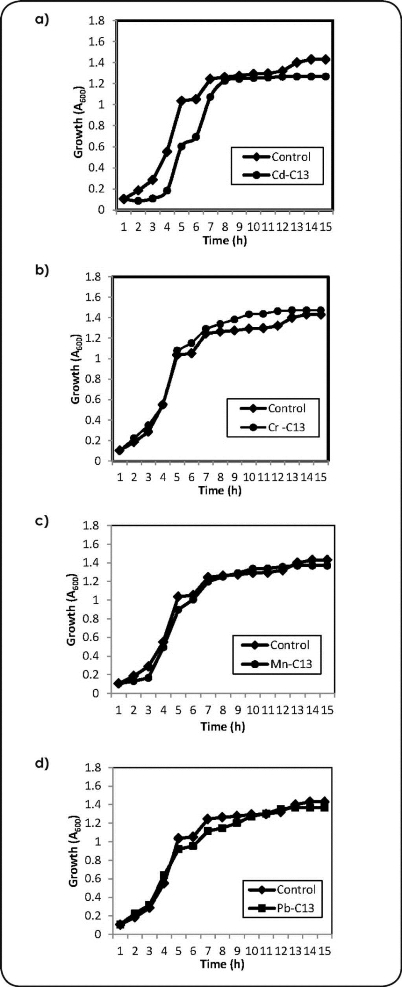
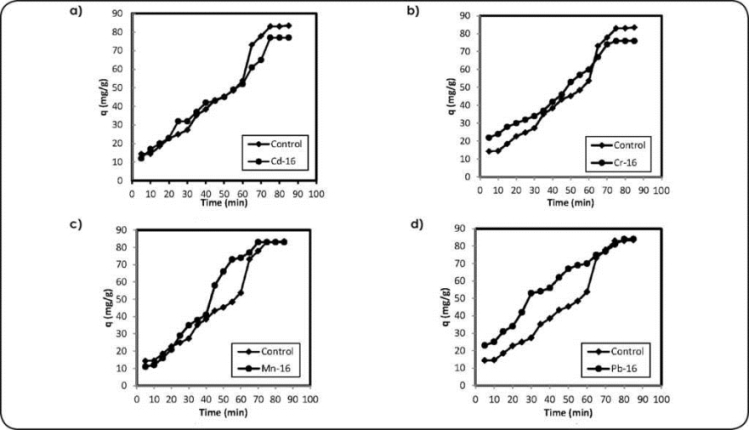
Figure 1 (a b,c and d). Effects of metals on cell growth to C13 and metal biosorption for Cd, Cr, Mn and Pb conditions: 50mg L-1 of metal concentration.
Identification and properties of isolates
To examine carbon source preferences, the isolates were grown on M9 minimal medium agar and supplemented with 0.2% of different sugars at 35 °C. As shown in Table I, two strains were able to use all the carbon sources. The bacterial strains that were utilized in the experiments described above were identified by DNA sequence analysis of 808 to 943 by fragments of 16s rDNA prepared by PCR as described in the Materials and Methods section. Comparison of those sequences using the BLAST program indicated 99% identity of the PCR products with the rDNA sequences of Bacillus sp. (Figure 3).
Table I Characterization of bacterial strains isolated from an activated sludge at a wastewater.
| Utilization of carbon source 1 | Metal resistance (mg L-1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strains | Gl | Fr | Ga | Xyl | Su | Cd | Cr | Mn | Pb |
| Bacillus sp (C13) | + | + | + | + | + | 50 | 50 | 50 | 50 |
| Bacillus sp (C16) | + | + | + | + | + | 50 | 50 | 50 | 50 |
1Carbon source used in this work were Glucose (Gl); Fructose (Fr); Galactose (Ga);Xylose (Xyl) and Sucrose (Su).
Effect of pH
The effect of the pH of a solution is a major factor that determines the biosorption property of removing metals ions from an aqueous system. The pH of solution has a significant impact on metal uptake since it determines the surface charge of adsorbent, solubility of the metals ions and the degree of ionization and speciation of adsorbate[24,25]. Cd, Cr, Mn and Pb adsorption increases linearly with in solution pH in the range of 7 to 10 as shown in Figure 4 for the isolated strain Bacillus sp. (C13 and C16). In contrast, decreases the Cd, Cr, Mn and Pb adsorption at low pH values is due to an increase in competition for adsorption sites by H+[26]. The results obtained in this study showed that the alkaline treatment is more effective in removing metals, as after 5 minutes the biosorption process removed 90% of the metals[27]. The possible reasons for this is that at pH less than 3, polymers are protonated and restrict entry of metallic ions, and at higher pH, the groups responsible for the retention of metals are negatively charged facilitating the binding of the metals ions[24,28-31]. This resulted in a more favorable electrostatic attraction capacity of Cd, Cr, Mn and Pb: a concentration of 50 mg L-1 of metal forces, and so enhances, cationic metal ion adsorption at a temperature of 27 ± 1 °C.
Effect of contact time
The contact time was evaluated as one of the important parameters affecting the biosorption efficiency. Figures 5 and 6 show the biosorption efficiency of Bacillus sp. (C13 and C16) at concentrations of 50 mg L-1 and at temperature of 27±1 °C for all the metals (Cd, Cr, Mn and Pb) as a function of contact time. The Cd>Mn>Cr>Pb uptake increases with a rise in contact time up to 80 minutes, and after that it is almost constant. The fast initial metal biosorption rate was attributed to the surface binding and the following slower sorption was attributed to the interior penetration. Different kinds of functional groups, with different affinities to metal ions, are usually present on the biomass surface. According to these results, a contact time of 120 minutes was set in order to ensure that equilibrium conditions are attained. Beyond 180 minutes equilibrium no longer exists and amount of metal adsorbed decreases with time up to 2,140 minutes and aging increases.
Isotherm and Kinetics Study
Several kinetic models are available to understand the behavior of biosorbents and also to examine the rate of the controlling mechanism of the adsorption process. The Langmuir constants b and qmax and the correlation coefficient R2 are given in Table II. The calculated value of Langmuir constant b showed that adsorption is favorable, and the linearized equation presented a good correlation of the removal capacity for Cd, Cr, Mn and Pb. Figure 7 shows the Langmuir adsorption isotherm. The plots of C/q versus Cf (mg L-1) of Bacillus sp. (C13 and C16) for Cd, Cr, Mn and Pb resulted in straight lines.
Table II Langmuir isotherm for Cd, Cr, Mn and Pb conditions: 50mg L-1 of metal concentration with alkaline treatment.
| Adsorption isotherm | C13 | C16 | ||||
|---|---|---|---|---|---|---|
| Parameters | Value | R2 | Parameters | Value | R2 | |
| qmax (mg/g) | b (mg/L) | qmax (mg/g) | b (mg/L) | |||
| Cd | 0.489 | 0.114 | 0.998 | 0.463 | 0.119 | 0.995 |
| Cr | 0.528 | 0.120 | 0.997 | 0.465 | 0.090 | 0.996 |
| Mn | 0.459 | 0.054 | 0.997 | 0.528 | 0.149 | 0.998 |
| Pb | 0.484 | 0.120 | 0.998 | 0.536 | 0.180 | 0.995 |
Langmuir Model: The Langmuir treatment is based on the assumptions that maximum adsorption corresponds to a saturated monolayer of solute molecules on the adsorbent surface, that the energy of adsorption is constant, and that there is no transmigration of adsorbate in the plane of the surface. The linear form of the Langmuir isotherm model is given by the following equation:

CONCLUSIONS
The strains showed a high capacity for the uptake of heavy metals (Cd > Cr >Pb>Mn) both in single and in mixed heavy metal solution. The biosorption process depends significantly the pH of the solution and is favored at around value of 7 to 10 and 24 hours pre-culture times. Thus, the presence of metal in the growth medium allowed the maintenance of tolerance at a level comparable with that observed in isolation. The study indicated that the dead biomass of Bacillus sp. could be used as an efficient biosorbent material for the removal of heavy metals in aqueous solutions. The Langmuir adsorption model used for the mathematical description of the biosorption equilibrium of the adsorption isotherm metallic ions onto alkaline treatment showed the best correlation (Table I) for the adsorption. Greater than 90% of adsorbed heavy metals were distributed both in cell wall and in cell membrane fractions. Therefore, this study reports a method that could be useful for bioremediation. Biosorption has received great attention during the last years, due to the potential use of microorganisms for cleaning metal-polluted water or wastewater streams. Biosorption, utilizing the ability of non-living biomass to accumulate heavy metals from wastewater, is considered as a more competitive, effective and economically attractive treatment method than bioaccumulation, because maintaining a viable biomass during the metal removal process can be rather difficult. The future prospect lies in the application of this microorganism for purposes like heavy metal remediation and potential use in extracting rare metals from dilute solution or removing toxic metals from industrial effluents. However, the application of these treatment processes is sometimes restricted, due to technological and economical constrains.











 nueva página del texto (beta)
nueva página del texto (beta)