Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Ingeniería, investigación y tecnología
On-line version ISSN 2594-0732Print version ISSN 1405-7743
Ing. invest. y tecnol. vol.12 n.3 Ciudad de México Jul./Sep. 2011
Laser Radiation CO2 Effects in Cement Paste at Different Hydration Stages after Preparation
Efectos de la radiación láser de CO2 en la pasta de cemento a diferentes etapas de hidratación después de su preparación
Moreno–Virgen M.R.1, Soto–Bernal J.J.2, Ortiz–Lozano J.A.3, Frausto–Reyes C.4, Bonilla–Petriciolet A.5, González–Mota R.6, Rosales–Candelas I.7 and Pineda–Piñón J.8
1 CICATA. Querétaro, Qro. México Instituto Tecnológico de Aguascalientes. E–mail: moreno_virgen@yahoo.com.mx
2 Instituto Tecnológico de Aguascalientes. E–mail: j2sb@cio.mx
3 Universidad Autónoma de Aguascalientes. E–mail: aortiz@correo.uaa.mx
4 CIO, Unidad Aguascalientes. E–mail: cfraus@cio.mx
5 Instituto Tecnológico de Aguascalientes. E–mail: petriciolet@hotmail.com
6 Instituto Tecnológico de Aguascalientes. E–mail: Rgmota73@yahoo.com.mx
7 Instituto Tecnológico de Aguascalientes. E–mail: ilianaroca@yahoo.com
8 CICATA. Querétaro, Qro. México. E–mail: arqjpp@yahoo.com
Información del artículo: recibido: agosto de 2009.
Reevaluado: abril de 2010.
Aceptado: octubre de 2010.
Abstract
In this work the changes occurring in cement pastes irradiated by 10.6¡mi CO2 laser at different stages of hydration after preparation are presented. Raman spectroscopy, X–ray diffraction and Scanning Electronic Microscopy (SEM) techniques were used to observe molecular structural changes. Intensity of cement paste Raman peaks after laser irradiation was monitored in samples irradiated 2, 3, 4, 5, 6, 7, 8, 9,10 and 11 days after their preparation. Applied laser power changed Raman peaks intensity at 187.5cm–1,563cm–1, 695cm–1, 750cm–1, 897cm–1,1042cm–1 and 1159cm–1 that correspond to compounds already present in cement pastes. X–ray diffraction, SEM images and changes in the Raman peaks confirm the recrystalization of cement paste compounds into new phases (alite and belite) after irradiation. The produced changes show a clear dependence on the applied laser power density and age of samples.
Keywords: cement paste, laser, characterization, hydration stages.
Resumen
En este trabajo se presentan los cambios ocurridos en la pasta de cemento irradiada con láser de CO2 a 10.6|im a diferentes edades después de su preparación. Las técnicas de Espectroscopía Raman, Difracción de Rayos X y Microscopia Electrónica de Barrido (SEM) se usaron para observar cambios en la estructura molecular. La intensidad de los picos Raman de las pastas de cemento después de la irradiación fue monitoreada en muestras irradiadas a 2, 3, 4, 5, 6, 7, 8, 9, 10 y 11 días después de su preparación. La potencia de láser aplicada cambió la intensidad de los picos Raman a 1875cm–1, 563cm–1, 695cm–1, 750cm–1, 897cm–1, 1042cm–1 and 1159cm–1, que corresponden a los compuestos ya presentes en la pasta de cemento. Las imágenes de difracción de rayos X y SEM, confirman la recristalización de los compuestos de la pasta de cemento en nuevas fases (alita y belita) después de la irradiación. Los cambios producidos muestran una clara dependencia de la densidad de potencia del láser aplicado y la edad de las muestras.
Descriptores: pasta de cemento, láser, caracterización, etapas de hidratación.
Introduction
Unique characteristics of laser radiation make it useful for material processing and characterization (Tirumala et al., 2005). Laser techniques have been already used on concrete surfaces for several tasks. CO2 laser can remove contaminated surface layers of concrete and modify the surface appearance as well as surface properties of cement–based materials. Laser treatments produce novel surfaces with textures, properties and appearance unique to treated materials (Lawrence et al., 2000). Effects of laser radiation on the structure of cement paste or concrete have been reported a little. Using commercially batched ready–mix concrete, a linear relationship was established for compressive strengths at 28 days (wet–cured) and 6 h (microwave–cured). The relationship is important because the impact of concrete mix adjustments is quickly appreciated, reducing the frequency and financial severity of rework or litigation (Tumidajski et al., 2003).
Under intense laser radiation, heat generation is expected from the absorbed light. The effects of elevated temperatures on concrete properties have been extensively studied. In general, concrete compressive strength decreases as temperature increases.
Kim et al. (1998) modeled the development of compression strength in concrete according to curing temperatures. They showed that concrete cured at high temperatures reaches higher strength at early ages, although it decreases with time. Weather conditions have a direct impact on cement hydration, setting, hardening and strength development. The temperature at which these processes occur affects concrete microstructure (Ortiz et al., 2008 and 2009). The absorption coefficients of aggregates are greater at high temperatures, with reference to saturation times, after 30 min and 24 hours decreases at high temperatures. Both these facts have consequences for workability of concrete (Ortiz et al., 2009). On the other hand, concrete cured at low temperatures starts with lower strength but it reaches similar or higher strength values after certain elapsed time.
Around 100°C the physiabsorbed moisture begins to evaporate and elasticity reduces by about 10–20%, but compressive strength remains unchanged. For temperatures above 300°C, the hydration water of silicates is released causing a contraction of cement paste but solid aggregates expand. Compressive strength decreases slowly between 450°C and 500°C, whereas at temperatures higher than 500°C it falls rapidly. Around 600°C, the crystals in the aggregate undergo a α–β–SiO4 conversion increasing their specific volume. Calcium hydroxide begins to dehydrate deteriorating the concrete structure. As temperature approaches 900°C, calcium carbonate decomposes losing all free or bound water and the compressive strength falls to zero (Wei et al., 2000).
Another factor that has a clear influence on compressive strength is the pore structure of porous media, which has been recognized as a vital parameter influencing the properties of cemented material, such as strength, fluid transport ability and thus durability (Fall et al, 2008).
High power laser treatments on concrete produce instantaneous heating in specific surface areas, inducing superficial structure changes in the concrete. These changes may be affected by the traverse speed and the depth of the laser interaction. CO2 laser radiation at 10.6 interacts with water molecules and its effect is stronger compared to other lasers as high power diode laser.
Raman Spectroscopy, a useful tool for material characterization, allows the detection of changes occurred in the laser treated materials. The sensitivity of certain materials to laser irradiation may be an advantage when the study involves laser–induced oxidation and/ or crystallization processes (Witke et al., 1998).
Alarcón et al. (2005) reported studies of cement paste exposed to fire at temperatures over 800°C heated by stages of 100°C for periods of 24 hours. They used thermal analysis techniques to study the effect of the temperature in the mineralogical composition of hydrated cement. This kind of analysis assumes that during heating, the cement paste suffers a continuous sequence of ±1 reaction of irreversible decomposition. They concluded that irreversible reactions of dehydration and decarbonation in cement paste can be used as reference to estimate the temperature reached by concrete during fire exposition.
X–rays diffraction and hydration heat techniques may be used to characterize the amount of hydrates in cement. These methods can only be used in certain stages of the hydration.
Hydration progressively reduces the intensity of the bands, but does not generate band a new locations. It is tentatively suggested that the fluorescence affect may be somehow associated with the status of the cement components as orthosilicates (Newman et al., 2005).
Furthermore, they involve some assumptions about the chemical nature of the cement mixture.
Scanning Electronic Microscopy (SEM) has been used to analyze changes in microstructure and hydrate grade in samples subjected to different types of cured. The analysis by SEM images carried out by Goncalves–Silva et al. (2002) may be considered a valid option in relation to other techniques; the analysis of cement–based materials is useful in the evaluation of mechanical properties, water absorption, air permeability and evolution of hydration degree.
New advantageous techniques of analysis based on the image information are possible using electronic microscopy. They propose here an alternative procedure to the segmentation of microstructures of cement and aggregates in concrete. The central idea consists of a particular use of the morphological operator watershed.
Characterization methods like X–ray diffraction and Raman spectroscopy are used in materials like cement minerals, cements and their reaction products (Skibsted et al., 2008). Raman spectroscopy has received attention in its application to the characterization of pure cement phases. Various configurations of instrumentation and laser excitation sources have been used. The study of Potgieter et al. (2006a) reported studies of pure synthesized cement phases and hydration of pure cement phases.
Also Potgieter et al. (2006b) reported the characterization of OPC (Ordinary Portland Cement), fly ash and slag, using UV–VIS, VIS and NIR excitation in micro–Raman spectrometry and their results were compared with previously published results. Also, they reported a summary of Raman shifts for non–irradiated cement in which they used as excitation lasers He–Ne, Ar and IR. They found that the Raman shifts for the pure mineral phases and those in the clinkers investigated with a NIR excitation source (1064nm) differed significantly from the shifts observed with VIS excitation with respect to the silicate phases. In this work we used a semiconductor excitation laser with a wavelength of 830nm to obtain Raman shifts of the irradiated cement.
Another study show a scanning electron microscope (SEM) pint–counting technique to study the hydration of plain Portland and blended cement pastes containing fly ash and or slag (Feng et al., 2004).
In this work we report surface changes measured in cement paste samples after being irradiated by a 10.6|im CO2 laser. The changes were monitored by Raman spectroscopy, X–ray diffraction and Scanning Electronic Microscopy (SEM). As shown by the reported results, the changes in cement paste depend on laser intensity and they can be used to know their initial state of hydration.
Materials and methods
Materials
Cement paste samples were prepared with an Ordinary Portland Cement (CPO) that corresponds to an ASTM C 150 type I cement and distilled water to eliminate any influence of trace impurities. The cement compressive strength obtained by the procedure described by ASTM C 109 resulted 32.5MPa at an age of 7 days.
A CO2 laser Synrad 40W was coupled to a 0.6cm focal length external lens to have a laser spot size of 0.1cm of ratio at sample surface. An area of 0.0078 cm2 of the paste surface was irradiated applying different laser powers of 20, 26, 30 and 33W.
Methods
The cement paste samples were mixed according to the ASTM C 305 "Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency" (ASTM C305–99, 1999) with a water/cement ratio of 0.5.
Laser radiation of cement paste samples started 48 hours after their manufacture, treatment consisted of a single laser scan at a speed of 1.27m/s and which reached a surface temperature of 170°C. Laser irradiation treatment was applied on samples at different ages. Ages considered started at two days old and they were increased by one day steps up to 11 days old.
Laser treated samples were characterized by Raman spectroscopy, x–ray diffraction and Scanning Electronic Microscopy (SEM). Profile intensity changes were recorded in the Raman spectra of the cement paste samples after CO2 laser radiation treatment. Raman spectra were taken with a Renishaw model 1000 Raman spectrometer (excitation wavelength of 830nm) for all samples. Several spectra (up to 5) were taken and averaged for samples of a given laser treatment and age.
X–rays diffraction spectra were obtained for cement paste samples before and after laser treatment with a Rigaku model Dmax–2100 x–ray difractometer.
SEM micrographs were taken from the surface of the cement paste samples, before and after laser treatment with a XL30 SEM equipped with an EDAX Spectrometer for X–ray dispersion elemental analysis.
Results and analysis
Both Raman spectra, from irradiated and not irradiated samples can be compared in figure 1. The Raman spectrum from two days old cement paste not exposed to CO2 laser irradiation presents weak peaks at 187 and the irradiated strong peaks are 187.5, 563, 695, 750, 897, 1042 and 1159cm–1. These peaks correspond to compounds usually found in cement as the one located at 187.5cm–1 due to the presence of Fe2O3; the peak at 563cm–1 indicates the presence of Si–Si bond, alumina (Al2O3) produces peaks at 695cm–1 and 750cm–1 from the Al–O bond. Peaks at 897 and 1042cm–1 reveal the presence of calcium silicates. Peak at 1159cm–1 indicates the presence of gypsum (CaSO4 · 2H2O).
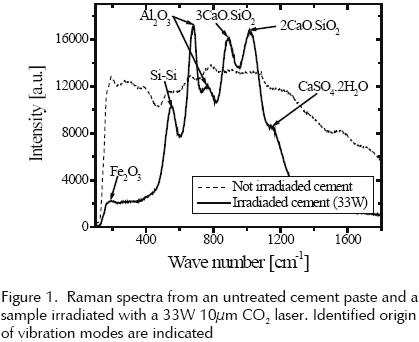
Fe2O3 and Al2O3 peaks do not exist independently in the cement paste but belong to agglomerate as C4AF (tetracalcium aluminoferrite) that are not chemically bonded but can be detected independently by means of Raman spectroscopy.
The series of spectra taken for cement paste samples of the same age (two days old), under different CO2 laser irradiation conditions are plotted in figure 2a. Similar series of Raman spectra were obtained for older samples in different stages of hydration, which were irradiated from three up to eleven days after preparation.
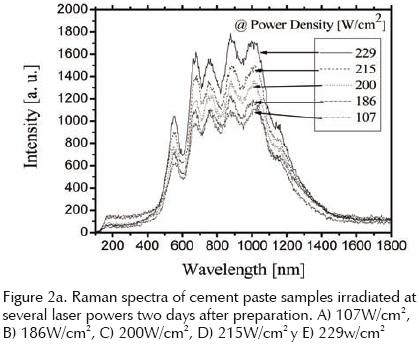
Although it is not a strongly marked trend, the intensity of the different peaks present in the Raman spectra, increase with the increment in power density of the CO2 laser applied with the exception of the peak located at 187.5cm–1 of Fe2O3 that shows a negative tendency. Raman Spectroscopy can detect only compounds or functional groups, chemically pure metals cannot be detected by this technique, which is why there is a negative trend in the growth of Fe2O3 peak with increasing radiation power, because of energy deposited in the sample by the laser is sufficient to break the covalent bond between the iron and oxygen.
The heats of formation (which are the same dissociation) of Al2O3 and Fe2O3 are respectively: –1675.7KJ/ mol and 820KJ/mol where negative sign indicates that this is a reaction that releases heat and being provided a number of additional energy cause an inhibition in the same and, therefore, will not break the covalent bond that links the Al and O, showing that less energy is needed to break the covalent bond that links the Fe and O, that breakup is possible by laser radiation.
This can be seen first in the Raman, noting a negative slope at the peak of that element, indicating that it is reducing the amount of the compound (Fe2O3), on the other hand, the SEM (elemental analysis) indicates a increase of Fe in the sample, the product of the dissociation of Fe2O3.
The intensity of the peaks in the Raman spectra taken at different days after preparation presents a linear trend versus age. In figure 2b this trend is clear from the slope of growth of the Raman picks of those samples as observed. Peaks at 750 and 1159cm–1 are the least affected by laser irradiation. All other peaks corresponding to Si–Si, Al–O, Ca–O, Ca–Si bonds have minor difference between their slopes that fall around 0.016.
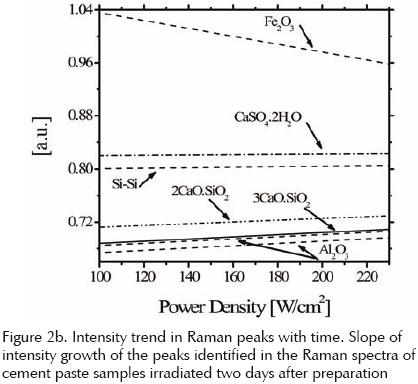
Figure 3, shows SEM micrographs from the surface of cement paste samples two days after preparation. Figure 3a shows cement paste without laser treatment. Figure 3b shows two clearly distinguished regions, left side shows non irradiated surface and right side shows laser irradiated surface. Figure 3c shows an amplified image of the CO2 laser irradiated zone were spheres smaller than ten microns in diameter can be seen.
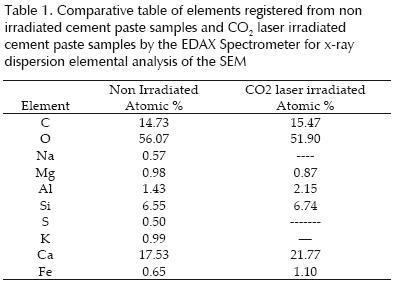
Table 1 presents the elemental composition for cement paste with an age of two days with 33W and without CO2 laser irradiation. In the laser irradiated surface, two phases can be seen (alite and belite), one without a defined morphology (belite) and the second one spherically shaped (alite) respectively. The elemental analysis of these two phases is shown in table 2 where both phases are compared for samples irradiated with different intensities.
Figure 4 shows the x–ray diffraction spectrum for cement paste samples, a) natural sample and b) CO2 laser irradiated, some identified peaks are indicated.
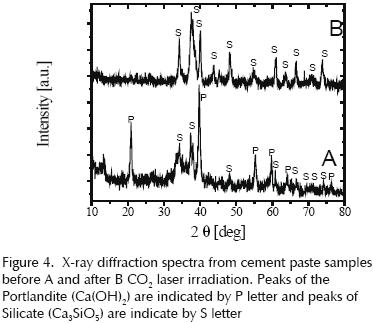
The following sample was irradiated two days after preparation and was analyzed by XRD at the age of 28 days.
The range used in this diffractogram is needed to identify the alite and belite.
Discussion
The SEM results reveal the formation of two phases in the CO2 laser irradiated area, with clearly differentiated morphology that we call them phase A (alite) and phase B (belite). The composition of CO2 laser irradiated samples show a different composition from the original cement paste as revealed by the elemental analysis whose results are displayed in table 1. There, it is clear that elements like Sodium (Na), Sulfur (S) and Potassium (K) present in the original sample were removed by the CO2 laser. The power density was sufficient to evaporate those elements, in fact, the laser has been used to clean the concrete surface, and in general, to remove surface impurities.
The elemental composition of the laser treated zone reveal the presence of calcium carbonate and calcium silicate.
Phase belite with irregular morphology and phase alite composed of spherical particles of diameter around 10μ as shown in figure 3c, also show different compositions.
As shown in table 2 where the elemental analysis of both materials allow to compare the composition of both phases for samples irradiated with intensities, of 28, 30, 31 and 32W, phase alite contains more calcium and less oxygen and carbon than phase belite, all other elements as Mg, Al, Si y Fe are similar in both phases. It is also observed that the ratio X/Ca were X is C, O and Si is higher for phase B.
The changes observed through the SEM images, can be related to the changes observed in the X–ray diffraction pattern shown in figure 4, where a laser induced crystallization process is revealed the laser radiation induces an increase in temperature and consequently increases the rate of cement hydration, producing more quantity of portlandite crystals.
The normal setting of Portland cement appears to result from the hydration of C3S and C3A and the formation of CSH phases and AFT. According to other studies reported (Ortiz, 2005), the setting of Portland cement is due to a re–crystallization of the primary microcrystalline ettringite crystals highly developed. The crystal phases revealed by SEM and X–ray analysis can be tracked by Raman spectroscopy because of the Raman spectrum provides information on the crystallinity of the material: if the Raman peak is narrower then the material is more crystalline, thus showing the recrystallization of the irradiated material.
The negative slope of the peak of Fe2O3 in the Raman spectra reflects the loss of this compound in the sample, that decreasing is because laser radiation is breaking the covalent bond between iron and oxygen, and it explains why the analysis performed with SEM elemental revealed an increase of iron produced by the dissociation of Fe2O3.
Further research is under way to relate these results to chemical reactions in cement and concrete, including aggregates such as sand and gravel to assess hydration process in cement products.
Conclusions
CO2 laser irradiation induced structural changes in cement paste that were revealed in the present study. Absorption of laser radiation by cement paste increases the local temperature high enough to recrystallize the surface material. Some material as Na, S and K were removed from the irradiated zone. Recrystalized material is mainly alite which shows a spherical morphology with less than 10u,m in diameter immerse in a connected net of belite. Used characterization techniques proved to be valuable when identifying these changes. The produced changes show a clear dependence on the applied laser power density and age of samples.
The laser induces an increase in the surface temperature of the cement paste why accelerates the production of crystals of portlandite, observed in the XRD, SEM and corroborated with the narrowing of the Raman peaks. Measured Raman peaks correspond to compounds already present in cement however; laser irradiation promotes the growth of crystalline phases.
Acknowledgements
The authors are grateful to CONACYT (49765–F), CO–FAA and SIP–IPN for their economic support.
References
Goncalves–Silva A. Classification of Microstructures by Morphological Analysis and Estimation of the Hydration Degree of Cement Paste in Concrete. XV Brazilian Symposium on Computer Graphics and Image Processing (SIBGRAPI'02). IEEE Computer Society, Brazil. 2002. [ Links ]
ASTM C150 Standard Specification for Portland Cement of 32.5 MPa, 1999 ASTM Standardas, Vol. 04.01, American Society for Testing and Materials, West Conshohocken, PA, USA. 1999. [ Links ]
ASTM C305–99 Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency, 1999 ASTM Standards, Vol. 04.01, American Society for Testing and Materials, West Conshohocken, PA, USA. 1999. [ Links ]
Tirumala–Rao B., Kumar R., Nath A.K. Processing of Concretes with a High Power CO2 Laser. Opt Laser Technol., 37(5):348–356. 2005. [ Links ]
Skibsted J., Hall C. Characterization of Cement Minerals, Cements and their Reaction Products at the Atomic and Nano Scale. Cement Concrete Res., 38(3):205–225. 2008. [ Links ]
Ortiz J.A., Aguado A., Roncero J., Zermeño M.E. Influencia de la temperatura ambiental sobre las propiedades de trabajabilidad y microestructurales de morteros y pastas de cemento. Revista Cemento y Concreto. Investigación y Desarrollo, 1(1):2–24. 2009 [ Links ]
Ortiz–Lozano J.A., Aguado de Cea A., Agulló–Fité L., García–Vicente T. y Zermeño de León M.E. Experimental Study of the Effect of Temperature on the Strength of Reddy Mixed Concrete. Theory. Materiales de construcción, 58(291):7–22. 2008. [ Links ]
Ortiz–Lozano J.Á. Estudio experimental sobre la influencia de la temperatura ambiental en la resistencia del hormigón preparado. Tesis (Doctoral) España. Pp.52–53. 2005. [ Links ]
Kim J.K., Moon Y.H., Eo S.H. Compressive Strength Development of Concrete with Different Curing Time and Temperature. Cement Concrete Res., 28(12):1761–1773. 1998. [ Links ]
Lawrence J., Li L. A Comparative Study of the Surface Glaze Characteristics of Concrete Treated with CO2 and High Power Diode Laser. Part I: Glaze characteristics. Mater Sci Eng., 284(1–2):93–102. 2000. [ Links ]
Ortiz J., Aguado A., Agulló L., García T., Zermeño M. Influence of Environmental Temperature and Moisture Content of Aggregates on the Workability of Cement Mortar. Construction and Building Materials, 23(5):1808–1814. 2009. [ Links ]
Witke K., Klaffke D., Skopp A., Schreckenbach J.P. Laser–Induced Transformation as a Tool for Structural Characterization of Materials by Raman Spectroscopy. J Raman Spectrosc., 29(5):411–415. 1998. [ Links ]
Alarcon–Ruiz L., Platret G., Massieu E., Ehrlancher A. The Use of Thermal Analysis in Assessing the Effect of Temperature on a Cement Paste. Cement and Concrete Res., 35(10):609–613. 2005. [ Links ]
Fall M., Samb S.S. Pore Structure of Cemented Tailings Materials Under Natural or Accidental Thermal Loads. Materials Characterization, 59(5):598–605. 2008. [ Links ]
Ortiz–Lozano, J.A., Aguado de Cea A., Zermeño de León M.E. y Alonso–Farrera F.A. Influencia de la temperatura ambiental en las propiedades del concreto hidráulico. Ingeniería. Revista académica de la FI–UADY 11(2):13–20. 2007. [ Links ]
Tumidajski P.J., Gong B., Baker D. Correlation Between 28 Day and 6–Hour Compressive Strengths. Cement and Concrete Research, 33(9):1491–1493. 2003. [ Links ]
Newman S.P., Clifford S.J., Coveney P.V., Gupta V., Blanchard J.D., Serafín F., Ben Amotz D., Diamond S. Anomalous Fluorescence in Near–Infrared Raman Spectroscopy of Cementitious Materials. Cement and Concrete Res, 35(5):1620–1628. 2005. [ Links ]
Potgieter–Vermaak S.S., Potgieter J.H., Belleil M., Deweerdt F., Van–Grieken R.. The Application of Raman Spectrometry to the Investigation of Cement Part II: A Micro Raman Study of OPC, Slag and Fly Ash. Cement Concrete Res., 36(4):663–670. 2006. [ Links ]
Potgieter–Vermaak S.S., Potgieter J.H., Van–Grieken R. The Application of Raman Spectrometry to Investigate and Characterize Cement, Part I: A review. Cement Concret Res., 36(4):656–662. 2006. [ Links ]
Wei–Tun Ch., Yun–Seng G. Concrete at High Temperatures Above 1000°C. Central Police University, Republic of Taiwan. 2000. [ Links ]
Feng X., Garboczi E.J., Bentz D.P., Stutzman P.E., Mason T.O. Estimation of the Degree of Hydration of Blended Cement Pastes by a Scanning Electron Microscope Point Counting Procedure. Cement Concrete Res., 34(10):1787–1793. 2004. [ Links ]
About the authors
Ma. del Rosario Moreno–Virgen. She obtained her BS degree (2003) in Instituto Tecnologico de Aguascalientes and her MS degree (2006) by the Instituto Tecnologico de Aguascalientes. Her doctoral degree by the National Polytechnic Institute, conferred by the Graudate Program in Advanced Technologies, CICATA–IPN, Querétaro Section. His scientific interest is focused on the matter–radiation interaction.
Juan José Soto–Bernal. Is currently a research professor at the Instituto Tecnologico de Aguascalientes; in addition, he is collaborating at the Centro de Investigaciones en Óptica, A.C. (CIO). He received his Ph.D. in optics from CIO (2001). His interest is focused in the characterization of materials, applied spectroscopy and laser applications. He is a member of the Researchers National System (SNI), level I.
José Angel Ortiz–Lozano. Graduated in Civil Engineering from the Universidad Autónoma de Aguascalientes (Mexico) in 1999, he was granted a doctorate in engineering by Universitat Politecnica de Catalunya (in Barcelona, Spain) in 2005, with a thesis focused on the effects of environmental temperatures on the mechanical properties of concrete, mainly on the compressive strength. Dr. Ortiz has published over five papers in international journals cited in the ISI Journal Citation Reports and over thirteen in national and international congresses. Dr. Ortiz works for the Universidad Autónoma de Aguascalientes as a full–time professor, at the moment he addresses three research projects with public financing, i.e. Consejo Nacional de Ciencia y Tecnologia de Mexico (CONACyT). Dr. Ortiz is member of the National System of Researches of the CONACyT in Mexico.
Claudio Frausto–Reyes. Claudio Frausto is currently a professor at the Centro de Investigaciones en Óptica, A.C. (CIO). He received his BS degree (1994), MS degree (1996) and Ph.D. degree (1999) from the Instituto Nacional de Astrofísica Óptica y Electrónica (INAOE). His scientific interest is focused on Raman spectroscopy applications and Fourier optics.
Adrián Bonilla–Petriciolet. Is currently a research professor at the Instituto Tecnológico de Aguascalientes; He received his BS degree (1999) from the Instituto Tecnológico de Aguascalientes and his Ph.D. degree (2005) from the Instituto Tecnológico de Celaya. His interest is focused in Thermodynamics and is member of the National System of Researches, level I.
Rosario González–Mota. Graduated from the Instituto Tecnológico de Aguascalientes as a Chemical Engineering and obtained a Ph. D. degree in Optical Engineering and Laser Technology from Centro de Investigaciones en Óptica A.C. Currently she is a professor at the Instituto Tecnológico de Aguascalientes. Her research interest is related to optical properties of materials.
Iliana Rosales–Candelas. In 1993 she obtained the bachelor degree title of Electronic Engineer in Universidad Autónoma de Zacatecas. In 2002 she obtained the degree of master in science in the Holography and Lasers area and currently is a Ph.D student in the Centro de Investigaciones en Óptica A. C., in León, Gto. Mexico, researching about Optical Polymers using UV–VIS and NIR spectroscopy.
Jorge Pineda–Piñón. Professor–Researcher of the CICATA Querétaro, since January, 2005. Master's degree on Architecture by the Escuela Superior de Ingeniería y Arquitectura of the Instituto Politécnico Nacional and doctor's degree in engineering by the Universidad Autónoma de Querétaro. Expertise areas: construction materials, high temperature solar furnace, and bioclimatic houses. Professional area: designer and constructor of houses. Professional experience: He was Technical Sub director of the Technological Research Center 1 "Walter Cross Buchanan" of the IPN. Publications: Six published book articles, three published paper in a scientific journal, two sent papers to scientific journals and one patent in progress.














