1 Introduction
Nanostructured materials, such as nanoparticles, are considered to be less than 100 nm in size, so they have very different physical and chemical properties at their natural or raw scale. Its properties are determined by the crystallographic structure of the nanoparticle surface, its size, and its shape. Semiconductor nanoparticles have electrical, magnetic, and optical properties due to quantum confinement, so they can be used in various applications such as luminescent materials, optoelectronic devices (lasers) [1], sensors, plasma television screens, light-emitting diodes white (LED) [2], fluorescent lamps [3] biosensors among others. As luminescent nanoparticles, they can be highly fluorescent when excited by ultraviolet light, so they can be used as an alternative for organic and inorganic staining.
In applications associated with luminescence, a high luminescence efficiency is usually required, which can be modified by the presence of impurifiers, by an active ion dispersed in the crystal structure of the matrix or by the type of crystal structure itself [4].
There are several compounds that are used as a matrix to obtain luminescent materials and that can be inpurified with oxides, phosphates, fluorides or lanthanides. Zinc sulfide (ZnS) is a semiconductor used commercially as a photoluminescent and especially if it is doped [5-6].
It has been investigated that the lanthanides or rare earths used as dopants for semiconductor nanoparticles, in addition to being luminescent compounds, reduce their environmental impact, are biocompatible, and have less toxicity and greater chemical and thermal stability [7-11].
Lanthanides have properties that allow tuning the optical properties of semiconductor materials.
An important feature of these materials is their narrow emission bandgap, which represents a great advantage for their use in flat panel displays, medical labeling, disease detection, and imaging [12], as well as in fluorescent probes and can be used in vivo, and in vitro [13].
Quantum confinement in semiconductor nanomaterials is important because it creates new optical, electrical, and mechanical properties of the materials. Therefore, the size and shape of the particles [14] have a dramatic effect on the density of electronic states and thus on the optical response.
In this work, we present a nanoprecipitation method for the synthesis of undoped and CeCl2 doped ZnS nanoparticles, to observe their optical properties, where nanoparticles or quantum dots with sizes around 10 nm can emit red and smaller sizes emit red. in orange, yellow, and green [15].
2 Materials and Methods
2.1 Synthesis
The synthesis method used to obtain metallic nanoparticles is based on nanoprecipitation reactions, which are carried out through controlled release processes of precipitating cations or anions at moderate temperatures. It is a simple and low-cost colloidal method of processing [16].
2.1.1 ZnS Nanoparticles
The nanoparticles were developed by a colloidal method and chemical precipitation [16] using zinc chloride (ZnCl2) and sodium sulfide (Na2S), purchased from Sigma Aldrich, as initiators. 0.039 ZnCl2 was dissolved in 50 mL of deionized water and the solution was magnetically stirred for 20 min at room temperature (RT). Subsequently, 1.22 g of Na2S were dissolved in 72 ml of distilled water and stirred magnetically for 20 minutes at room temperature.
The resulting solution was added dropwise to the previously prepared solution. A solution of 0.5 g of PEG in 10 mL of deionized water was prepared, as a passivating agent and added to the ZnS solution until complete dissolution was achieved.
2.1.2 Doped ZnS Nanoparticles
Using zinc chloride (ZnCl2) and sodium sulfide (Na2S) as initiators and CeCl3 as a doping agent. A solution of 0.7039 g of ZnCl2 and 1% w/v CeCl3 was dissolved in 50 ml of deionized water and the solution was stirred magnetically for 20 min at room temperature. Separately, 1.22 g of Na2S was dissolved in 72 ml of distilled water, and the solution was magnetically stirred for 20 minutes at room temperature. The resulting solution was added dropwise, to the previously prepared solution. 0.5 g of PEG solution was added as a passivating agent to obtain the precipitate (Figure 1)
2.1.3 Dispersion and Filtration
The solution was subjected to ultrasonic dispersion at 45 kHz for 30 min (TI-H-5, Elma, Germany) and then filtered using 0.22 µm Millipore membrane filters.
In this type of synthesis, the nanoparticles are dispersed within a dispersing medium (water). They are prepared from colloidal solutions of nanoparticles with a surfactant coating that controls the surface energy by intermolecular forces to prevent their aggregation and increase their size and shape and modify their optical luminescence properties [17].
2.1.4 Centrifugation and Washes
The resulting precipitate from each sample was centrifuged and washed [18].
2.1.5 Drying of the Nanomaterial
The precipitate was dried at 60 °C for 6 h to remove any organic residue, water, and other by-products formed that might evaporate at this temperature.
After drying, the precipitate was crushed with an agate mortar to obtain a fine semiconducting nanoparticle powder.
2.2 Characterization of Nanoparticles
2.2.1 X- Ray Diffraction Pattern (XRD)
The synthesized nanoparticle samples were examined by X-ray diffraction (XRD), Bruker-AXS, model D8-advance coupled to a 1.5406 A° copper anode X-ray tube, using a diffracted beam monochromator to select the radiation. Kα, to analyze the crystalline structure of the nanomaterial and estimate the size of the crystal. The diffractogram obtained shows a series of maxima called diffraction patterns, which correspond to the constructive interference of waves elastically scattered by the atomic planes of the crystal [19].
The position and intensity of these peaks provide information about the crystal structure. To determine the average size of the crystals in the analyzed sample, the Debye-Scherrer equation was used considering the main diffraction peak in the (111) plane, the broadening of the maximum diffraction peak defines the size of the crystal.
2.2.2. Scanning Electron Microscopy (SEM)
Through the technique of scanning electron microscopy or SEM (JEM-6390 LV Scanning Electron Microscope, Jeol, Japan) and energy-dispersive X-ray spectroscopy (EDS), the microstructure of the nanoparticles and the redistribution of elements in the sample were determined.
2.2.3. Optical Properties
The excitation and emission wavelengths depending on the size and shape of the nanoparticles, as well as the synthesis conditions, were considered [20]. The optical properties were characterized by employing a spectrofluorimeter (Edinburgh Instruments FS5, United Kingdom). and country). To determine the intensity of the photoluminescence of the granules of the nanomaterial, and obtain the excitation and emission PL spectra of the nanoparticles.
3 Results
3.1 X- ray Diffraction Analysis
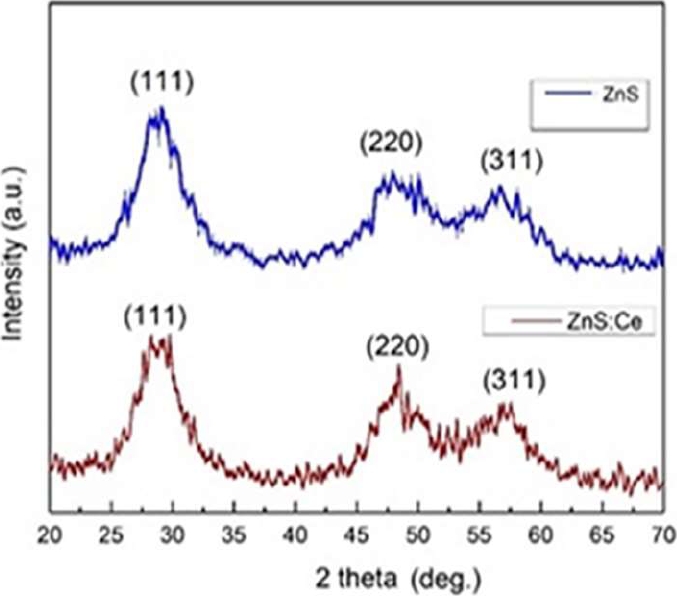
The diffractograms of the samples were obtained in the three main peaks, corresponding to the peaks that are assigned to the planes (111), (220), and (311) of the cubic phase of ZnS, which is observed in Figure 2. Small shifts were observed in the peaks of the diffraction pattern of the doped sample, so it can be thought that there is a good integration of the doping material in the parameters of the lattice due to the concentration of CeCl3.
Using the Debye-Scherrer equation (1), information on the average size of the synthesized nanoparticles and their internal structure was obtained [20]:
where τ gives the average diameter of the nanocrystals in the direction perpendicular to the related planes. The values of the total width at half of the main peak maximum (FWHM) of the undoped and doped samples and the estimated values of the nanoparticle size are presented in Table 1.
3.2 SEM Analysis
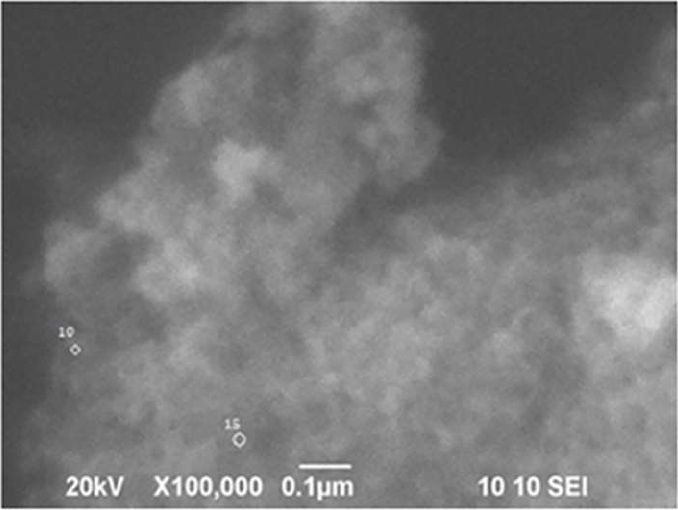
In the SEM micrographs, the morphology of the pure and doped ZnS nanoparticles was verified, being found to be very similar. A homogeneous surface could be expected, however, the surface may have defects due to the crystal growth process or due to agglomeration presenting an inhomogeneous surface. SEM micrographs show, that quasi-spherical nanoparticles with sizes less than 100 nm can be observed (Figure 3).
3.3 Photoluminescence Studies
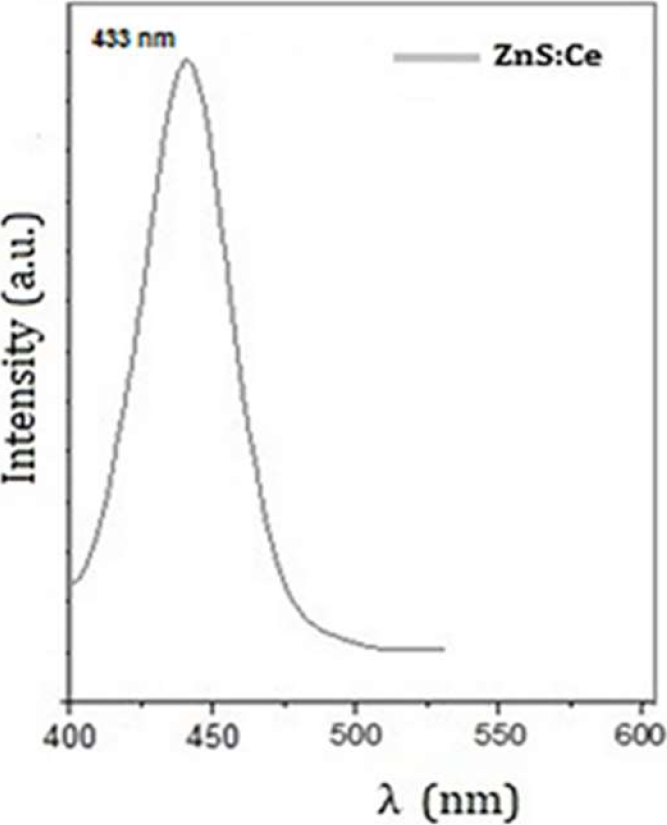
The photoluminescence spectra of pure and doped nanoparticles are presented. The excitation wavelength is 330 nm and the emission wavelength in the blue is 368 nm for the pure nanoparticles as shown in figure 4 (a) y (b).
It can be observed that for Ce+3 doped nanoparticles there is a shift in the wavelength towards blue at 447 nm and with 2 times more luminescence intensity than pure nanoparticles.
4 Conclusions
Undoped and Cerium-doped ZnS nanoparticles were successfully synthesized using a precipitation reaction that requires a relatively short time for its reaction and was carried out at RT, obtaining nanoparticles with sizes between 2-100 nm. It was observed that the size of the nanoparticles depends on the passivating or stabilizing agent, so these sizes can be changed by modifying the concentration of the passivating agent and the concentrations in the precursors.
The ZnS nanoparticles present a cubic structure as observed in the X-ray diffraction patterns. The resulting sizes after applying the Debye-Scherrer equation are 2.25 nm and 2.98 nm for the doped material.
The PL analysis shows an emission with a wavelength of 368 nm for the undoped nanoparticles applying UV light and under an excitation spectrum of 330 nm, while for the doped nanoparticles the light emission was presented with a shift at 433 nm under an excitation spectrum of the same wavelength.
This favors their potential application of the doped nanoparticles in optoelectronic devices and for their possible use in imaging for disease detection. Lanthanide ion doped nanoparticles have higher luminescence intensity almost twice as much as undoped nanoparticles. From the analysis of the quantum confinement effect in the literature it is known that it is the nanoparticles smaller than 10 nm that meet the characteristics of intense light emission and long decay time.











 nova página do texto(beta)
nova página do texto(beta)