Introduction
The brown alga Sargassum has become a very important marine resource in recent times, since global changes in the sea have generated massive arrivals on different coasts of the world, including México (Casas et al., 1990; Rodríguez et al., 2016). Brown algae are a source of alginates that have properties which allow the production of viscous solutions or the formation of gels, which can be used in different industries such as for textiles, paper, pharmaceuticals, bandages, capsules, dental impression material, immobilization of organisms and plant growth promoters, among many (Di Filippo et al., 2018).
Most of these studies about the polysaccharides from brown seaweeds in México are focused on the giant kelp Macrocystis pyrifera, because it is the most abundant and provides the highest alginate yield. Hernández et al. (2012) published a detailed process for the extraction of alginate from M. pyrifera, which was scaled up to pilot plant level. That method was considered as the control process to carry out this research. The chemical composition and biological activity of Sargassum horridum has previously been described for beds in Bahía de La Paz, Baja California Sur, the months with maximum values being found as follows: alginate yield 8.6 % in August, viscosity 73 mPa s (measured in 1 % alginate solution at 22°C with a viscometer (NDJ-1) at 60 rpm using the appropriate spindle) in September and gel strength 1906 g cm−2 in May, maintained for the rest of the months at around 800 g cm−2 (Di Filippo et al., 2018).
The hypothesis of this research is that alginate production from S. horridum can be adapted from the process used for M. pyrifera, reducing the consumption of water and reagents, and therefore reducing the production cost and increasing the alginate yield and quality.
Materials and methods
Samples of S. horridum were collected from San Juan de la Costa, in Bahía de La Paz, Baja California Sur, México, in June 2018. The algae were cut near the holdfast with a knife, at 1-2 m depth (n = 30 of 1 m2) and transported in mesh bags to the laboratory. They were washed with tap water and sun-dried. Subsequently, they were ground in a mill and sieved to homogenize the particle size to 30 mesh (0.5 mm2).
Sodium alginate production process
All the experiments were carried out in triplicate. The volumes described for the control are those used for M. pyrifera in the process described by Hernández et al. (2012), which includes the following steps: hydration, acid pre-extraction, alkaline extraction, dilution, filtration, precipitation, conversion of calcium alginate to alginic acid, conversion of alginic acid to sodium alginate, and drying.
Hydration
Ten grams of dried and ground algae were hydrated for 12 h with different volumes of 0.1 % formaldehyde solution at a ratio of 1: 9 (alga: formaldehyde) coded as H control, 1: 8 (H8), 1:6 (H6), 1:5 (H5) and 1:4 (H4). After this time, the algae were filtered, and the residual liquid was measured. Since the volume of liquid incorporated into the tissues was constant, hydration was carried out with the minimum ratio of 1: 4 for the next experiments.
Acid pre-extraction
The algae previously hydrated at 1:4 was placed in different volumes of distilled water: 1:20 control (Pre-control), 1:15 (Pre15), 1:10 (Pre10) and 1:5 (Pre5). The pH was adjusted to 4 by adding 1 N hydrochloric acid solution. The algae were maintained under constant stirring with a magnetic stirrer, for 15 min, filtered and transferred to the next stage.
Alkaline extraction
The algae, previously hydrated and treated with acid as described above, were placed in water at different ratios (alga: water): 1: 20 (Ex control), 1: 15 (Ex15) and 1: 10 (Ex10). Then, 10 % sodium carbonate solution (w/v) was added until the pH was adjusted to 10, while maintaining the beaker in a water bath at 80 °C, with an external motor stirrer (CAFRAMO®) with a three-blade propeller, at a speed of 800 rpm for 2 h.
Dilution and filtration
The alginate solution produced was diluted with distilled water to 1: 55 (Control), 1: 20 (Di20), 1: 15 (Di15) and 1: 10 (Di10), based on the initial weight of the algae, and mixed with 10 g of diatomaceous earth (Celite®) as a filtration aid. The mixture was then filtered with a layer of diatomaceous earth over a filter paper, contained in a Buchner funnel under vacuum.
Precipitation and drying (laboratory level method)
The clarified alginate solution was precipitated with alcohol in a proportion of 1: 2 to assure that all the alginate in solution was precipitated. Finally, the alginate fibres produced were oven-dried at 55 °C for 12 h and placed in a desiccator to avoid moisture. The percentage of alginate was calculated based on the initial weight of the alga: R = (dry weight of alginate/dry weight of alga) × 100. At industrial level, the alginate is not precipitated with alcohol, because of the high cost, so it is precipitated as calcium alginate and then converted to alginic acid and finally to sodium alginate. The next experiments followed those industrial steps.
Quality control of the alginate obtained
Viscosity
The viscosity of the samples was obtained by preparing a 1 % alginate solution and measuring the viscosity with a viscometer NDJ-1, measuring at 60 rpm and 22 °C, with the appropriate needle (1-4), before and after adding sodium hexametaphosphate which sequesters any residual calcium (Hernández et al., 1999).
Gel strength
The gel strength of alginate was measured by preparing a 1 % alginate solution in distilled water and filling a dialysis membrane tube (5 cm in diameter × 2.5 cm in height); the tubes were immersed in a 10 % calcium chloride solution for 24 h to form the gels. After that time, the gel strength was measured with a texture analyser (TA.XT Plus), programmed to carry out a penetration of 2 cm at a speed rate of 5 s (breaking point) (Camacho & Hernández, 2012).
Precipitation to calcium alginate, alginate bleaching, and conversion to sodium alginate
A new batch of 10 g of alga was processed until the filtration stage. The clarified alginate solution, instead of being precipitated with alcohol, was precipitated with 37.5 mL of 10 % calcium chloride for every 10 g of dry alga used. The resulting alginate fibres were immersed in 20 mL of water, adding 5 % sodium hypochlorite (commercial chlorine) at different volumes: 0.0 (control, T1), 0.5 (T2), 1.0 (T3), 2.0 (T4) and 3.0 mL (T5). The bleached alginate fibres were subjected to three acid washes for conversion to alginic acid as follows: the fibres were suspended in 20 parts of water for one part of algae; the pH was adjusted to 2 with 1 N hydrochloric acid solution and stirred for 15 min. The sample was drained off and the acid washing was repeated twice, adjusting the pH to 1.8 each time. Sodium alginate was obtained by adding 75 mL of water and 75 mL of alcohol and neutralizing with sodium carbonate until adjusting to pH 10, with constant agitation (Arvizu et al., 2002).
Alginate color
The color of a 1 % alginate solution was measured using a UV Visible Spectrophotometer (Thermo Scientific™ GENESYS 10S) at a wavelength of 510 nm (as a percentage of absorbance). Color was compared with commercial sodium alginate (71238 SigmaTM and AlgimarTM) and with the Munsell charts (2009), to assign a name to the colors.
Alginate purity
One gram of calcium chloride was dissolved in 100 mL of methanol-water solution (40-60 %). The resulting solution was added to 100 mL of 0.5 % alginate solution, while gently stirring. The precipitate was removed using a fine filter and washed with methanol-water solution (20-80 %). A second washing was carried out with methanol water solution (40-60 %). The precipitated alginate was dried in an oven at 105oC for 2 h. The alginate was maintained in a desiccator for one hour and weighed. The alginate content (purity) was computed as: (weight of the precipitated / weight of the initial alginate on dry basis * 100) (Reyes, 2005).
Modification of the optimized control process with minimum water consumption (TCont), changing the pre-extraction acid treatment for a neutral pre-cooking stage (PCS)
To avoid the use of hydrochloric acid in the process and reduce the contamination from wastewater, experiments were performed with substitution of the acid pre-extraction by a neutral pre-cooking stage. The algae were treated in a water bath at 75 °C for 2 h, with constant agitation with an external motor (CAFRAMO®) and three-bladed propellers at 500 rpm. The algae were subjected to the following stages: hydration: H4 (1 : 4 alga : water); neutral pre-cooking: 1 : 7; extraction: Ext10 (1 : 10); dilution: Di10 (1 : 10); and filtration.
Statistical analysis
Analysis of variance (ANOVA with α = 0.05) was used to test the best process conditions, based on the alginate yield, viscosity (1 % solution) and gel strength, obtained in each experiment after modifying the consumption of water in each of the stage (Zar, 1984), included in the program STATISTICA version 7.0 (2004).
Results and discussion
Hydration, acid pre-extraction, alkaline extraction, dilution and filtration treatments
The statistical analysis showed no significant difference (p < 0.05) among any of the treatments for the first four steps. When varying the hydration volume, down to the proportion 1 : 4 (alga : 0.1 % formaldehyde), the alginate yield (average 8.3 %), viscosity (average 89.3 mPa s) and gel strength (average 1611 g cm−2) were maintained without significant changes (Figure 1); therefore, the minimum amount for algae rehydration is one part of alga with four parts of formaldehyde solution. With this change, the formaldehyde solution, was reduced by 55 %.

Figure 1 Effect of hydration volume with 0.1 % formaldehyde on the yield (%), viscosity (mPa s) (measuring to 60 rpm) and gel strength (g cm−2). Hcont: control (1: 9 alga: water), H10: 1: 10, H8: 1: 8, H6: 1: 6, H5: 1: 5, H4: 1: 4
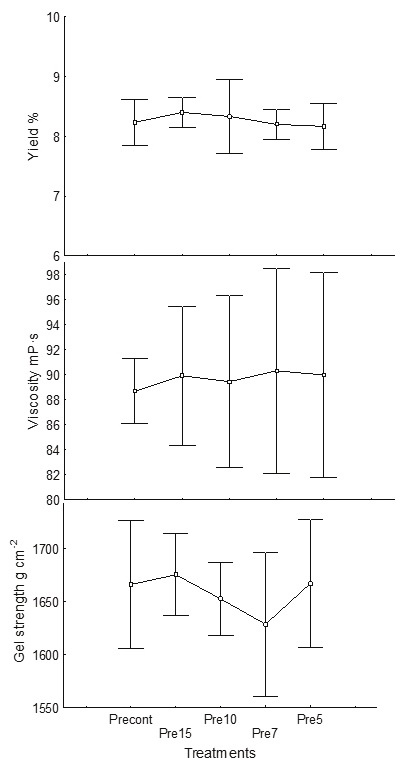
No significant effect of reducing the volume of water down to the proportion 1 : 4 (alga : water) was found during the acid treatment, with no significant difference (p < 0.05) found for the yield 8-8.6 %, viscosity 89.6 mPa s or gel strength 1641-1668 g cm−2 (Figure 2). Hernández et al. (2012) proposed that for M. pyrifera, one part of dry algae requires up to nine parts of water (1: 9); however, for S. horridum, one part of algae only requires four parts of water (1: 4). This can be explained because the cell wall does not allow excessive water entry; for this reason, even if more than four parts of water were used, Sargassum does not absorb any more. Haug (1964) suggested that formaldehyde is necessary to fix phenolic compounds that are in the cell wall, otherwise a dark brown alginate is produced. This reduction also reduces water contamination since less formalin is used at this stage. With the proposed rehydration volume, a saving of 55 % of water was achieved (Table 3).
Acid pre-extraction
In this stage, ion exchange of Ca2+, Mg2+ and K1+ occurs, and the phenolic compounds are eliminated. The reaction with the acid allows the alginate mixture to transform into alginic acid and prepares the algae for the alkaline extraction to facilitate the removal of soluble sodium alginate. Using one part of algae to five parts of water, a saving of 50 % is obtained. Also, 44 % of hydrochloric acid was saved and no significant differences in alginate yield, viscosity or gel strength were found.
Alkaline extraction
In the alkaline extraction step, it was possible to reduce the water used from 1: 15 to 1: 10 without significant differences (p < 0.05), maintaining the average yield as 8.1-8.5 %, viscosity as 83.2 mPa s and gel strength as 1638-1664 g cm−2 (Figure 3). Also, during this step, the amount of sodium carbonate was reduced by 22 %.

Figure 3 Effect of volume of water in the alkaline extraction step on yield (%), viscosity (mPa s) (measured at 60 rpm) and gel strength (g cm−2). Excont: control (1: 20 alga: water), Ex15: (1: 15), Ex10: (1: 10)
Before this stage, the algae do not yet show any type of change in color or shape, only the cell wall has been hydrated and is swelled. For the extraction step, the volume can be reduced to 10 parts of water for one part of dried seaweed since this species does not produce high viscosity and it is still possible to stir the solution at that viscosity. The water reduction represents a saving of 37 %. Reduction of the water volume allows a saving of 22 % of sodium carbonate. The extraction parameters proposed by Hernández et al. (2012) were maintained, which consisted of increasing the pH to 10, temperature of 80 °C and a time of 120 min. No significant differences in viscosity or gel strength of alginate were obtained by reducing the volume of water used. At the end of this treatment, the viscosity of the paste was 300-800 mPa s.
During dilution, the water was reduced from 1 : 20 to 1 : 10, maintaining (p < 0.05) the yield (8-8.6 %), viscosity (90.5 mPa s) and gel strength (1665-1678 g cm−2) (Figure 4); this volume saves 72 % of water in this step. The experimental dilutions did not present any significant difference in the parameters evaluated. Total water saving at this stage was 72 %. This is the step in which more water is saved since the viscosity that occurs after extraction is not as high as when M. pyrifera is processed. With S. horridum, the cell wall contains less alginate (8.6 %) (Di Filippo et al., 2018) and the alginate produced is of lower viscosity. Therefore, to carry out the filtration, a smaller volume of water is needed for filtration (1: 10). The total water saving in the whole process with the modifications described above was 62.22 % (Table 1).

Figure 4 Effect of volume of water in the dilution step on yield (%), viscosity (mPa s) (measuring to 60 rpm) and gel strength (g cm−2). Dicont: control (1: 55 alga: water), Di20: (1: 20), Di5: (1: 15), Di10: (1: 10)
Table 1 Comparison of the water used in the control process of Hernández et al. (2012), with Macrocystis pyrifera and the process using less water, calculated to process 1 kg of dried seaweed Sargassum horridum
| Step | Control process | New process | ||
| Proportion | Volume of water (L) |
Proportion | Volume of water (L) |
|
| Hydration | 1 : 10 | 9 | 1 : 4 | 4 |
| Acid pre-extraction | 1 :10 | 10 | 1 : 5 | 5 |
| Alkaline extraction | 1 : 16 | 16 | 1 : 10 | 10 |
| Dilution | 1 : 55 | 55 | 1 : 15 | 15 |
| Total water used | 90 | 34 | ||
Comparison of the control (Tcont) vs the modified process using less water and changing the acid pre-extraction for neutral pre-cooking (Tnew)
The average alginate yield was significantly higher (p > 0.05) in the new process (Tnew, 13.5 %) than in the control treatment (Tcont, 8.3 %) (Figure 5).

Figure 5 Comparison of yield in the control process (Tcont) and in the process using less water, changing the acid pre-extraction for neutral pre-cooking (Tnew)
For viscosity, there was no significant difference between the two processes; for Tnew on average it was 86.87 mPa s while with Tcont it was on average 88.67 mPa s (Figure 6).

Figure 6 Viscosity in the control process (Tcont) and in the process using less water, changing the acid pre-extraction for neutral pre-cooking (Tnew)
The gel strength was significantly (p > 0.05) higher in the new process (Tnew 2442 g cm−2) than the control (Tcont 1666 g cm−2) because the optimized extraction conditions generate less depolymeration of the alginates, that is, the bonds are not broken and the polymer chains remain long, thus producing more rigid gels (Figure 7).
Color
Bleaching of the alginate with sodium hypochlorite (NaClO) resulted in a significant change in coloration, based on the Munsell charts (2009), for different concentrations of NaClO (Table 2). Sodium alginate Sigma® was used as a control, since it has the lowest absorbance value (0.03) at a wavelength of 510 nm and is a low viscosity product. The treatments with sodium hypochlorite did not affect the alginate viscosity which was in the range 80-90 mPa s (Figure 8); the gel strength was not affected either, being maintained at high values of 2400 g cm−2 (Figure 9).
Table 2 Color characteristics of alginates by absorbance and comparison with Munsell charts
| Alginate solution (1 %) |
Absorbance (510 nm) |
Munsell charts | ||
| Hue | Color | |||
| Treatment with 5 % NaClO (mL) | ||||
| T1 | 0.0 | 2.609 | 7.5 YR (2.5/3) | Very dark brown |
| T2 | 0.5 | 1.499 | 5 YR (4/4) | Reddish brown |
| T3 | 1.0 | 0.849 | 5 YR (5/8) | Yellowish red |
| T4 | 2.0 | 0.232 | 10 YR (6/6) | Yellowish brown |
| T5 | 3.0 | 0.196 | 2.5 Y (7/4) | Pale brown |
| AlgCICIMAR® | 0.068 | 10 YR (8/4) | Very pale brown | |
| Sigma® | 0.03 | 2.5 Y (8/1) | Transparent white | |

Figure 8 Comparison of the viscosity of alginates for different treatments with sodium hypochlorite in the bleaching stage, the measurements were made with a viscometer NDJ-1 at 60 rpm, at a constant deformation gradient
Contrasting the control process (Tcont) with the modified process using less water and changing the acid pre-extraction for neutral pre-cooking (Tnew)
After verifying that reducing the volume of water in all the alginate extraction stages did not affect the rheological properties, experiments were conducted to change the acid pre-extraction stage for a neutral pre-cooking stage in which the algae were kept in a water bath at 75 °C, under constant agitation with an external motor at 500 rpm for 2 h.
The change in this stage had beneficial results. Neutral washing or neutral pre-cooking eliminates more phenolic compounds; this was seen in the wastewater, since it had a darker brown color and higher viscosity, compared to the process with acid. This washing step was carried out with seven parts of water for one part of seaweed.
Yield
The results were beneficial using this new process, increasing the yield from 8.3 % to 13.5 % (62 %). This yield was higher than that reported by Di Filippo et al. (2018) and the maximum yield of 13.7 % reported by Rodríguez et al. (2008) for S. sinicola.
The yield in the present study with the pre-cooking modification had a maximum of 13.5 %; however, it was lower than for other species such as S. ilicifolium (23 %), S. carpophyllum (32 %), S. siliquosum (41 %) (Ragaza & Hurtado, 1999), S. tenerrimum (18.32 %) (Redekar & Raje, 2000), S. oligocystum (20.5 %), Sargassum sp. (20.9 %) and S. filipendula (17 %) (Camacho & Hernández, 2012). The yields obtained in this study with the modification of neutral pre-cooking are higher than those reported for Dictyota caribaea (7.4 %), Padina perindusiata (5.4 %) (García et al., 2012) and those for S. horridum that were carried out in the same season of the year by Di Filippo et al. (2018).
Neutral pre-cooking helps to soften the cell wall, giving the alginate a better chance of being successfully extracted; it also improves the color since at this stage many phenolic compounds are removed, and the final alginate is lighter than that produced with acid pre-extraction.
Viscosity
For the process with less water and neutral pre-cooking, a normal low viscosity was maintained, ranging between 82 and 89 mPa s. This result is similar to those reported for Sargassum by Rodríguez et al. (2008) who obtained viscosity of 41-191 mPa s, and higher than the values reported by Di Filippo et al. (2018) of 38 to 73 mPa s, and by Camacho and Hernández (2012) of 12 to 21 mPa s. Although the yields were different, the viscosity were not significant different among treatments. This suggest that, under the extraction parameters, the alginate chain remain with approximated the same length and therefore the pre-cooking has no significant effect on the viscosity.
The viscosity of alginates used in the food and pharmaceutical industries is variable, and there is some demand for alginates with low viscosity, for example, as a stabilizer for bottled beverages, for gelatinizing, thickening, deflocculating and in cream ice creams, among other products. In the pharmaceutical industry, alginates are used to stabilize cosmetics such as face masks, and products that remove heavy metals from the human body. Therefore, use could be found for S. horridum alginates with varying viscosity, according to the industry needs.
Gel strength
The process using less water and neutral pre-cooking showed a significant positive effect on gel strength, with high values (2416 to 2472 g cm−2) exceeding all previous reports, including that of Di Filippo et al. (2018) who reported a minimum in June (815 g cm−2) and maximum in May (1906 g cm−2). Lower values (709 to 866 g cm−2) were reported by Camacho and & Hernández (2012). Draget et al. (2002) suggested that the alginate from S. sinicola has better stability than M. pyrifera alginate, because of the higher quantity of the guluronic acid units and biaxial bonds (GG) of the polymer chain. The increased gel strength, compared to the control process, may be due that cooking process preserved the length of the guluronic acid chains, which are related to the production of stronger gels (Murillo & Hernández, 2007).
Alginates with a high gel strength (above 750 g cm−2) have a wide range of industrial uses. Murillo & Hernández (2007) showed that GG blocks predominate in the alginate from Sargassum; for this reason, the gels produced have greater strength. An example of use is in microcapsules, used for the slow release of products, which need a high gel strength to maintain the stability of beneficial bacteria, and thus improving the growth of land plants (Yabur et al, 2007).
Color
The color that best suits the industry is that of alginates produced from treatment with 3 mL of sodium hypochlorite. According to Munsell (2009), the color obtained is classified as pale brown; although it is not as white as the alginate from M. pyrifera, it is very close.
Conclusions
The optimized method to process the alga Sargassum to obtain sodium alginate includes the following steps and suggested proportions of alga: water: hydration: 1: 4; neutral pre-cooking: 1: 7 (75 °C, 2 h); extraction: 1: 10 (pH to 10, 80 °C, 120 min); dilution: 1: 10; and vacuum filtration. Alginate precipitation uses 37.5 mL of 10 % calcium chloride for every 10 g of dry alga. Calcium alginate fibres are bleached with 3 mL sodium hypochlorite per 10 g of alga. Conversion to alginic acid uses three washes of 10 % hydrochloric acid at a ratio of 1: 10 until pH 2; the second and third washes use a proportion of 1: 5, adjusting the pH to 1.8 with constant stirring for 15 min. Neutralization to sodium alginate is carried out in 1: 1 alcohol: water and the pH is adjusted to 10 with sodium carbonate. Finally, alginate fibres are pressed, dried, and milled to dust.
Reducing the volume of water in all the alginate extraction steps does not affect the rheological properties. This new process increases the yield from 8.3 % to 13.5 % (62 %). Neutral pre-cooking helps to soften the cell wall, and the alginate is better extracted; it also improves the colour, since at this stage some phenolic compounds are removed, and the final alginate is lighter than when using the acid pre-extraction.











 nueva página del texto (beta)
nueva página del texto (beta)