Introduction
Colombia is the fourth milk producer country in South America with more than 6500 million of liters of milk produced by year (2015). The 8% of this production is dedicated to the cheese industries. From the cheese process, two products are reclaimed. The first one is the cheese curd, which is the base of the cheese and will continue to proceed after coagulation. The second one is Cheese Whey (CW) that represents the 85-95% of the milk used, which not go on in the process (Guinée, 2011). It contents 55% of milk nutrients like lactose, soluble proteins, lipids and mineral sales (Brandelli et al., 2015). Due to its high organic matter content: 50-70 kg COD/ m3 (Demirel et al., 2005), cheese whey represents a legitimate potential substrate for the Anaerobic Digestion (AD) (Comino et al., 2012).
AD process can be seen as a triple action process, it allows reducing a polluting discharges (Yang et al., 2016; Garfí et al., 2016) and obtaining on one hand, a new renewable energy as biogas production (Mao et al., 2015) and on the other hand, getting benefice as a bio fertilizer (Kataki et al., 2016; Mukhlesur et al., 2014). AD is a biological process whose main operation condition is to be realized in a total lack of oxygen. This bio conversion is put up in four steps: hydrolysis, acidogenesis, acetogenesis and methanogenesis. Each reaction is realized by different bacterial populations. The microorganisms that act in the AD process can be grouped into two parts. First, they are the Volatile Fatty Acids (VFA) producers (hydrolysis and acidogenesis) and the second one is composed by VFA consumers (acetogenesis and methanogenesis) (Liu et al., 1997). The main component of CW is lactose (80% of dry solids) which is a component rapidly and easily degradable (Heldal et al., 2014). Lactose degradation causes a high production of acids due to acidogenesis bacteria, mostly converted to butyrate (Kaan et al., 2015). These acids may be consumed by the methanogenic arqueas acting in the methanogenesis step. Nevertheless, there is a difference of growing rate between the bacterial populations. In fact, the growing rate of methanogenesis arqueas is lower than the acidogenesis bacteria, it can provoke inhibition of AD process (Wang et al., 2014).
Furthermore, CW presents a considerable lack of Total Alkalinity (TA) which prevents to overcome the acidification problem. In this way, the AD process of CW can be seen as a complex process which needs a permanent control. For this reason, it requires the stability monitoring. This stability can be supervised with the following parameters: pH whose favorable range is between 6.5 and 8 (Poh, 2009), and buffer capacity which express as the ratio VFA/TA, and which guarantee the inexistence of an acidification when is below 0.4 (Kong et al., 2016). However, with an acid substrate as CW, buffer capacity tends to be elevated because of its high VFA concentration and its lack of alkalinity.
As a result, so as to ensure the steady state of the AD of an acid substrate, alkalinity addition is required (Heldal et al., 2014), but some parameters could be managed to overcome this fault (Venetsaneas et al., 2009). Therefore, the use of a suitable co-substrate which may be provides the CW currencies, is presented as an advantageous alternative. It deals with the anaerobic co-digestion (ACOD). It was proved that fresh dairy manure (FDM) is a good co-substrate for cheese whey (Kavacik et al., 2010) because of its high alkalinity (140 meq/L) and COD (120mgO2/L); (Comino et al., 2012).
Reactor type used in AD could influence the process performance (Dareiori et al., 2015; Bertin et al., 2013). In continuous operations, two main types of reactors have been amply studied: Upflow Anaerobic Sludge Blanket (UASB) and the Continuous Stirred Tank Reactor (CSTR). UASB reactors reach short Hydraulic Retention Times (HRT) (from 1 to 10 days) allowing organic matter removals around 85% (Gavala et al., 1999) to 95-97% (Rico et al., 2015). According to CSTR reactors, studies have shown that the design allowed a favorable homogenization of the medium. Authors reported biogas yield between 1.51 m3 biogas/m3 reactor*day (Kavacik et al., 2010) and 1.37 m3 CH4/ kg COD (Rico et al., 2015), however, a low organic matter degradation of 26% was obtained. Nowadays, there is an increase focus on using low cost technologies that could accomplish similar yields. The Plug Flow Reactor (PFR) is visualized as an alternative to carry out anaerobic digestion process thanks to the agitation kept without requiring an energy supplement. Successful studies with acid substrates as pineapple and food wastes were done using this reactor configuration. The first waste, obtained an organic matter yields around 60-65% for pineapple AD (Namsree et al., 2012). In the case of food wastes AD, using as a co-substrate, rice husks, which reach the degradation of 82% of input organic matter (Jabeen et al., 2015). Additionally, PFR design offers the possibility to implement a phase’s separation along the reactor. In the AD, it can be separated two bacteria groups whose respectively conditions are different: the producers and consumers of VFA (Liu et al., 1997). VFA producers need an acid medium whereas the consumers necessitate an alkaline medium. Thus, a separation could be successful for the bacteria growing. The PFR allows a microorganisms’ distribution along the reactor (Namsree et al., 2012). This last study reveals that is possible to implement AD process with an acid substrate in a low cost reactor. The AD of cheese whey in PFR reactor has not been evaluated. Besides, to improve the bacteria communities’ co-existence, boosting their favorable growing conditions, the use of the effluent recirculation can be a profitable issue. It was recently verified with chicken manure that recirculating the effluent fermentation, allows increasing the pH and the system alkalinity so as so to raise the organic loading rate (OLR) (Wu et al., 2016; Zamanzadeh et al., 2016). Studies about effluent recirculation use on CW anaerobic process are limited. The aim of this research was to implement an AD process of CW in a PFR, investigating the behavior and stability of the process, through the use of effluent recirculation and of fresh dairy manure as co-substrate. In order to review the effective separation of phases along the reactor, acidity medium was analyzed.
Materials and methods
Substrate and inoculum
The first step of this study was to do a physic-chemical characterization of the CW from a dairy industry located in Santander state, Colombia (Lat: N 7°8’ 19, 2’’ Long W 73°7’ 31, 298’’). This company treats a volume of 1600 liters of milk per day, which originates from the state’s producers. This study last 5 weeks during which, samples were collected twice per week. CW samples were recollected directly from the storage tank after cheese making and storage at 4°C in Biotechnology Laboratory, Universidad Industrial de Santander (Colombia). The main objective was to ensure that the CW characteristics do not present significant changes. The Grubb’s test was done, which is a statistical test to analyze the extremes values, looking if they are acceptable with respect to the others, or if they may be considered as aberrant values. Then, a complete characterization was done: Volatile Solids (VS), Total Solids (TS), pH, VFA, TA, proteins, lipids and carbohydrates composition. As co-substrate Fresh Dairy Manure (FDM) was implemented. It was recovered in a slaughterhouse located in Santander state, Colombia (Lat: N 7°12’ 14, 029’’ Long W 73°7’ 50, 47”). The co-substrate was, then characterized with following parameters: Volatile Solids (VS), Total Solids (TS), pH, VFA, TA, and proteins, lipids and carbohydrates composition. The inoculum used was FDM recovered in the same slaughterhouse than the co-substrate. Before starting reactors load, inoculum was pre-incubated at 25°C with the aim to reduce residual organic material. Inoculum composition was 117g COD/L, 34.0 g TS/kg and 60% of VS. Inoculum pH was about 7.5 ± 0.03. On this inoculum, a trophic groups count (more probable number of cells of inoculum concentration (MPNcells/gVS) which reported the presence of 2.8.104 acetoclastic methanogenic arqueas, 2.3.104 of hydrogenotrophic methanogenic arqueas and 2.9.104 methanogenic arqueas of methanol (Alzate et al., 2016).
Equipment
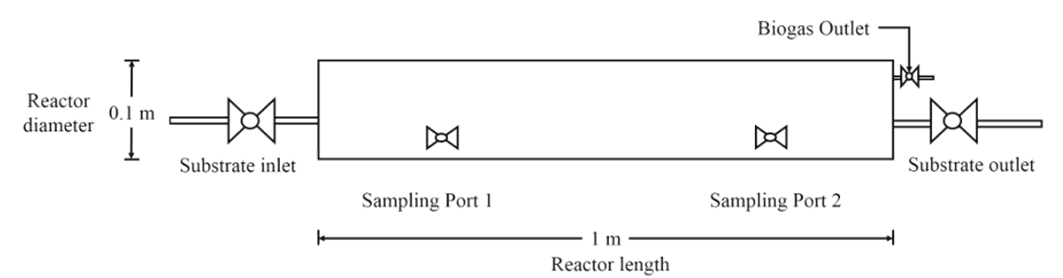
Anaerobic mono-digestion processes of cheese whey and its co-digestion were performed in cylindrical polyvinyl chloride reactors keeping a length/diameter ratio of 9 where the length was of 1 meter, and the diameter measured 0.11 m with a total volume of 8.1 L (operating volume 4.8 L). The reactors dimensions were taken from literature (Martí, 2012). The reactors were kept at ambient temperature (26 ± 2°C). The temperature of the reactor was measured in the outlet of the process. The inlet and outlet of the reactors were closed with hermetic valves. In order to sample along of the reactor, two valves were installed as it is shown in Figure 1.
Biogas production measurement
Biogas production was quantified by the liquid displacement method of a saturated saline solution. The produced biogas was directly measured from the reactor under constant pressure (Angelidaki, 2011).
Physical and chemical analysis
Total Chemical Oxygen Demand (COD), Total Solids (TS) and Volatile Solids (VS) were measured according to standard methods procedure 5220D and 2540G respectively (APHA, 2005). pH was measured using a pH-Meter (Metromb6h 691). Volatile Fatty Acids (VFA) and Total Alkalinity (TA) were determined via titration (Taylor et al., 2015). All those variables were measured per duplicate. During the continuous process, every five days, all these analysis were done in outlet samples, so as to control the global processes. Moreover, samples from port 1 and port 2 (Figure 1) were design for the purpose of verify the comportment of the bioprocess in the axial direction of the reactor via pH and VFA measurement. Methane concentration in the biogas at the end of the study, was determined by gas chromatography with a gas chromatographer (AT 6890A Agilent Technologies) outfitted with a thermal conductivity detector (TCD) in series with a Flame Ionization Detector (FID).
Operations conditions
Set-up As a start-up step, the system was submitted to a batch process to attain the steady state. For both reactors, the inoculum/substrate ratio used was 1.5 ginoculum/gsubstrate. Besides, for the ACOD, a volumetric relation was maintained (CW: FDM 65:35) based on the studies done by Fernández et al., (2015). The system steady state was determinate when the biogas production reached a stationary daily production and the volatile solids conversion exceeded the 75%. This period was stopped when a VS conversion of 75 % was reached.
Continuous Process steps. The reactors were set up with a continuous feeding. The OLR was increased by steps of 0.5 g VS/L day to the acidification of the process. First, an OLR of 2 kg VS/m3 was set up. For the AD and ACOD, HRT was calculated with the operation volume, resulting 30 and 35 days respectively. In a first step, in order to analyze the recirculation impact, both processes were fed only with substrate (CW and mixture of CW and FDM). Then, the feed were changed, thus, 40% of recirculation were implemented keeping OLR constant (Namsree et al., 2012).
Behavior description of the anaerobic process stability according to VFA content
With the aim of represent the existence of a methane or an acid phase along the reactor length, the next mechanism was proposed.
Where C0.33 and C0.66 correspond to the VFA concentration at length of 0.33 and 0.66 m respectively. Also, Cinh represents the inhibition concentration of the system and 𝑛 is an arbitrary number. Dividing the Ec 1 by the Cinh and reorganizing terms we obtain
In that case, the values for β denote the predominance of the methanogenic or an acidogenic phases along the reactor, as is shown below
Results
Substrates
Variation of the CW’s composition
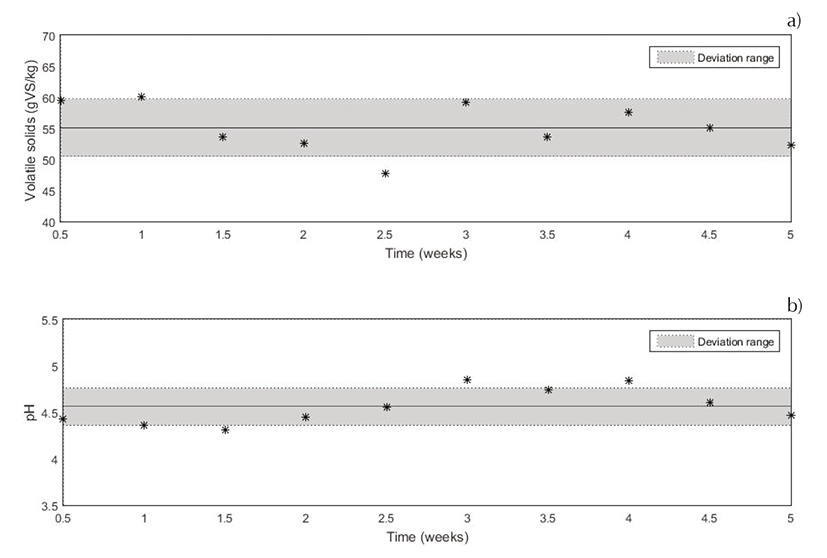
Along five weeks, VS and pH, for one dairy industry were studied. In Figure 2, it is shown the evolution of VS and pH during this 5-weeks study. During this 5-weeks study, pH values were kept between 4.37 and 4.75. These values showed that this CW was acid due to the cheese process. In fact, the cheese process implements the coagulation of milk by fermenting, that produce and acid CW. Volatile solids were maintained between 50.47 and 59.74 g VS/ kg. It could be conclude that using a CW obtained from a same cheese fabrication process, with milk from the same farms, the characterization of it was constant in the time. To observe differences between each variable, Henry and Grubbs’ test was applied. This analysis helps to ensure the reproducibility of the process, and besides, that the moment when the CW was recollected did not affect those variables. It is shown that there were not significant differences along time. It was proved that this whey characterization did not present notorious changes.
Physic-chemical characterization of the substrates and mix CW: FDM
In Table 1, substrate, co-substrate and the mix CW: FDM (65:35) characteristics are presented. Results indicated that CW and FDM have a valuable organic matter content to improve an anaerobic process. Their potential can be highlight by the high value of Theoretical Biomethane Potential (BMPth). Moreover, the VS/TS ratio is encountered in the range between 0.52 and 0.98 that specify the high organic matter content easily biodegradable. The C/N ratio is considered as optimal between 15 and 45 (Garfí et al., 2016). It can be observed that CW has a lower C/N ratio; as a consequence the co-digestion of CW with FDM could enhance the nutritional equilibrium in the substrate composition. The VFA values demonstrate that the organic matter is soluble and available to be easily metabolized in acetate, and posteriorly in biogas (Raposo et al., 2006). Then, the process stability depends on the pH. The buffer capacity that provides the co-substrate helps the pH to increase (from 4.6 for the CW until 6.9 for the mix) and foster the anaerobic process. Besides, the substrate composition is fundamental for the microbial development. An unbalance could induce a vulnerable hydrolysis and a reduction of the methane production. Carbohydrates presented 55.3 and 69.2% p/p, for CW and FDM respectively. The anaerobic degradation of these macromolecules depends on the relation between the growing rate of acidogenic and methanogenic metabolic (Yang et al., 2015). The carbohydrates present a rapid hydrolysis which generates a strong acidification by reducing the methanogens, as well as the AD of CW could sensitive to an acid inhibition. Though, CW is rich in proteins, in this way, favors the buffer capacity during the fermentation. Finally, the poor lipids content of CW lets the medium less exposed to the risks of acidification and adsorption that can provoke sludge flocculation and the biomass washing (Neves et al., 2009). To conclude both substrates have various components useful for the anaerobic digestion. The mix would be helpful to improve each substrate characteristic.
Table 1 Substrates compositions
| Variables | Substrate | Co-substrate | Mix |
|---|---|---|---|
| Cheese Whey | Fresh Dairy manure | CW: FDM | |
| Total solids (g ST/kg) | 65.37 | 139.61 | 91.30 |
| Volatile solids (g VS/kg) | 55.03 | 101.71 | 72.59 |
| VS/TS | 0.84 | 0.73 | 0.79 |
| pH | 4,6 | 8.2 | 6.9 |
| VFA (mg Ac. Acid/L) | 4020 | 8300 | 4300 |
| Carbohydrates (%) | 55.3 | 69.2 | - |
| Proteins (%) | 23.0 | 3.11 | - |
| Lipids (%) | 1.9 | 23.5 | - |
| Empirical formula | C47H44O2N2 | C32H47O20N | - |
| C/N | 11.9 | 23.5 | - |
| PMBth (m3CH4/kgVS) | 0.781 | 0.509 | - |
Set up step
This stabilization period lasted nearly a month (26 days) with the purpose of obtaining a favorable medium process to start the continuous operation. For both systems, the pH was kept constant between 7.25 and 7.8 (favorable range for anaerobic processes) in AD and ACOD systems. During set up step, the buffer capacity and organic matter conversion stability were important criteria to prove the success of the medium acclimation. Both processes reached a similar conversion of the organic matter 77 ± 0.01. As well, biogas productions at ambient temperature were equal to 479.9 L and 489.6 L biogas/ kg VS for AD and ACOD respectively. These results followed the expected comportment for biogas production showing values that could be included in the range of other studies that operated with some similar conditions. Previous studies realized with co-digestion of CW with dairy manure using UASB and CSTR reactors at temperatures of 35-37°C. In these, they were reached biogas productions between 245.45 L biogas/kg VS (Carlini et al., 2015) and 838.4 L biogas/kg VS (Fernández et al., 2015).
Process
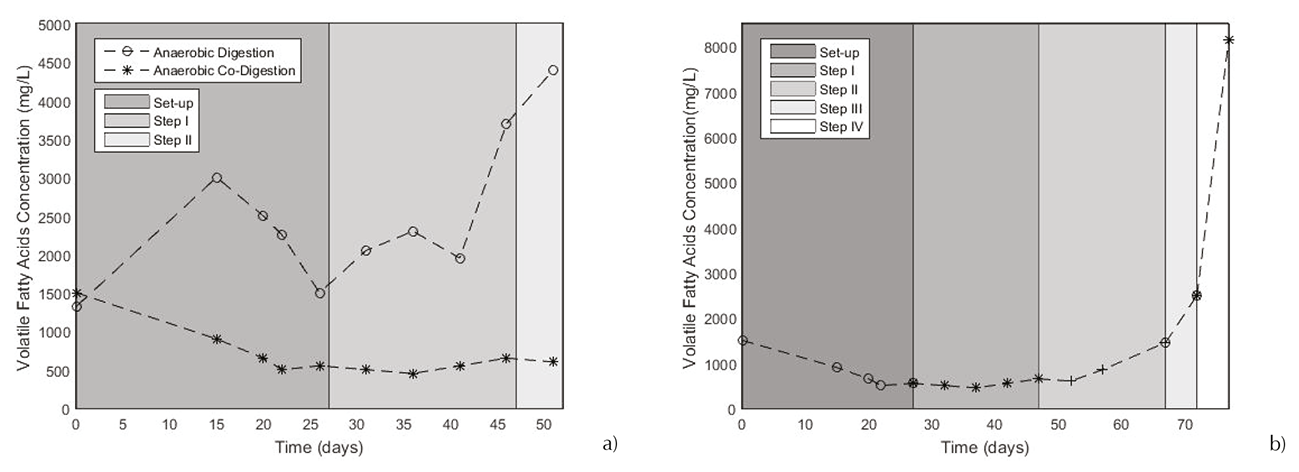
The continuous processes were undertaken initiating with an OLR equal to 2 g VS/L. Starting without the effluent recirculation, after a 20-days period, the feed volume changed due to the recirculation of 40% of the effluent. The system response to the recirculation showed a notorious difference between both bioconversion processes. VFA production in anaerobic processes is an important factor because represents the substrate organic matter degradation. In Figure 3a, was presented VFA production for AD and ACOD systems. Using an acid substrate as cheese whey, the process is harder to control, since this substrate has a high content of lactose. Lactose is a sugar which produced rapidly VFA during the acidogenesis phase (Comino et al., 2012). Dealing with AD process, VFA concentration increased as soon as began the continuous process. Always this concentration was higher than 1000 mg/L, when other author reported a VFA concentration of effluent that was kept lower than 500 mg/L (Fernández et al., 2015). It is supposed that this accumulation provoked an inhibition of the methanogenic archaeas growing. Recirculation implementation was not efficient because the medium was already acidified. The inhibition of AD process can be underline by the concentration nearly of 5000 mg/L in the step II that could represent the inhibition by acids accumulation (Xu, 2014). Studying the ACOD process, during steps I, II and III, VFA concentration remained consistently below 2000 mg/L. This fact can demonstrate the ACOD performance due to the effective work of the methanogenic arqueas which reached to consume the acids. Nevertheless, as the AD process, the medium inhibition is explained by the high and rapidly production of AGV (Figure 3b). This rapid conversion makes the methanogenic arqueas growing difficult because their quantity was not sufficient to support an OLR of 3 g VS/L day (Step IV). It was reported a VFA concentration of 8150 mg/L at the end of the process.
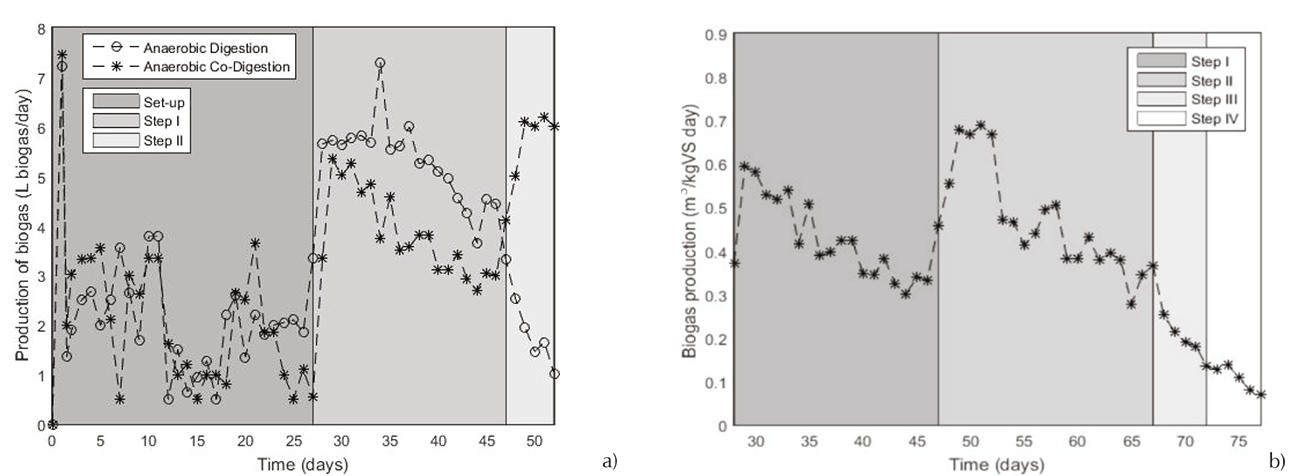
Biogas production in the AD process is the criterion which allows ensuring the yield of the system. For the AD, the biogas production during this first step is represented in the Figure 4. As it can see, the daily production had a high increase beginning the continuous process but it decreased after ten days. It was observed a biogas production of 535 L biogas/kg VS day for AD system. As well, the recirculation establishment did not help the AD system to regenerate. There are in the literature, studies with a higher OLR than 2 kg COD/m3*day. Yet, the biogas yield was not relevant: 0.095-0.151 m3 CH4/kg COD for an OLR of 9 kg COD/m3*day (Rugele, 2013) due to the VFA accumulation. The same comportment was observed for the ACOD during the step I; however, the rapidly rising of biogas production proved that the recirculation improves considerably the ACOD microbiology. That means that the effluent recirculation helps to balance microbial communities in the reactor (Wang, 2014). The recirculation allows feeding the reactor with a medium more alkaline. It alternative enables to maintain equilibrium of phases in the reactor. The lack of alkalinity mentioned in the AD results was resolved adding a medium with a higher alkalinity (Wu et al., 2016). The ACOD process is seen as a solution to overcome the potential problem of destabilization (Heldal et al., 2014). In the Figure 4, it can be observed for the steps I, II and III that the ACOD system replied to changes (implementation de la recirculation and augmentation of the OLR) with a rising of the biogas production. For steps I and II, biogas production was respectively equal to 0.401 and 0.409 m3 biogas/ kg VS. Authors investigated the recirculation influence and this use permits to increase the OLR from 2 until 3 g VS/L day avoiding the inhibition (Nordberg et al., 2007). The daily biogas production average of this ACOD process equaled to 427.8 and 469.2 L biogas/kg VS day respectively for the steps I and II. Another authors reported, a daily biogas yield average in a range between 0.502 and 1.055 m3 biogas/m3*day at a temperature between 25 and 34°C (Kavacik et al., 2010). Finally, it can be concluded that the OLR of 3 g VS/L day was the break point of the ACOD process, due to the VFA accumulation, therefore the drop in the biogas production.
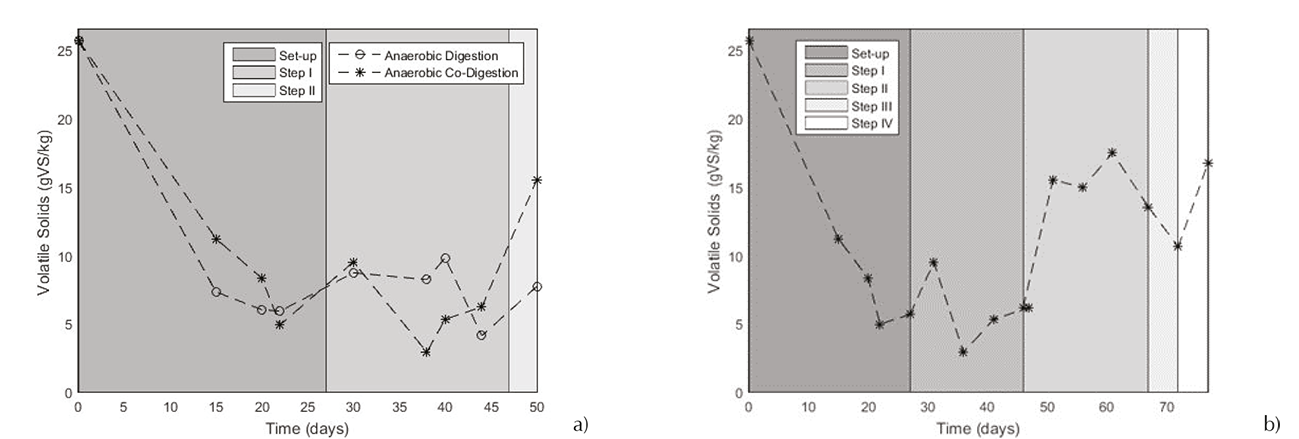
The organic matter degradation was analyzed by measuring the VS content. In Figure 5, were shown the systems comportments. It can be observed that the best yield of degradation was reached in the step 1 with 93%±0.4 for both systems. Since the step 2 (establishment of the effluent recirculation), the degradation underwent a decrease. In this phase (Steps II, 2g VS/L day with recirculation) a decrease of 3 and 4% was observed (for AD and ACOD respectively). This was a consequence of the recirculation, which rise the input organic matter (Namsree, 2012). During the step 2, it can be conclude that the degradation was sustained constant. In those cases, a high degradation can be traduced in a high biogas production (Ge et al., 2011). For the ACOD system, in steps III and IV (Figure 5b), degradation of VS of 85 and 77% respectively, was observed. These results, demonstrate that the combination of the reactor design and the effluent recirculation use allow exceeding yields reported in the literature. It was found a degradation of 60% with the same mix (CW: FDM 65:35; Comino et al., 2012), as well that it was reported a yield of 67.2% for an OLR of 2.7 g VS/ L. (Rico et al., 2015; Kavacik et al., 2010). Experiments did not surpass to degrade the 42% of the influent VS at mesophilic temperature (35-37°C). As an additional factor, it is important to mention that in this study, the methane content in the biogas obtained at the end of the step III was 68%.
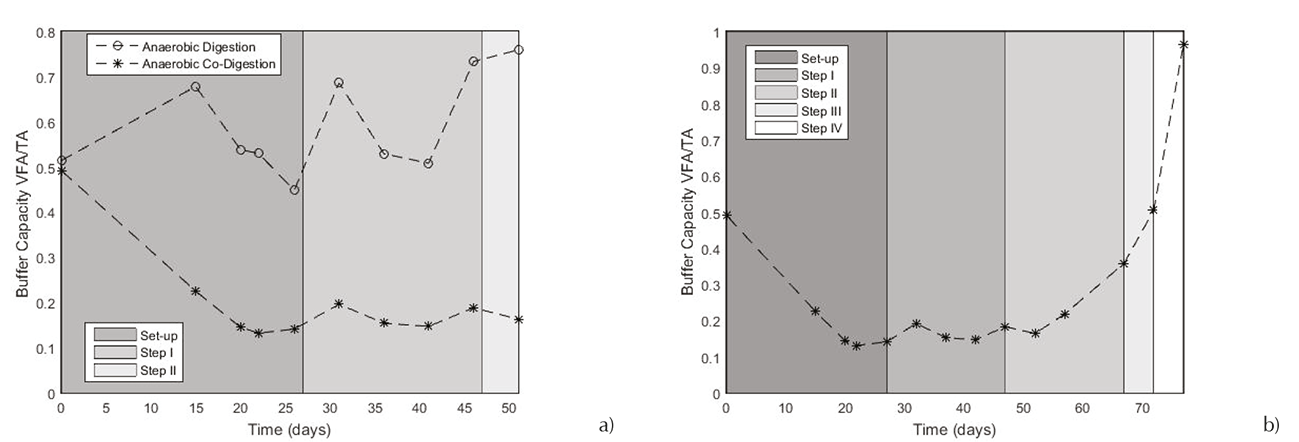
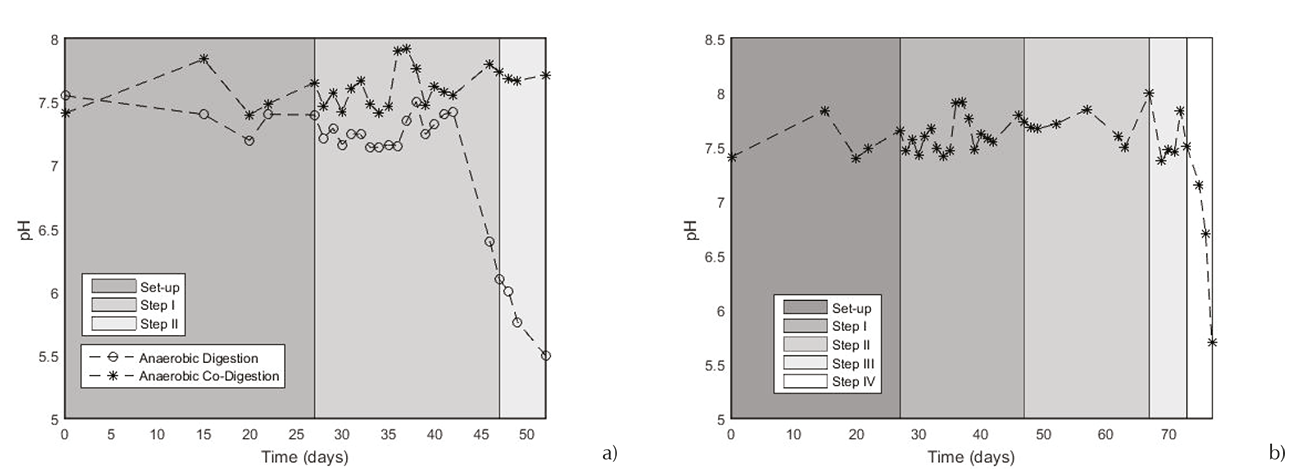
The process stability can be evidenced by the buffer capacity and pH analyses. Respect to the buffer capacity (Figure 6a), it can be mentioned that the cheese whey bioconversion is a process which may encounter some stabilization problems. The AD process failed after nearly 46 days. That can be supported when the buffer capacity approached 0.8. Secondly, was studied the ACOD process, comparing its comportment. In the Figure 6b, the buffer capacity of ACOD showed a better behavior. Since the twentieth day (batch period) until the seventy-second day, it was maintained between 0.1 and 0.5 which means that the system revealed a very good stability. In this day, the buffer capacity exceeds 0.5. It can be interpreted as the process inhibition by a high concentration of acids with a low total alkalinity too low to compensate this acidity.
Gradient of pH and VFA
This analysis was realized by measuring the pH and the VFA concentration at three different points along the reactor (first point 0.33 m, second point 0.66 m and output 1 m from the inlet). Due to the rapid inhibition of the AD system, this examination was done only with the ACOD process of CW with FDM. In the Figure 7, it can be observed the pH evolution during this process, in the different points. During the first step of continuous process (without recirculation), the pH was kept constant at around 7.4 ± 0.4 (Figure 8a). Nonetheless, as soon as the recirculation was implemented, it was brought to light at the 25 days de ACOD, a notorious decrease of pH (6.53 ± 0.01) in the first part of the reactor. In the second point, the pH reached a value of 7.5 and was maintained to the outlet. This phenomenon can be traduced as the inception of a phase separation. Five days after, still with 2 g VS/L day with recirculation, pH value was of 5.75 ± 0.02 in the first part by keeping an alkaline outlet which enables to make sure the proper functioning of the process. This fact explains that with a large VFA production, it was deducted that the methanogenic archaeas activity was satisfactory to consume the majority of these acids produced, attaining a biogas production favorable (0.409 m3 biogas/kg VS). The pH value from the second point revealed the existence of the methanogenic phase, which needs alkaline conditions to ensure the archaeas activities. In the Figure 9, it can be seen the co-existence of these phases. When the reactor began to be operated with the OLR of 2.5 g VS/L day with recirculation, it was observed a behavior change, by reducing the conditions of neutrality of the methanogenic phase. Lastly, with the last OLR (3 g VS/L day with recirculation), the acid phase has been extended along the reactor (pH values below 6). The low values of pH reduced severely the biogas production, by pointing out the acidification of the ACOD process. The acidogenic phases overcame the methanogenesis (Figure 9).
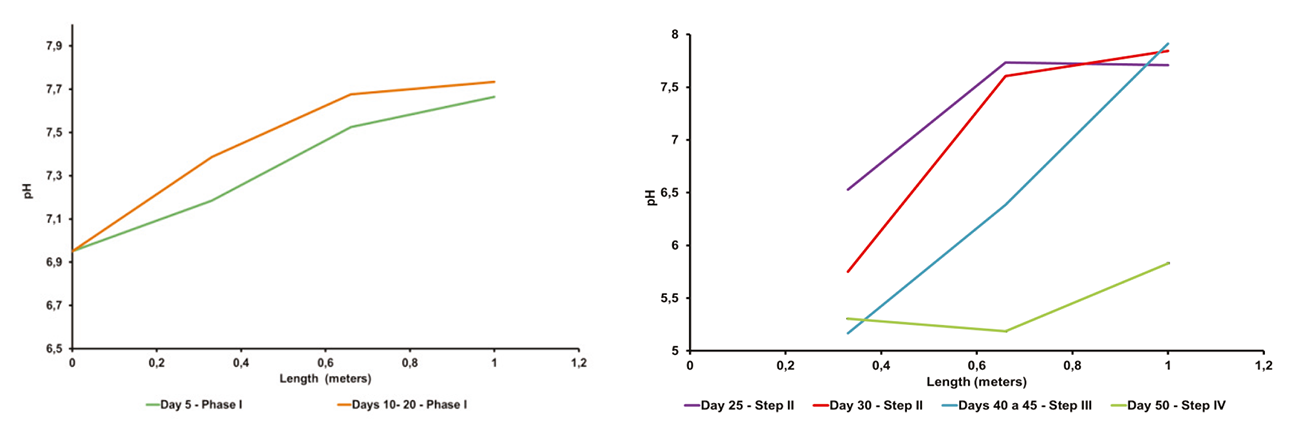
Figure 8 a) Evolution of pH of the ACOD process in the reactor axial direction for the first step without recirculation, b) in the other phases with recirculation: Steps II, III and IV
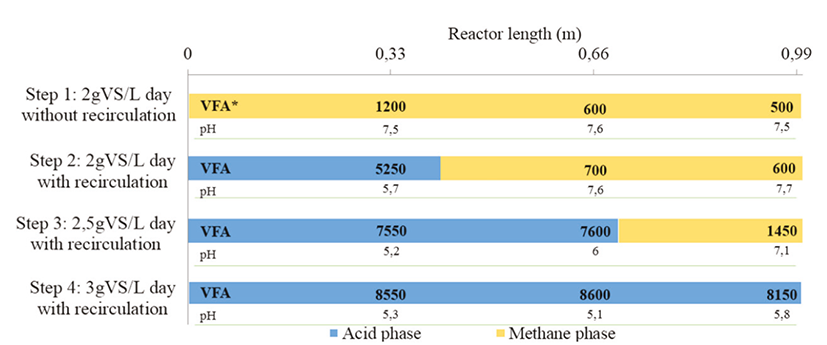
Figure 9 Bars showing zones of dominant acidogenic and methanogenic phases in plug flow reactor operating in different steps of the continuous process (*VFA= mg/L)
In that context, it was possible to apply the Ec. 2 to describe in a simple way the behavior of the anaerobic process according to the content of VFA along the reactor. Equation 2 can predict the proper functioning of the process represented in the fact that acidogenic and methanogenic phase coexists. In the best of the cases, the first part of the Ec. 1 should be near to cero, and the second part near to the value of the 𝐶 𝑖𝑛ℎ . This is linked to the fact that in the acidogenic phase there are a high concentration of VFA, in the other hand, in the final section of the reactor, the concentration of VFA should be reduced because of the activity of methanogenic archaeas. Consequently, the values of 𝛽 could be interpreted as it is shown in Ec 2.
In the study, the inhibition concentration of the system was considered equal to 5000 mg/L of VFA. In the first step, the dominance of methane phase is underlined by the values of 𝛽 between 1.38 and 1.64. The phases’ co-existence may be observed with 𝛽values of 0.81 and 0.52. Finally, the inhibition process was substantiated by the 𝛽 values of -1.07 and -1.34.
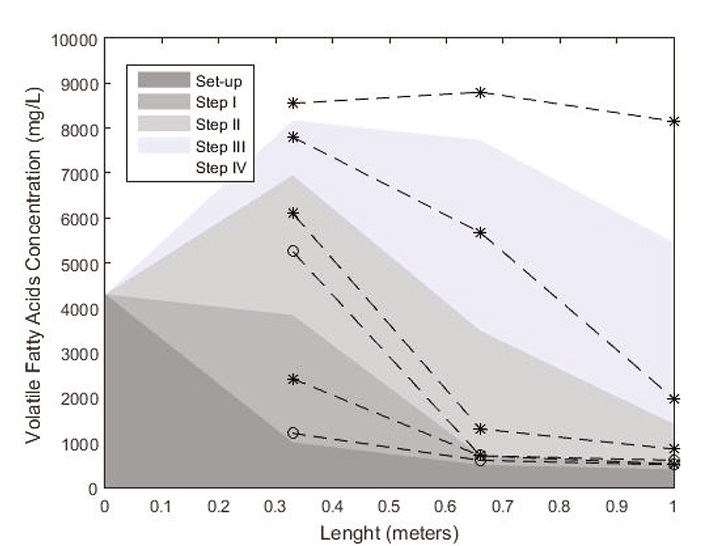
The Figure 10 shows the variability of the VFA concentration in the reactor axial direction. These results confirmed the pH analysis. With an OLR of 2 g VS/L day with recirculation, the reactor was feed with an initial VFA concentration of 4300 mg/L, increased to 6100 mg/L in the first part of the reactor and in the outlet, the concentration was equal to 850 mg/L. These last results prove that was implemented an acids consummation and that the reactor design allows a phase separation. In the first part, the hydrolysis and acidogenesis phases occurred and in the second part, were the acetogenesis and methanogenesis. These conditions were testes with AD of sludge wastewater (Liu et al., 1997). This separation permits a considerable enhancement of the process stability (Stamateleou, 2012). In fact, the two groups of bacteria have not the same necessity of growing conditions. By separating the phases, the bacteria growing and thus, their activity is improved (Saddoud et al., 2007).
The process destabilization can be explained by the VFA variability along the PFR. Indeed, by increasing the OLR (Step III), it was perceived an augment of the acids in the first part of the reactor (7800 mg/L), and in the second part, the consummation was no longer efficient as the concentration of 5675 mg/L was. In compliance with these last values, it can be affirmed that the process began to be destabilized. In the last step, it was improved completely the inhibition of the process along the reactor, with a nearly constant value of 8150-8500 mg/L, this fact demonstrated the presence of only one phase: acidogenesis in the reactor (Figure 9).
Finally, it is important to point out that anaerobic processes are sensitive to temperature changes. Traditionally, the AD is carrying out at 37°C because it is the optimal temperature for the methanogens growing and reaction rate of the hydrolysis and acidogenesis is faster. In real conditions, digesters must be able to function given low and fluctuating ambient temperature (Perrigault et al., 2012). In this study, the AD process was implemented at ambient temperature to simulate the local conditions and avoid the rapid production of VFA. The results obtained, in term of biogas production, during this investigation were comparable to others studies realized in mesophilic conditions (35-37°C), that suggests an acclimation of the bacteria at ambient temperature. To apply AD processes at mesophilic temperatures, it requests a supplementary energy source (approximately 20% of the heat produced is destined to the auto-consummation). In various cases, this demand was completed by the own biogas production of the reactor. Therefore, it results that, even if these systems produce best quantities of biogas, this entire production cannot be totally exploited.
Conclusion
In this research, anaerobic digestion from cheese whey was implemented in a tubular reactor under local conditions. Experiments demonstrated that the AD of an acid substrate tends to inhibit due to the accumulation of VFA (5000 mg/l) in the system. To counteract those problems, it was proved that the combination of recirculation and co-substrate use, allow improving the process stability. In this sense, the anaerobic co-digestion of cheese whey with fresh dairy manure system demonstrated better performance in terms of stability (pH kept between 7.5 and 7.9 and a VFA/TA ratio lower than 0.4), by reaching a biogas production of 0.409 m3 biogas/kg VS with an OLR of 2 kg VS/m3*day. In that case, the reactor’s design permits to highlight the existence of two main phases (acid phase and methane phase). Finally, this study suggests that the plug flow digester could be benefic for an acid substrate anaerobic treatment without supplementary energy requirements.











 nueva página del texto (beta)
nueva página del texto (beta)