Introduction
Corrosion is an aggressive effect over material performance and degradation under working environments, which is a natural inevitable process. Corrosion is a severe problem that affects several industry fields, such as infrastructure, transportation, utilities, production and manufacturing, as it causes premature failure of metallic components (Koch, 2002); typically for ferrous metals, manifesting as unsightly red rust or paint/coating blistering. The estimated worldwide losses due corrosion exceed $1.8 trillion, annually (Schmitt et al., 2009).
One of the most common means to reduce corrosion is through the application of organic coatings (Akinci & Yilmaz, 2011; Bierwagen, 1998; Huai et al., 2011; Mathivanan & Radhakrishna, 1998). Coatings are generally exposed to different environments and working conditions, hence they may require a set of properties such as adhesion, high hardness, high toughness, high corrosion and oxidation resistance, among others. Recently, nanoparticles have been incorporated into matrices in order to improve corrosion protection, barrier properties, filling pores and voids in the matrix, and increase overall mechanical properties, including wear resistance (Abdullayev et al., 2013; Barna et al., 2005; Guin et al., 2015; Gusev et al., 1998; Lam & Lau, 2006; Li et al., 2013; Sababi et al., 2014; Wang & Zhang, 2010a).
Regarding corrosion performance, Hamdy and Butt (2007) were able to successfully evaluate the effect of chromate conversion coatings for the corrosion protection of aluminum alloys. It was observed that vanadia treatments improve the corrosion resistance due to the formation of highly protective vanadium oxides, as Vanadium combines through the pores of Al-oxide layer to form thick Al-V-oxide layer. Due to the buffering action of vanadium, it plays an important role to reject the chloride ions from the material surface. Feng et al. (Wang et al., 2010c) proposed nano-Al2O3 particles incorporation to composite coatings in order to improve the corrosion and oxidation resistance. Moreover, Montemor (2014) reviewed the most recent self-healing and smart coating alternatives for enhanced corrosion protection. Poor adhesion between substrate and coating may cause spalling and delamination of the coating, which can be largely controlled by relieving residual stresses from the coatings (Harish et al., 2010). For instance, epoxy coatings containing 1.0 wt.% of Zn, SiO2, Fe2O3, and halloysite clay nanoparticles have been applied to steel substrates, reducing the corrosion rate by 11 to 910 times after immersion in a 3 % NaCl solution for over 28 days (Shi et al., 2009). Alkyl-based waterborne coatings filled with ZnO improved abrasion and scratch resistance, and showed little changes on the surface topography after salt-spray evaluations (Dhoke et al., 2009). Furthermore, a study varying the nano-TiO2 particle size (10-150 nm) demonstrated that smaller nanoparticles increase corrosion inhibition efficiency and lower permeability to corrosion agents (Deyab & Keera, 2014). Similarly, other investigations have combined diverse particle additives achieving a synergistic effect (Lu et al., 2016).
Wear resistance of polymer coatings has also been improved with nanoparticle reinforcements, as has been observed by several authors (Bahadur et al., 1994; Cho & Bahadur, 2005; Jia et al., 2006; Wang et al., 2003; Wang et al., 2010b; Xian et al., 2006). It has been found that physical characteristics are not significantly affected; due to the particles small size, nanoparticles do not affect the transparency or the gloss of coatings. Furthermore, nanocoatings can thus be used to maintain the surface appearance and durability as well. Polymer coatings form transfer films of metal counterfaces upon friction, this effect is enhanced with nanoparticle additives (Friedrich & Schlarb, 2011). For example, Barna et al. (2005) investigated the incorporation of diverse oxide-based nanostructures to improve hardness and mechanical performance of coatings, thereby improving their wear and scratch resistance.
A synergistic effect has been observed when different nanoparticles have been combined (Jia et al., 2006; Wang et al., 2010b; Xian et al., 2006). Wang et al. (2003) for instance, reported a synergistic action in the tribological behavior of polyamide composites made with carbon fiber and micro-size MoS2 filler. The aim of our study is to develop a nanocomposite for corrosion and wear protection of steel substrates, with an epoxy vinyl ester coating and nanoparticles reinforcements; varying filler fractions and mixtures of Zn and TiO2 nanostructures, and determine the presence of a synergistic corrosion and wear protection effect.
Experimental details
Materials
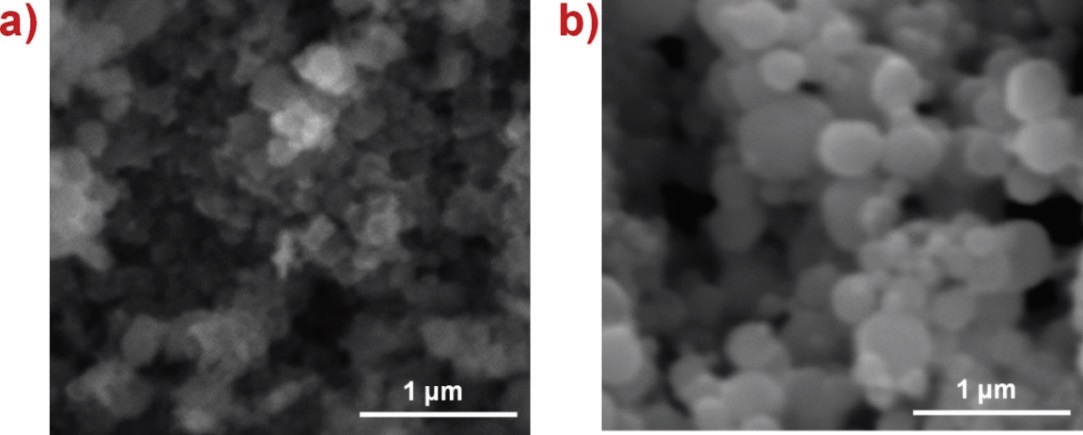
The epoxy vinyl ester resin Derakane Momentum 411-350, Cobalt Naphthenate (Cobalt 6 %), and Methylethylketone Peroxide (MEKP) NOROX MEKP-925H catalyst were obtained from Ashland. Nanoparticles of Zn ( < 50nm, spherical) and TiO2 ( < 21nm, spherical) were obtained from Sigma Aldrich. Scanning electron micrographs of these nanoparticles are shown in Figure 1.
Polymer nanocomposite coatings preparation
Steel substrates of an AISI 1045 steel measuring 150 mm by 100 mm with 2.0 mm in thickness were sanded and washed with acetone to remove any rust and dirt from the surface. Next, surfaces were sanded with 220-grit sandpaper to an Ra of 0.48 µm. Nanocomposite coatings at various concentrations and combinations of Zn and TiO2 nanoparticles within an epoxy vinyl ester resin were prepared as shown in Table 1. This resin is widely used for coatings and as a matrix for composite materials. In order to break large agglomerates, nanoparticles were first dispersed in the neat resin using an Ultra Turraz T10 homogenizer for 5 min, followed by 2 min sonication with a Cole-Parmer 750-Watt ultrasonic homogenizer at room temperature (25 °C). Then, 0.05 parts per hundred (phr) of Cobalt Napthenate 6 % and 1.00 phr of methyl ethyl ketone peroxide (MEKP) catalyst were added to the resin mixtures in order to harden the composite. Curing time of resin was 30 ± 10 min at 35 °C. Lastly, polymer nanocoatings were uniformly applied with a brush and allowed to cure for 24 h. The thickness of coatings was 150 µm, measured by an Elcometer 456 coating thickness gauge.
Salt spray corrosion test
Coated samples were scribed per ASTM 1654, and placed in an automatic salt chamber at a 60 ° angle using a Harshaw/Filtrol salt spray cabinet for 480 h. Five samples were prepared and teste for each nanocomposite coating, according the Dixon statistical methodology (Dean & Dixon, 1951; Rorabacher, 1991). Tests were run according to ASTM B-117. The tests were conducted at 35 °C with a spray of 5 % NaCl solution, pH of 6.5 - 7.2, and 96 % relative humidity. Samples were scanned and analyzed according to ASTM D-7087 and monitored after 96, 168, 288, 336, 408, and 480 h., respectively, measuring the increase in corroded area from the scribe mark.
Tribological evaluation
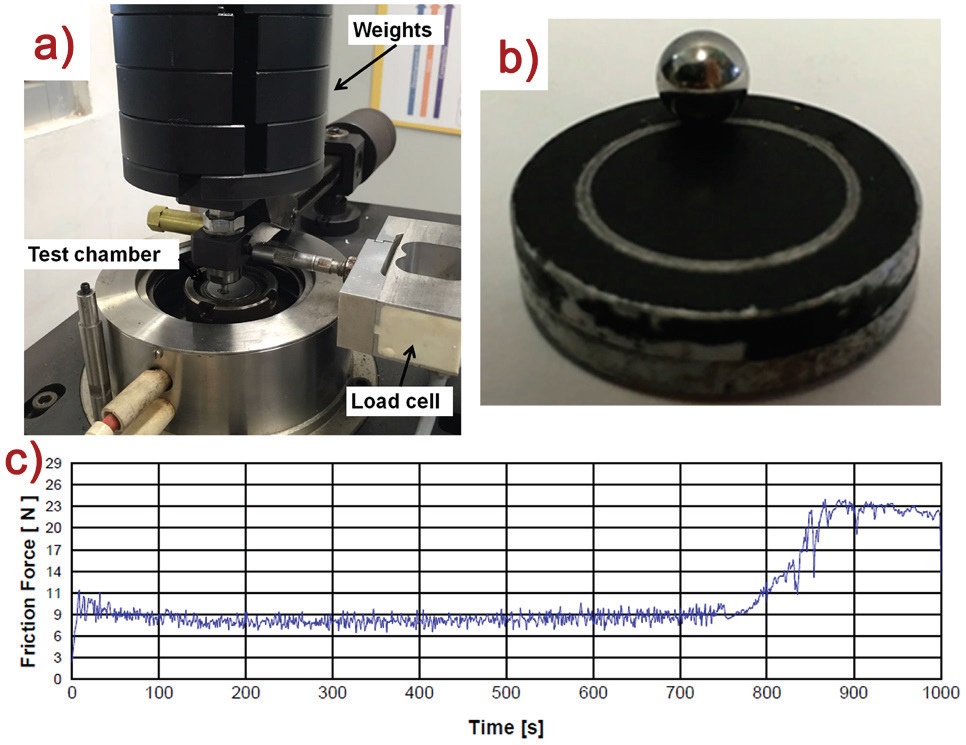
Tribological properties of nanocomposite coatings were determined with a ball-on-disk tribotester (Figure 2a), under ASTM G-99, to determine the wear resistance with an accelerated test under point contact conditions. Here, the coatings are subjected to a load of 25 N, speed of 238 rpm, over a total distance of 100 m and wear track diameter of 16 mm (Figure 2b). With this method an increase in the friction force is observed when the coating layer breaks, and thus the duration of the coating is obtained. Higher duration and wear resistance of coatings is beneficial for delaying corrosion initiation. Figure 2c shows the friction force through time; it could be seen that at 750 s, the coating layer is broken, and friction force increases.
Results and discussion
Corrosion results
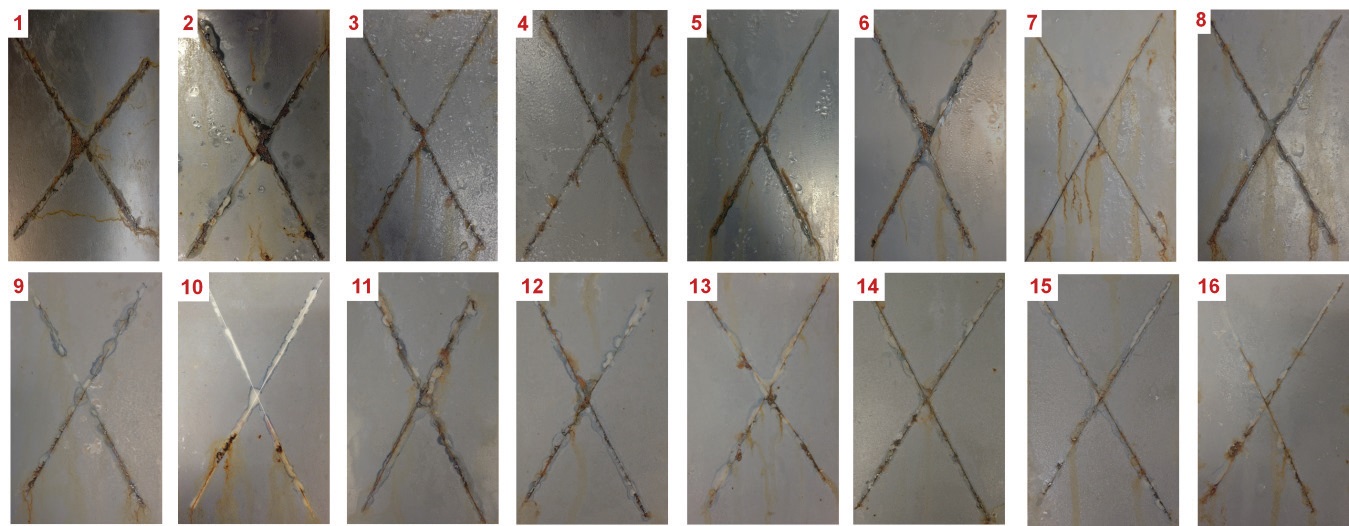
Figure 3 shows the general appearance of scribed coated steel surfaces prior to the salt spray test. Images of samples after exposure for 96 h, and 480 h are shown in Figure 4 and Figure 5, respectively.
As expected, the highest degree of corrosion is found for the neat resin specimen (sample 1). Corrosion products are found around the scribes for all samples; even at 96 h, as shown in Figure 4. In general, in both images, Figure 4 and Figure 5, the least amount of rust is found on the surfaces of samples combining different proportions of mixtures of Zn and TiO2 nanoparticles, compared to samples with only one type of nanoparticle, this was observed qualitatively.
Figure 6 and Figure 7 depict the growth of the corroded area with respect to the scribed mark obtained through image analysis performed with 2D CAD system, in accordance to ASTM D-7087. Images were taken and analyzed at 96, 168, 288, 336, 408, and 480 h, and compared to the neat resin coating. The epoxy vinyl ester resin presented a rapid increase in corroded area (150 %) after 96 h of exposure, after which the increase was lower. At 480 h the neat resin had an increase of 200 %, whereas all nanoparticle concentrations and mixtures showed better corrosion protection. In Figure 6 results showed an increasing corrosion protection rate up to 0.5wt.%, after which a reverse trend was present, most likely due to filler agglomeration, particularly for TiO2, as has been observed from previous research and work in our group (Peña et al., 2014, 2015, 2016). In general, this nanoparticle showed better performance compared to Zn. For instance, at 0.1 and 0.5 wt. % TiO2 (Figure 6a) had a lower increase of corroded area at 480 h; 115 % and 44 %, respectively. This could be attributed to increased barrier properties against oxygen and water (Chen et al., 2006; Deyab & Keera, 2014), and due to filling the coatings’ pores and voids (Moazeni et al., 2013). The observed increase at 1.0 wt. % TiO2 after 288 hrs under corrosion evaluation could be attributed to certain tendency of the nanoparticles to agglomerate, due to filler fraction, and form clusters, which diminished the protection in the coating. Zn nanoparticles (Figure 6b) showed a rapid increase on corroded area after 96 h, similar to the neat resin, and an increase on area failed from scribe of ~200 %, 107 %, and 116 % at 0.1, 0.5, and 1.0 wt. %, respectively at the end of the test.
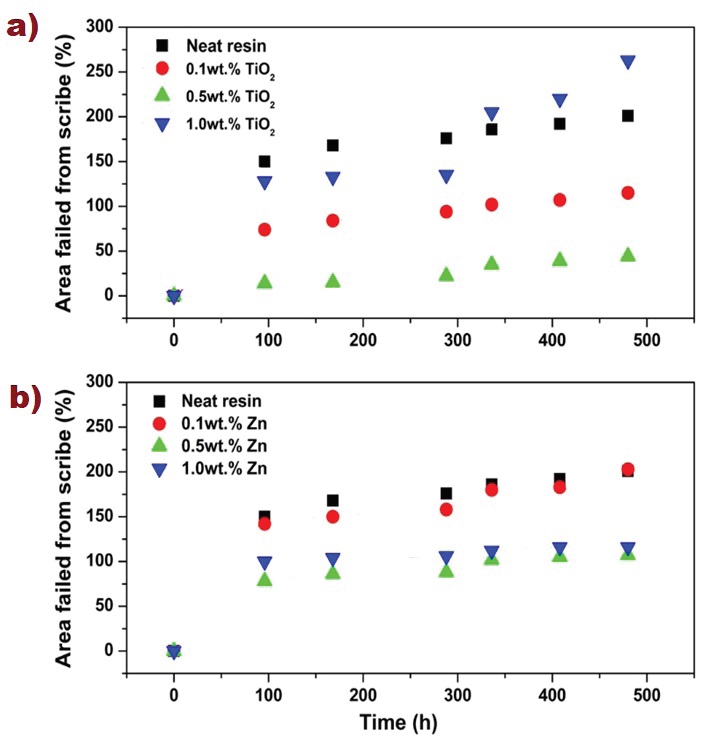
Figure 6: a) Comparison of the area failed from scribe versus time for TiO2 nanocomposites, b) Zn nanocomposite coatings
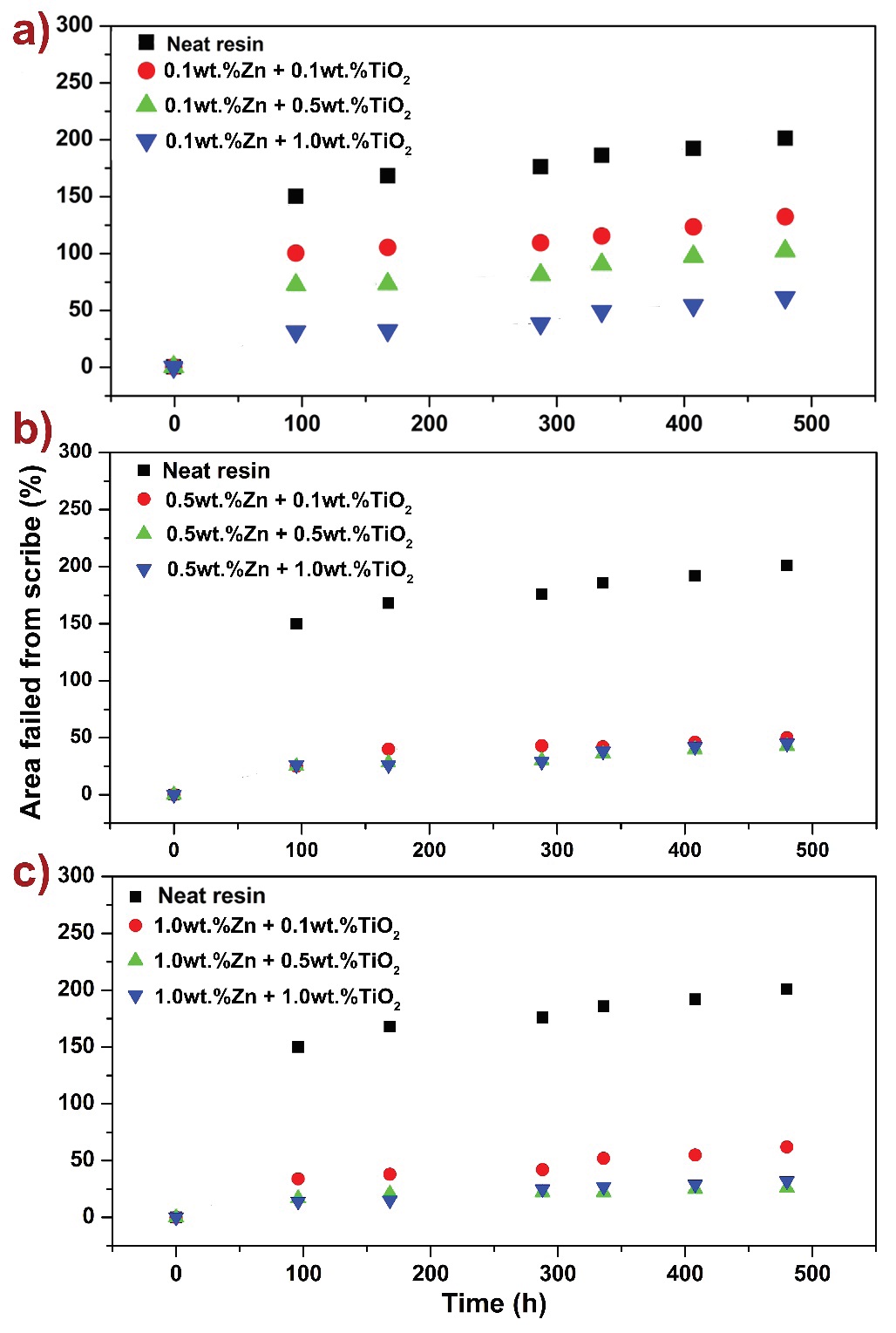
Figure 7: a) Comparison of the area failed from scribe versus time for 0.1 wt. % Zn nanocomposite coatings with varying TiO2 concentration, b) comparison of the area failed from scribe versus time for 0.5 wt. % Zn nanocomposite coatings with varying TiO2 concentration, c) comparison of the area failed from scribe versus time for 1.0 wt. % Zn nanocomposite coatings with varying TiO2 concentration
A synergistic effect was observed when both Zn and TiO2 nanoparticles were incorporated into the epoxy vinyl ester resin (Figure 7). At a fixed concentration of 0.10 wt. % Zn (Figure 7a) the corroded area was lower with higher TiO2 content; 31 % and 61 %, at 96 h and 480 h, respectively. An increased corrosion protection was found at a fixed 0.5 wt.% concentration of Zn (Figure 7b), where increase of ~ 26 % where found at 96 h exposure, and up to ~ 50 % increase was observed at 480 h, with increasing TiO2 filler fraction. Similar trends are observed at 1.0 wt. % Zn nanocomposite (Figure 7c); here the lowest corroded area was found with 0.5 wt. % TiO2, starting at 14 % and ending at 26 %. Thus, the best protection was observed for coatings with mixtures of 1.0 wt. % Zn and 0.5 wt. % TiO2.
These results can be attributed to the combined effect of TiO2 and Zn enhancing barrier properties and lowering of different sizes of pores and voids volumes on polymer coatings. Therefore, this shows that the addition and combination of Zn and TiO2 resulted in a synergistic effect, taking advantage of both nanoparticles’ corrosion reducing mechanisms.
Tribological results
Ball-on-disk tribotester was used for evaluations of tribological performance. Figure 8 depicts the synergistic effect of nanoparticle filler fraction and the coating duration under a constant load of 25 N. This result is determined when the coating breaks and the friction force increases, as shown in Figure 2c. Figure 8a shows that duration of the coating is improved with Zn and TiO2 nanoparticles with up to 0.5 wt. % up to 211 s, compared to 43 s for the unfilled polymer coating. When both nanoparticles are combined (Figure 8b) the duration increases up to 1000 s, with an improvement greater than 2000 %, compared to the pure epoxy vinyl ester polymer resin.
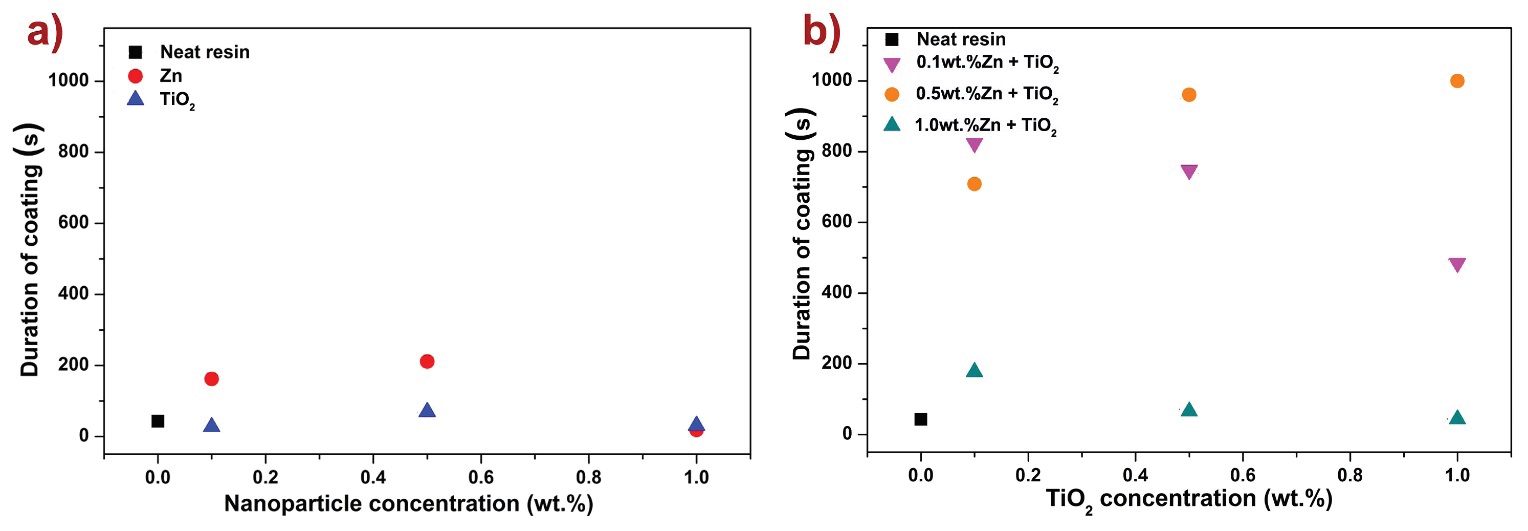
Figure 8: a) Duration of polymer nanocomposite coatings with varying concentrations of Zn and TiO2 nanoparticles, compared to the neat resin, b) duration of polymer nanocomposite coatings of 0.1, 0.5, and 1.0 wt. % Zn with varying concentrations of TiO2 nanoparticles, compared to the neat resin
Figure 9 shows the depth wear rate in µm/s obtained taking the displacement of the tribosystem that occurs in the z-axis (perpendicular to the disk surface) measured by the equipment and dividing it by the duration of the coating. For instance, in Figure 9a both Zn and TiO2 nanocomposites showed a decrease in depth wear rate compared to the neat resin with concentrations of 0.1 wt. % and 0.5 wt. %. The lowest wear rate, with a reduction of 89 %, is found for 0.5 wt. % Zn %. These enhancements can be explained as follows: it has been proposed that during the wear process nanoparticles could be slightly detached from the polymer matrix and provide a rolling bearing effect, enhancing the protection to the polymer nanocomposite coating (Friedrich & Schlarb, 2011). Furthermore, hardness of polymer nanocomposites increases with nanoparticle content, also lowering wear (Chen et al., 2006; Lingaraju et al., 2011; Zhi et al., 2001). However, at higher concentrations of Zn and TiO2 nanocomposites (1.0 wt. %) the wear rate increases, hence, the time for oxide to appear (layer duration). This may be due to nanoparticle agglomeration in the matrix. In Figure 9b, where 0.1 wt. %, 0.5 wt. %, and 1.0 wt. % Zn nanocomposite coatings were prepared with varying concentrations of TiO2; it was observed that all combinations decreased depth wear rate demonstrating a synergistic effect between the selected nanoparticles with improvements up to 95 %. Consistent with Wang´s study (Wang et al., 2010b), it is proposed that the small sized TiO2 aid in the filling of pores and voids of the matrix providing a load-bearing effect, whilst Zn nanoparticles provide a rolling-bearing effect, thus further reducing wear rate of polymer nanocomposites
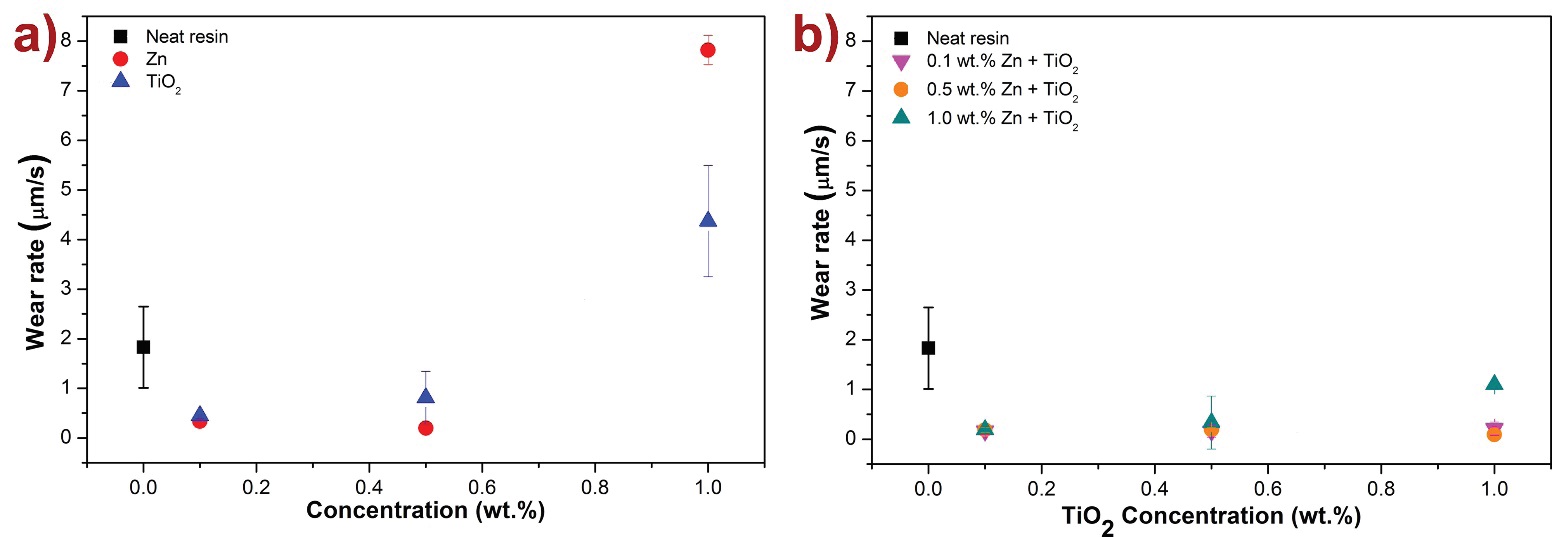
Figure 9: a) Depth wear rate of polymer nanocomposite coatings with varying concentrations of Zn and TiO2 nanoparticles, compared to the neat resin, b) depth wear rate of polymer nanocomposite coatings of 0.1, 0.5, and 1.0 wt. % Zn with varying concentrations of TiO2 nanoparticles. Lower wear rates are observed when the two nanoparticles are combined
Conclusions
Epoxy vinyl ester nanocomposite coatings were prepared with Zn and TiO2 nanofillers at various concentrations. The salt spray corrosion test showed that separately, both Zn and TiO2 improved the corrosion protection of steel substrates. However, when nanoparticles were combined, a synergistic effect was observed with lower percentage of corroded area, with the coating of 1.0 wt. % Zn and 0.5 wt. % TiO2 having the best performance. This is attributed to smaller void volumes and enhanced barrier properties by adding TiO2 and Zn. Similar results were obtained by the tribological tests performed with a ball-on-disk tribometer. Individual nanoparticles provided an increase on wear resistance, but when both are combined the results are enhanced. In this case, the proposed tribological mechanism is a load-bearing effect coupled with rolling-bearing mechanism by removal of nanoparticles during the wear process.











 text new page (beta)
text new page (beta)