Introduction
Leachates are formed as consequence of the waste moisture and the percolation of liquids through wastes during the stabilization process. Leachates exhibit high amounts of both Total Organic Carbon (TOC) and Chemical Oxygen Demand (COD); therefore, leachates contain considerable amounts of organic and inorganic compounds, heavy metals, high pH variability, high contents of total and dissolved solids, nitrogen as N-NH3 and high chloride concentrations, among many others. Such composition on leachates depends on the nature of the wastes (pH, age, temperature, type of cover material) and the waste stabilization phase (Borzacconi et al., 1996; El-Fadel et al., 2002).
Different processes have been tested to treat leachates, both biological (aerobic or anaerobic processes) and physicochemical (Warith and Sharma 1998; Rivas et al., 2003; Tatsi et al., 2003; Rivas et al., 2004). The concentration of pollutants from leachates varies with time; consequently, a single treatment can hardly be successfully employed. In general, it can be said that leachates from young landfills show high concentrations of organic matter and biodegradability indexes (BI = BOD5/COD) over 0.4, making it possible to efficiently treat them by biological processes; nevertheless, these treatments are not efficient for leachates from old landfills, with biodegradability indexes below 0.02 (Warith and Sharma, 1998; Robles, 2005).
The older the landfill is, the biodegradable organic fraction in the leachate decreases. This results in a very low BOD5/COD ratio because the fatty acids and other readily biodegradable substances turn into methane in the landfill (Lau et al., 2001). Most organic compounds from stabilized landfills are refractory; thus, the biological processes to treat these leachates have very limited effectiveness (Lau et al., 2001; Yoo et al., 2001). Their refractory nature involves the necessity of using alternative methods to the biodegradation processes to reduce the pollutant loads in these effluents.
Determining the most appropriate treatment for a landfill leachate is complicated, since it is liquid wastes with high content of organic and inorganic substances. Extensive research has been made looking for the appropriate treatments for leachates; however, due to their heterogeneous composition and the variable volume produced, the results obtained with a leachate treatment cannot be directly extrapolated to another one. Therefore, the leachates from a landfill must be individually evaluated and subjected to treatability tests to find the appropriate treatment system.
There are numerous references about physicochemical treatments of leachates, with drawbacks such as the cost of chemicals and the production of sludge difficult to dispose (Yoo et al., 2001; Rivas et al., 2004; Kurniawan et al., 2006). However, old leachate treated by the physicochemical process allows achieving high removal efficiencies, which, would not be possible to reach by biological treatment due to their low biodegradability index. It has been considered the physicochemical treatment as complement for the biological process.
The coagulation-flocculation and flotation processes are designed to remove suspended particles from the liquid phase; at low pH, they remove small particles, which provide the leachate with color. However, the leachates in general -while subjected to successive filtration inside the landfills- have low concentrations of suspended organic matter; in consequence, the efficiency of the coagulation-flocculation process is low, from 25 to 50% of COD removal (Yoo et al., 2001; Gálvez et al., 2005), unless working with high doses of coagulants. The latter would cause the removal by wiping action in a low pH values, thus small colloidal particles providing the leachate with color -such as humic acids- are removed; this allows COD removals of 40 to 70% (Batarseh et al., 2007; Orta y Monje, 2006).
The adsorption and Fenton intensive oxidation processes can remove both particulate and dissolved matter. The experience in organic matter removal superior to the coagulation-flocculation processes -of 50 to 70% COD removal- can be found in the literature (Wang et al., 2003; López et al., 2004; Xing et al., 2008; Abdul et al., 2009; Foul et al., 2009; Derco et al., 2010).
The sludge produced with the Fenton process has low density and, in consequence, is difficult to remove by gravity; thus, using doses of coagulants after Fenton has been tested, showed improvements for sludge removal (Zhang et al., 2006; Cortez et al., 2009; Deng, 2007). However, the improvement in the sludge decantation does not imply a significant improvement in the organic matter removal (Yoo et al., 2001; Pala and Erden, 2004; Batarseh et al., 2007; Wu et al., 2010). Operative procedures to reduce sludge generation have also been assayed decreasing the volume of sludge produced, from 75 to 30% (Mahmud et al., 2011). At the same time, they got better COD and color removal, of 68% and 87%, respectively, with the conventional Fenton process, and of 84% and 93%, respectively, through modified Fenton process, which consist of the addition of Fenton reactants by stages and sludge recirculation. Moravia et al. (2011) got COD and color removal up to 76.7% and 76.4%, respectively, with a similar process and the sludge generation was of 32.1%.
Filtration is an alternative for removal the sludge generated during the Fenton process. Moravia et al. (2011) used microfiltration and got 75% in organic removal and 95% in color. Méndez et al. (2010b) compared sedimentation with filtration after the Fenton process. They compared COD and color removal efficiencies obtained with sedimentation (1 h of hydraulic retention) and with filtration, both after the Fenton process, and they proved that the latter is more efficient than the first one; they got up to 77% COD and 87% color removals with the filtration process, against 73% COD and 84% color for sedimentation.
Leachates treated with the Fenton process still present high loads of organic matter; therefore, they cannot be directly discharged and require a complementary process. If the biodegradability index is low, the process cannot be biological, but physicochemical, yet not coagulation-flocculation. Since the Fenton process removes suspended particles that otherwise would be removed by the coagulation-flocculation process, an adsorption process with granular activated carbon was used for this experiment. Despite there are experiences of both individual processes, with removals of 60 to 70% each, it is expected that the adsorption process after Fenton will be more efficient, since it would eliminate partially degraded molecules with the Fenton process.
The aim of this study was to determine the efficiency of the leachate adsorption process when treated by Fenton/filtration/adsorption.
Materials and methods
Characterization. Ten leachate-sampling campaigns were carried out every two weeks from October 2009 to February 2010. The samples were collected at three evaporation ponds from the Mérida, Yucatán landfill and the following parameters were determined: pH, Color, Turbidity, Conductivity, Alkalinity, Total hardness, Chlorides, BOD5, Total COD, Soluble COD, TOC, Fats and oils, SMBA, N-NH3, N-org, TKN, Total P, Sulfides, O2, TSS, VSS, TS, TVS, Redox, Fe, Mn, Zn, Na, K, Cd, Pb, Cr, Cu, Ni and Ag. The characterization was carried out according to the standard methods (APHA-AWWA-WPCF, 1999).
Fenton oxidation (Optimal time). Jar tests (Phipps & Bird) of the Fenton process were performed. The reagents were mixed for one minute at 300 rpm; later the speed was slowed to 30 rpm and stirred for 20 minutes. Samples were taken at 5, 10, 20, 40, 60, 80, 100 and 120 minutes of sedimentation, they were filtered and COD and color were determined. The optimal value of pH used was the same of a previous study on the same landfill leachate (pH = 4, with concentrated H2SO4, 97% w/w) (Méndez et al., 2010a). The Application of the optimum dose of Fenton reagent according to the COD was that found by (Méndez et al., 2010b) for the same landfill leachate: [Fe2+]/[H2O2] = 0.6; [COD]/[H2O2] = 9.
Filtration. To compare the decantation and filtration processes after Fenton, five tests were performed (pH = 4; contact time = 60 minutes [Fe2+]/[H2O2] = 0.6; [COD]/[H2O2] = 9). COD and color were determined from the raw leachate, the supernatant and the sludge filtered through Whatman paper 40. The amount of sludge was determined as well with an Imhoff cone after one hour of decantation.
Adsorption. The leachate treated by Fenton was filter pressed through 4 µm pore filter paper with an Interfilter press, model 20-18, and stored in a 200 L container to use it in the adsorption process. Subsequently it was pumped into an acrylic column 75 cm long and 20 cm in diameter, packed with 60 cm of lignite macroporous granular activated carbon. The carbon surface area was 348.61 m2/g, with relative density of 0.38 and the adsorption cross section area was of 0.162 nm2.
An ascending flow of 170 mL/min was pumped and color and COD were measured at the column’s input and output during the time required for colmatation. The COD and color removals were plotted according to the time.
Results and discussion
Characterization.Table 1 shows the results of the leachate characterization. Due to the way of landfill operation, leachates of different ages were mixed in the evaporation ponds, favoring high variability of the parameters shown in Table 1. Therefore, causing a mixture of features of the leachates produced during acid fermentation and the methanogenic phase.
Table 1 Features of the leachates produced in the Mérida, Yucatán landfill
| Parameter mg/L | Measurement | Range | Parameter mg/L | Measurement | Range |
|---|---|---|---|---|---|
| pH1 | 8.3 | 7.9 - 8.5 | O2 | 0.72 | 0.15 - 1.30 |
| Turbidity2 | 108 | 95 - 130 | TSS | 95 | 42 - 153 |
| Conductivity3 | 17.9 | 17.1 - 18.5 | VSS | 69 | 38 - 101 |
| Alkalinity * | 4305 | 548 - 11107 | TS | 12,545 | 10,064 - 16,214 |
| Total hardness * | 955 | 720 - 1196 | TVS | 3813 | 2546 - 5260 |
| Chlorides | 3156 | 2489 - 3654 | Redox | 19 | -133 - 123 |
| BOD5 | 1098 | 236 - 2580 | Fe | 64.05 | 7.92 - 164.4 |
| Total COD | 5346 | 4268 - 7610 | Mn | 0.81 | 0.12 - 1.49 |
| Soluble COD | 4895 | 3161 - 7490 | Zn | 3.20 | 0.43 - 5.80 |
| TOC | 2857 | 2283 - 4380 | Na | 11,850 | 1632 - 28180 |
| Fats and oils | 29 | 4 - 62 | K | 10252 | 1636 - 23100 |
| MBAS | 6.49 | 0.88 - 13.80 | Cd | 0.0069 | 0.001 - 0.158 |
| N-NH3 | 1210 | 795 - 2303 | Pb | 0.236 | 0.016 - 0.900 |
| N-org | 208 | 82 - 320 | Cr | 6.98 | 4.74 - 14.35 |
| TKN | 1419 | 1004 - 2515 | Cu | 0.214 | 0.056 - 0.388 |
| Total P | 37.32 | 7.04 - 75.12 | Ni | 0.349 | 0.319 - 0.387 |
| Sulfides | 405 | 30 - 705 | Ag | 0.039 | 0.037 - 0.039 |
* Measured as CaCO3,
1 No unit,
2 NTU,
3 mS/cm
The cover material is a type of regional soil called sahcab, of karstic nature (CaCO3) and fragile structure. When the cover material is subjected to compacting during the landfill’s building stage, porosity reduced, thus producing a double effect on it: it acts as a filter retaining the larger particles and reacts with the leachate, causing the dissolution of its carbonates. This is reflected in the low TSS concentration and pH and alkalinity values obtained by Méndez et al. (2002).
It can be seen that most of the solids in leachate are dissolved, whereas only 0.76% of the total solids are suspended. This result indicates that the coating material (sahcab) at the Merida landfill retains most of the suspended solids. In consequence, the use of a physicochemical process such as coagulation-flocculation to treat these leachates would be inefficient.
Consistent with previous results, it can be observed that most of the organic matter -measured as soluble COD- is dissolved (91.56%). This figure is similar to that reported by (González et al.. 2001) in leachates from landfills in Veracruz and Mexico City, Mexico.
The pH is relatively high compared to other leachates, which is related to the sahcab-leachate interaction. (Słomczyńska and Słomczyński, 2004) reported leachate pH values between 5.4 and 7 in six Polish and two American landfills. The high alkalinity may be due primarily to the type of covering material, which provides the leachate with calcium and magnesium carbonates and bicarbonates. The relatively high ammonia-nitrogen concentration, added to the alkalinity, gives the leachate a high buffer capacity. High sodium and potassium levels, as well as hardness, are also related to the dissolution of the cover material.
Metals are found in significant concentrations compared to those reported by (Jensen and Christensen, 1999). The authors reported chromium average concentrations of 0.0064 mg/L in four leachate samples, while in the Mérida, Yucatán landfill the average concentrations of chromium founded were of 6.98 mg/L. The presence of metals in the leachate indicates that, during the acidogenic phase, low pH values solubilized them; when going through a layer of cover material, pH increases again, but not to the values required to cause the metals precipitate. This explains the heavy metals high concentrations and high pH values found in the leachate sample at the same time.
Fenton oxidation
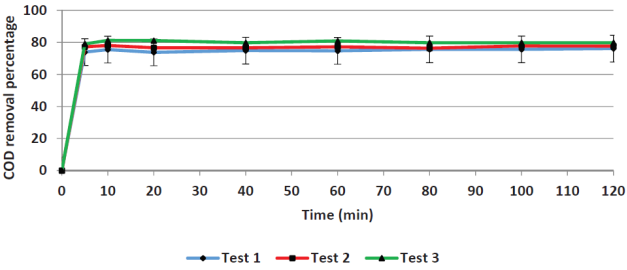
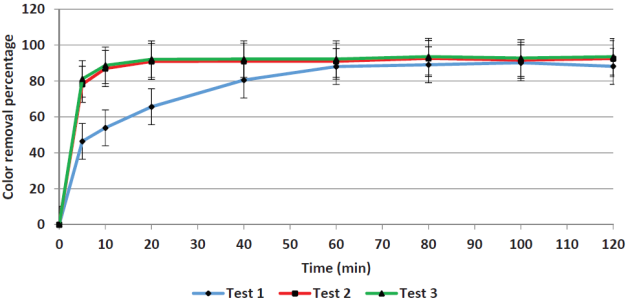
Contact time. Figures 1 and 2 show the removal efficiencies of COD and color plotted as a function of contact time in. For COD removal, the limit efficiency is obtained at five minutes, whereas for color removal the limit is obtained at 60 minutes. The average value of the initial COD was 7660 mg/L and the remaining COD was 1767 mg/L. The 60-minutes mark was chosen as adequate to remove a greater amount of the remaining color (primarily iron) to reduce the load for the next process (adsorption).
Yoo et al. (2001) arrived at similar results when applying the Fenton process to leachates; they obtained removal efficiencies that became asymptotic after 20 minutes. Other researchers have been reported different optimal reaction times as seen in Table 2, in which optimal values ranging from 5 to 120 minutes. The Fenton reaction kinetics is controlled by the Fe2+ concentration since it acts as a catalyst.
Table 2 Obtained results compared to other Fenton oxidation trials
| Parameter | Unit | Mérida, Yucatána | Italyb | Delaware, USAc | Hong Kongd | Spain 1e | Istanbulf | Spain 2g |
|---|---|---|---|---|---|---|---|---|
| Characterization of the untreated leachate | ||||||||
| pH | pH units | 8.57 | 8.2 | 6.67 | 8.5 | - | 7.3 | 7.1 |
| Conductivity | mS/cm | 21.83 | 45.35 | - | - | - | - | 47.1 |
| Alkalinity | mg/L | 6115.96 | 21470 | 4050 | - | - | 9850 | - |
| BOD5 | mg/L | 647 | 2300 | - | 75 | 475 | 12200 | 7100 |
| COD | mg/L | 9080 | 10540 | 8596 | 1500 | 8100 | 20700 | 6500 |
| TOC | mg/L | 2266 | 3900 | 2124 | 470 | - | - | - |
| BOD5 / COD | - | 0.071 | 0.218 | - | 0.050 | 0.059 | 0.589 | 0.54 |
| Optimal conditions used for the Fenton treatment | ||||||||
| Reaction time | minutes | 20 | 120 | 30 | 40 | - | 5 | 60 |
| pH | pH units | 4 | 3 | 2.5 | 6 | 3.5 | 3.5 - 4.0 | 3 |
| H2O2 | mg/L | 600 | 3300 | 2550 | 200 | 34000 | 2000 | 6500 |
| Fe2+ | mg/L | 1000 | 275 | 2792 | 300 | 558 | 1000 | 650 |
| [Fe2+]/[H2O2] | - | 1.67 | 0.08 | 1.10 | 1.50 | 0.02 | 0.50 | 0.10 |
| [COD]/[ H2O2] | - | 15.13 | 3.19 | 3.37 | 7.50 | 0.24 | 10.35 | 1.00 |
| Fenton process efficiency | ||||||||
| COD | % | 77 | - | 61 | 38 | 80 | 85 | 75 |
| TOC | % | 71 | - | - | - | - | - | - |
| BOD5 | % | 44 | - | - | - | - | - | 98 |
| 0.100 | 0.5 | - | - | - | - | - | ||
a Present study, 2012.
Table 2 shows a summary of results after the application of the Fenton process to leachates. The COD, TOC, BOD5 removals and the biodegradability index variation (BOD5/COD) are compared. There is a wide variation in contact time, pH optimal values and Fenton reagent dose. Contact time varies from 5 to 120 minutes, pH ranges from 2.5 to 6 and Fenton reagent dose goes from 200 mg/L to 34,000 mg/L for H2O2 and 275 mg/L to 2792 mg/L for Fe2+. The relationship between the catalyst and the Fenton reagent ([Fe2+]/[H2O2]) varies from 0.02 to 1.67, while the ratio of the organic load and the oxidizing agent ([COD]/[H2O2]) for the leachate from Mérida, Yucatán landfill ranges from 15.13 to 0.24 (Rivas et al., 2004). Considering the variations of these relationships, Méndez et al. (2010b) found that the optimal values for the leachates from the city of Mérida, Yucatán are: [Fe2+]/[H2O2] = 0.6; [COD]/[H2O2] = 9. Therefore, the optimal dose of Fenton reagent can be established with the COD value.
Filtration
A by-product of the Fenton process is a low-density sludge, which is difficult to remove by decantation. For this reason, the filtration process was evaluated for the removal the produced sludge. Table 3 shows the results of the comparison for both the Fenton process and the filtration stage.
Table 3 Comparison of characteristics of untreated and treated leachate for the Fenton process followed by filtration
| Sample | COD (mg/L) | Color (U Pt-Co) | Sedimentability (mL/L) (1 h) | |
|---|---|---|---|---|
| Raw leachate | 8510 | 10600 | N/A | |
| Decanted leachate | 1 | 2390 | 1780 | 920 |
| 2 | 2250 | 1920 | 920 | |
| 3 | 2320 | 1680 | 930 | |
| 4 | 2300 | 1470 | 900 | |
| 5 | 2230 | 1680 | 910 | |
| Average | 2298 | 1706 | 916 | |
| Filtered leachate | 1 | 1950 | 1120 | N/A |
| 2 | 2090 | 970 | N/A | |
| 3 | 2050 | 900 | N/A | |
| 4 | 1880 | 1220 | N/A | |
| 5 | 1720 | 1390 | N/A | |
| Average | 1938 | 1392 | N/A | |
The average COD of the supernatant after the Fenton process and one-hour of sedimentation was 2298 mg/L, with standard deviation of 63 mg/L. This is slightly greater than that obtained with the filtration process, with an average 1938 mg/L and standard deviation of 165 mg/L. Similarly, the filtration also produces further color reductions, up to 1392 U Pt/Co, with standard deviation of 147.2U Pt/Co. Neverthless, by means of sedimentation only 1706 U Pt/Co was achieved in average, with standard deviation of 196.1 U Pt/Co.
Adsorption
Results obtained from the adsorption trials are shown in Figure 3. Based on the removal curve (leachate flow and COD removal), both, the L leachate/kg carbon and the kg CODrem/g carbon were calculated. These parameters are necessary to determine the amount of carbon required to treat any volume of leachate. While granular activated carbon can be washed and/or regenerated, the volume of wastewater would be greater than the volume of treated leachate, with similar pollutant loads; thus, this practice is discouraged, and saturated activated carbon should be considered a waste to dispose.
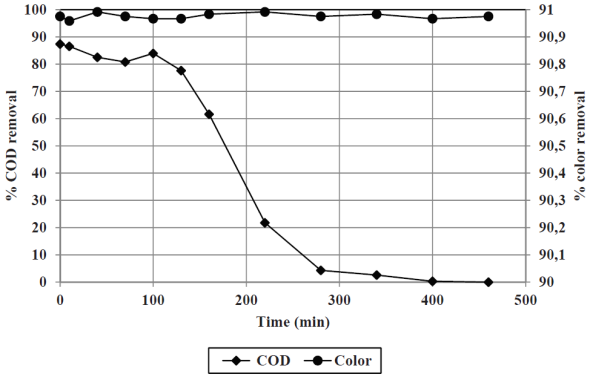
Figure 3 Color and COD removal from a leachate treated by Fenton/filtration, subjected to macroporous activated carbon adsorption
With the application of the adsorption process, COD removals over 80% and color removals over 95% are achieved, compared to the leachate treated by Fenton/filtration. Once exhausted the COD removal capacity, the activated carbon still removes color, mainly derived from excess of iron.
Fenton/filtration/adsorption
The results of the Fenton/filtration/adsorption treatment train are shown in Table 4. COD removals of 99.9% were obtained, of which 90.8% was removed by Fenton/filtration and only 9.1% by adsorption. Regarding color, removals of 100% are achieved, of which 95.7% correspond to the Fenton/filtration stage and 4.3% to the adsorption process.
Table 4 Results of the treatment train Fenton/filtration/adsorption
| Parameters | Raw leachate | Leachate after Fenton | Leachate after filtration | Leachate after adsorption |
|---|---|---|---|---|
| Color (U Pt-Co) | 26,160 | --- | 1120 | 5 |
| COD (mg/L) | 17,450 | --- | 1610 | 11 |
| BOD5 (mg/L) | 580 | --- | 335 | 8 |
| BI | 0.033 | 0.190 | 0.208 | 0.727 |
| pH | 9.0 | 2.7 | 2.7 | 8.7 |
| TS (mg/L) | 42,408 | 43,820 | 34,452 | 4624 |
| TSS (mg/L) | 925 | 11,380 | 330 | 153 |
The TS removal efficiency by Fenton/filtration/adsorption was 89.1%, of which 18.8% corresponded to Fenton/filtration and 70.3% to adsorption. TSS removals were of 83.5% after the full treatment, 64.3% corresponding to Fenton/filtration and 19.2% to adsorption. The TDS from the leachate treated by Fenton/filtration/adsorption (4471 mg/L) represent 96.7% of the TS. However, the organic matter -measured as COD and BOD5- was significantly lower (11 and 8 mg/L, respectively). Therefore, TDS are mainly composed of inorganic matter.
Although the COD, BOD5 and color removals during the adsorption process are much lower than Fenton/filtration, additional removal obtained allows to the effluent reach the values required to meet with Mexican regulations in force. In the adsorption process, pH raises from acidic (2.7) to alkaline (8.7) values, thus, achieving the Mexican Standards. The TSS content in the effluent is slightly above the Mexican and International Standards. The maximum value allowed for TSS according to NOM-001-SEMARNAT-1996 (SEMARNAT, 1996) in discharge waters is 60 mg/L; therefore, the effluent from the treatment used is slightly above this limit and exceeds the limit fixed by the European Union (35 mg/L) (EEU, 1991). The BOD5 value of the treated leachates is lower than that established by the Mexican Standard NOM-001-SEMARNAT-1996 for any kind of receiving body. Concurrently, the COD and color values obtained are lower than those established in the “Ley Federal de Derechos” (Provisions Applicable to National Waters) (SEMARNAT, 2009). Nevertheless, more detailed analyses should be conducted for the remaining substances in the treated leachate in order to ensure that they are environmentally safe.
The biodegradability index increases from 0.033 (raw leachate) to 0.727 (treated leachate), although the remaining organic loads are so low that there is no necessity to subject the leachates to subsequent biological treatments.
Conclusions
The cover material used in the landfill acts as filters and also reacts with the leachates; this reaction helps with the presences of low concentrations of suspended solids (0.76%), high pH and high alkalinity. Also, almost all the organic matter (96%) measured as COD is soluble.
Most particulate matter in the leachates are in soluble or colloidal form at small sizes (<50 microns), which avoid to get a high low efficiencies in clarification processes such as coagulation-flocculation and flotation.
The optimal contact time of the Fenton process was 5 minutes for COD removal and one hour for the color removal.
The filtration process was more efficient than sedimentation for the removal of the produced sludge during the Fenton process in terms of COD and color removal efficiency.
The adsorption efficiency with macroporous carbon was 99.9% for COD and 100% for color, corresponding to values of 11 mg/L for COD and 5 (U Pt-Co) for color, which complemented the process Fenton and allows to comply with Mexican standards for wastewater discharges.
The biodegradability index increased from 0.033 in the raw leachate to 0.727 in the treated leachate.
The BOD5 value of the treated leachate is lower than those established in the Mexican Standard NOM-001-SEMARNAT-1996 for any kind of receiving body. As well as the COD and color values obtained are lower than those established in the “Ley Federal de Derechos” (Provisions Applicable to National Waters, 2009).











 nueva página del texto (beta)
nueva página del texto (beta)