Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.52 no.8 Texcoco Nov./Dez. 2018
Crop science
Dry and wet management effects on the post-harvest quality of three Rosa hybrida cultivars
1Facultad de Estudios Superiores Iztacala, UNAM. Tlalnepantla, México.
2Colegio de Postgraduados. Recursos Genéticos y Productividad-Fisiología Vegetal, Campus Montecillo. Texcoco, México.
3Departamento de Agronomía, Universidad de Almería, España.
4Departamento de Horticultura, Universidad Autónoma Chapingo. Chapingo, México.
Water shortage is one of the main agricultural issues and alternative techniques are necessary to reduce its consumption. During the cut flowers post-harvest management, the excessive use of clean tap water is common after harvest, during the selection-packing process and during transportation to avoid dehydration of the flower stems. The studies about the benefits from this repeated hydration practice are scarce. The objective of this study was to evaluate the effect of the management with water (wet) and without it (dry) after harvest (phase I), during cold storage (phase II) and the vase life of rose cultivars (Rosa hybrida) Blush, Freedom and Topaz cultivars which have commercial importance. We hypothesized that the effects of the dry management of the rose stems modify the life in vase regard their cultivar. During phase I, a batch of cut flowers was kept in dry conditions and another placed in containers with clean tap water. At phase II (the stems remained at 1±1 °C and 85 % RH for 7 d) each batch divided in two and kept in a dry and wet condition. Then, vase life was evaluated on the stems at room temperature (20±3 °C and 56 % RH). The evaluated variables were the fresh biomass of the floral stem, floral opening, opening of the stomata pores, colony forming units (CFU, bacteria) and the incidence of Botrytis sp. The experimental design was completely randomized, with 12 treatments consisting of three cultivars and four management combinations each; the experimental unit was a floral stem from which ten were included per treatment. The management differences during phase II had a greater effect than in phase I. The three cultivars had similar reactions to both types of management during phase I, but dry handling during phase II significantly increased vase life. The effect of wet or dry management in phase I and II, in the vase life of rose stems, is partially dependent on the cultivar.
Keywords: Botrytis spp.; floral opening; stomatal pore opening; colony forming units; rose stems; vase life
La escasez de agua es uno de los problemas principales en la agricultura y técnicas alternativas son necesarias para reducir el consumo. Durante el manejo postcosecha de flores de corte el uso excesivo de agua potable es común después de la cosecha, durante la selección-empaque y durante el transporte para evitar la deshidratación de los tallos florales. Los estudios de los beneficios de esta práctica de hidratación reiterada son escasos. El objetivo de este estudio fue evaluar el efecto del manejo con agua (húmedo) y sin ella (seco) después de la cosecha (fase I) y durante el almacenamiento refrigerado (fase II) en la vida de florero de los cultivares de rosa (Rosa hybrida) Blush, Freedom y Topaz, con importancia comercial. La hipótesis fue que el efecto del manejo seco de los tallos de rosa modifica la vida en florero en dependencia del cultivar. En la fase I un lote de flores de corte se mantuvo en condición seca y otro se colocó en contenedores con agua potable. En la fase II (los tallos permanecieron a 1±1 °C y HR 85 % por 7 d) cada lote se dividió en dos y se mantuvieron en condición seca y húmeda. Luego, la vida en florero se evaluó en los tallos a temperatura ambiente (20±3 °C y 56 % HR). Las variables evaluadas fueron biomasa fresca del tallo floral, apertura floral, apertura del poro estomático, unidades formadoras de colonias (UFC; bacterias) e incidencia de Botrytis sp. El diseño experimental fue completamente al azar, con 12 tratamientos formados por tres cultivares y cuatro combinaciones de manejo; la unidad experimental fue un tallo floral y se incluyeron diez por tratamiento. El tipo de manejo durante la fase II tuvo efecto mayor que en la fase I. La reacción de los tres cultivares fue similar a ambos tipos de manejo durante la fase I, pero el manejo seco durante la fase II aumentó significativamente la vida en florero. El efecto del manejo húmedo o seco en la fase I y II, en la vida en florero de tallos de rosa, es parcialmente dependiente del cultivar.
Palabras clave: Botrytis sp.; apertura floral; apertura del poro estomático; unidades formadoras de colonias; tallos de rosa; vida de florero
Introduction
Flower growers use hydrating solutions or clean tap water to maintain the cut flowers turgor during their postharvest handling. In this process, the stems can remain hydrated (wet management) or without hydration (dry management) for some time, during transport for packaging or warehouse (Rudnicki et al., 1986; Arévalo-Galarza et al., 2012). Clean water is increasingly scarce, and its cost has increased. Therefore, it is necessary to establish techniques that reduce its consumption during cut flower postharvest handling.
The rose (Rosa hybrida) is one of the ornamental species with the largest planted surface in Mexico, its production amount almost 7 million gross in 2015 (SIAP, 2017). Traditionally, cut rose producers place the flower stems intermittently in containers with tap water or preservative solutions throughout the post-harvest handling, which implies an excessive water expenditure. Dry handling during storage has advantages, such as the efficient use of spaces is because more stems are stored per unit area in cold store, thus lower costs due to reduced labor and water savings (Macnish et al., 2009; Mosqueda-Lazcares et al., 2011).
However, some species are not tolerant to dry storage, such as dahlia (Dahlia hybrida), freesia (Freesia hybrida), gerbera (Gerbera jamesonii) and gypsophila (Gypsophila elegans) (Nowak and Rudnicki, 1990). This is the case of lisianthus stems (Eustoma grandiflorum) stored at 2±1 °C, which decreased their vase life by 42 % when kept in humid management, but 54 % when stored in dry condition. The effect of the combination of the wet or dry condition before storage (phase I) and during storage (phase II) on the quality of the cut flowers is still unknown.
The objective of this research was to evaluate the effect of water management (wet) and without it (dry) during phase I and phase II with refrigeration on the vase life of Blush, Freedom and Topaz rose cultivars. These results could optimize postharvest handling and reduce water management. The hypothesis was that the effect of the dry management on rose stems modifies their life in vase, compared with the humid handling relative, which is dependent upon the cultivar.
Materials and methods
Plant material and treatments
Forty flower stems from each of the Blush, Freedom and Topaz rose cultivars grown in a commercial greenhouse were harvested at 7:00. The average stem length was 70 cm. Immediately (phase I) the stems of each cultivar were separated into two lots of 20 stems each. The first batch was wrapped in kraft paper, placed in black polyethylene bags and kept in a greenhouse for 4 h (SFI), the second batch was placed in containers with tap water (pH 7.5, EC 563 µS cm-1); the water covered 10 cm of the base of the stem, and remained 4 h at the greenhouse (HFI). The average environmental conditions in the greenhouse were: 22±3 °C and 77 % RH.
Afterwards, the two batches from the three cultivars were transported to a laboratory, each stem was weighed and had 5 cm from its base removed. Each batch was subdivided into two and stored at 1±1 °C and 85 % RH for 7 d (FII), one in containers with tap water (HFII) and other, wrapped in kraft paper and stored in black polyethylene bags (SFII). As a result, there were four management conditions or treatments (SFI+SFII, SFI+HFII, HFI+SFII, HFI+HFII) per cultivar.
At the end of the storage, 5 cm of the base of the stems were removed to help re-establish the water flow in the stems. The foliage was partially eliminated, leaving in each stem two trifoliate leaves and three pentafoliate. Subsequently, flower stems were individually placed in a 200 mL container with tap water, and randomly distributed in a room with a 12:12 h (illumination of 10 µmol m-2 s-1) photoperiod, at 20±3 °C and 56 % RH to be evaluated.
Life in vase (VF) and floral opening
The flower stems VF was evaluated, which corresponded to the number of days it took for any of the following symptoms of senescence to appear: necrotic spots on the periphery of the petals, turgor loss, stem bending, falling petals and yellowing or abscission of the leaves. Also, from the third day on, the apical diameter of the flower buds, the relation between the floral opening and the maximum opening were evaluated, which, according to De La Cruz et al. (2015) are 67.2 mm for Freedom, 88.7 mm for Topaz and 90.3 mm for Blush cultivars.
Fresh biomass (BF), water absorption and evapotranspiration rate
Each floral stem was daily weighed, and their percentage biomass gain or loss of was calculated. The water in each container was weighed and the water absorbed (mL g-1) was assessed (Rezvanypour and Osfoori, 2011). The total weight of the stem and the water absorption rate were used to calculate the evapotranspiration rate (TE) daily with the following Equation:
where TE: evapotranspiration rate (g g-1); BFn: biomass of the floral stem on day 1, 2, 3, n; BFo: stem biomass on the previous day; PSn-1: biomass of the solution on the previous day; PSn: weight of the solution on day 1, 2, 3, n; Bit: initial biomass of the floral stem.
Colony forming units (CFU)
On the first and fourth day of VF, the CFUs were quantified at the vase water of each treatment; the control was the tap water without the floral stem. This evaluation was carried out by duplicate and by treatment on each evaluation day. For this, 1 mL vase solution was placed in the center of a plate for total aerobic counts (Petrifilm 3M™), kept at room temperature for 72 h and then the CFU was assessed.
Stomatal pore opening and number of stomata
The number of stomata and the opening of the stomatal pores was assessed on the first and fourth VF day on epidermal impressions of the second pentafoliate leaf. For this, a layer of transparent cosmetic varnish was applied between the ribs, allowed to dry for 30 min, the layer was detached and mounted on a slide, with the print side facing the microscope. Photographs were taken with a 6.3X objective in a photomicroscope (III, Carl Zeiss) with integrated digital camera for microscopy (PAXcam 3). The stomas number was quantified per square millimeter and the stomatal pore area was measured in the photographs taken with the 40X objective in the same microscope. Pores segmentation was done with the GIMP, 2.8.4 software, and the area was obtained with the Image tool (3.40) (Willcox et al., 2002).
Botrytis sp. incidence
The incidence of Botrytis sp. by the seventh evaluation day in vase was determined via a visual scale that considered four damage levels: 0) absence of visible symptoms, 1) necrotic points on the petals (maximum three on one petal or five on several petals), 2) necrotic spots on maximum three petals, 3) brown spots at the apex of the petals and loss of turgor, 4) brown spots spread over most of the surface, including the center, wilting and falling petals (Figure 1). Floral stems turning level 3 were considered at the end of their VF, even if their leaves were turgid. The identification of Botrytis sp. was done through the isolation, purification and identification of the genus using the Barnett and Hunter (1998) keys.
Experimental design and statistical analysis
The experimental design was completely randomized with a 3×2×2 factorial arrangement (three varieties × two treatments in phase I × two treatments in phase II). The experimental unit was a floral stem, and ten of them were included as repetitions. The evaluated variables were: life in vase, fresh biomass of the floral stem, floral opening, evapotranspiration rate and Botrytis sp. incidence. In addition, the number of stomata was counted and stomatal opening measured at the central area from five leaves, 15 fields were analyzed (three per sheet). The CFU were analyzed from a solution of two vases, by treatment, randomly selected. The data were analyzed with ANOVA and the significant differences were determined with the Tukey test (p≤0.05); besides, the interactions between the factors were obtained.
Results and discussion
Life in vase and floral opening
On average, the VF of Topaz cultivar was the highest (14 %, p≤0.05) amongst the three evaluated cultivars (Table 1). In both postharvest phases of the stems in dry handling, out of the three cultivars had VF at least 20 % higher than with their similar in wet management. The cultivar-phase relation was highly significant and showed especially that in Blush and Topaz cultivars the dry condition during phase II was determinant for longer VF. Wet handling in both phases statistically reduced the VF of the Freedom cultivar (Table 1). Storage in humid condition (HFII) kept the stems hydrated and maintained the metabolic activity of the tissues, the floral opening continued and reduced the VF after storage. This was the case of the flower buds of lisianthus (E. russellianum) stored in tap water, at 2±1 °C, from 1 to 3 weeks, which continued growing; however, when placed in a vase exhibit a premature 25 % buds drop (2.8 buds per floral stem) compared to dry handling, which resulted in the opening of 3.7 buds per floral stem (Ahmad et al., 2012).
Table 1 Vase life (days) of three rose (Rosa hybrida) cultivars with wet (H) or dry (S) management, before (Phase I, FI) or during (Phase II, FII) storage.
| Factor e interacción | Cultivar† | ||
| Blush | Freedom | Topaz | |
| Promedio | 7.35±0.44 b | 7.30±0.38 b | 8.37±0.32 a |
| Cultivar en FI | |||
| H | 6.90±0.23 b | 6.85±0.25 b | 7.70±0.13 b |
| S | 7.80±0.36 a | 7.75±0.25 a | 9.05±0.20 a |
| Cultivar × FI | NS | NS | NS |
| Cultivar en FII | |||
| H | 6.25±0.14 b | 7.05±0.29 b | 8.25±0.19 b |
| S | 8.45±0.23 a | 7.55±0.25 a | 8.50±0.26 a |
| Cultivar×FII | ** | ** | ** |
| Cultivar en FI y FII | |||
| HFI + SFII | 7.70±0.15 b | 7.50±0.34 a | 8.70±0.30 b |
| HFI + HFII | 6.10±0.23 c | 6.20±0.25 b | 7.80±0.13 c |
| SFI + SFII | 9.20±0.29 a | 7.60±0.37 a | 9.40±0.22 a |
| SFI + HFII | 6.40±0.16 c | 7.90±0.35 a | 7.60±0.22 c |
| Cultivar × FI × FII | ** | ** | ** |
†Mean values (± standard error) in a column within the same factor interaction, with different letters are statistically different (Tukey, p≤0.05). H: wet; S: dry. Phase I: time from cutting to storage (4 h); Phase II: time in cold storage (7 d), ** significant (p≤0.01); NS: not significant.
The floral opening index from the Topaz cultivar was 10 and 20 % higher than that of the Freedom and Blush. The cultivar × phase I relation had no effect on the floral opening; in contrast, the cultivar × phase II relation was highly significant, showing that floral opening in the Freedom stems was favored by the wet condition, unlike the Topaz cultivar (Table 2).
Table 2 Floral opening index of three rose (Rosa hybrida) cultivars in wet (H) or dry (S) management before (Phase I, FI) and during storage (Phase II, FII).
| Factor e interacción | Cultivar† | ||
| Blush | Freedom | Topaz | |
| Promedio | 0.68±0.03 c | 0.76±0.05 b | 0.84±0.06 a |
| Cultivar en FI | |||
| H | 0.69±0.03 a | 0.79±0.03 a | 0.88±0.04 a |
| S | 0.68±0.02 a | 0.73±0.04 a | 0.79±0.05a |
| Cultivar × FI | NS | NS | NS |
| Cultivar en FII | |||
| H | 0.70±0.02 a | 0.84±0.02 a | 0.67±0.03 b |
| S | 0.67±0.03 a | 0.67±0.03 b | 1.00±0.02 a |
| Cultivar × FII | ** | ** | ** |
| Cultivar en FI y FII | |||
| SFI + SFII | 0.65±0.03 b | 0.61±0.04 c | 1.00±0.03 a |
| SFI + HFII | 0.71±0.02 a | 0.84±0.02 a | 0.72±0.02 b |
| HFI + SFII | 0.63±0.05 b | 0.73±0.04 b | 1.00±0.03 a |
| HFI + HFII | 0.70±0.03 a | 0.85±0.04 a | 0.74±0.04 b |
| Cultivar × FI × FII | * | * | * |
†Mean values (± standard error) in a column within the same factor interaction, with different letters are statistically different (Tukey; p≤0.05). NS: not significant; * significant (p≤0.05), ** significant (p≤0.01).
The opening index is related to the maturity of the flower bud, the sugars reserves and water absorption necessary for the expansion of the petals (Ichimura and Shimizuko-Yumoto, 2007). The stems from the Freedom cultivar had lower fresh biomass (38.2±0.94 g) and VF than those from the Blush and Topaz cultivars; but its water absorption was intermediate (0.38 mL g-1) between those two. This indirectly confirmed that the carbohydrates reserves in the stem and petals are important for flower opening (Evans and Reid, 1988).
Total fresh biomass, absorption rate and evapotranspiration
Flower stems from the three cultivars increased their total biomass (102 %) the first day in the vase, the highest increase occurred in the treatments from the Freedom cultivar (108 %) which included the SFI-SFII conditions, and Topaz (109 %) at HFI-SFII and SFI-SFII conditions. From the second day on, the total biomass continuously decreased in all treatments. The stems in the two phases were maintained in dry condition, SFI-SFII significantly decreased less their wet biomass relative to those handled in wet conditions, HFI-HFII. These results are explainable since during the water storage stems were kept hydrated, but the stomas had 20 % greater opening compared with the stems stored in dry conditions. The open stomata maintain the transpiration gradient that favors the absorption of water at the base of the stems. On day 7, the total biomass of the Blush and Freedom cultivars was between 85 and 90 %, and in the Topaz cultivar remained between 92 and 95 %; so the latter showed greater floral openings and VF. In the stems of the three cultivars, weight losses greater than 15 % was related to evident wilting.
The absorption rate after storage was statistically higher (0.37, 0.48 and 0.65 mL g-1) in the stems of the treatments included the SFI-SFII condition compared to HFI-HFII (0.26, 0.29 and 0.48 mL g-1) in the Blush, Freedom and Topaz cultivars. The higher absorption in SFI-SFII was probably because the stress generated a lower water potential gradient compared to the one generated with the previous hydration in one of the two phases. Seven days after starting the vase period floral stems from the Topaz cultivar had 30 % more water absorption compared with Blush and Freedom (0.21 mL g-1), which also had lower VF. When the water absorption rate is greater than that of transpiration, there is a positive water balance; the total biomass is maintained with minor changes for longer, and the VF increases. In contrast, if transpiration exceeds absorption, due to metabolic activity, vascular occlusion or both, there is a water deficit that, in turn, causes premature dulling or bending of the flower peduncle (neck bend) (Reddy and Singh, 1996; Macnish et al., 2009; Hussen and Hassen, 2013).
Colony forming units (CFU)
The floral stems of the three cultivars in wet management in both phases on the first day of the VF had more CFU (136 to 189 CFU mL-1), than those that had dry handling (63 to 134 CFU mL-1). The same behavior was present in the evaluation on the fourth day, since it showed a significant increase in CFUs in the stems that remained wet in all the phases (Figure 2).
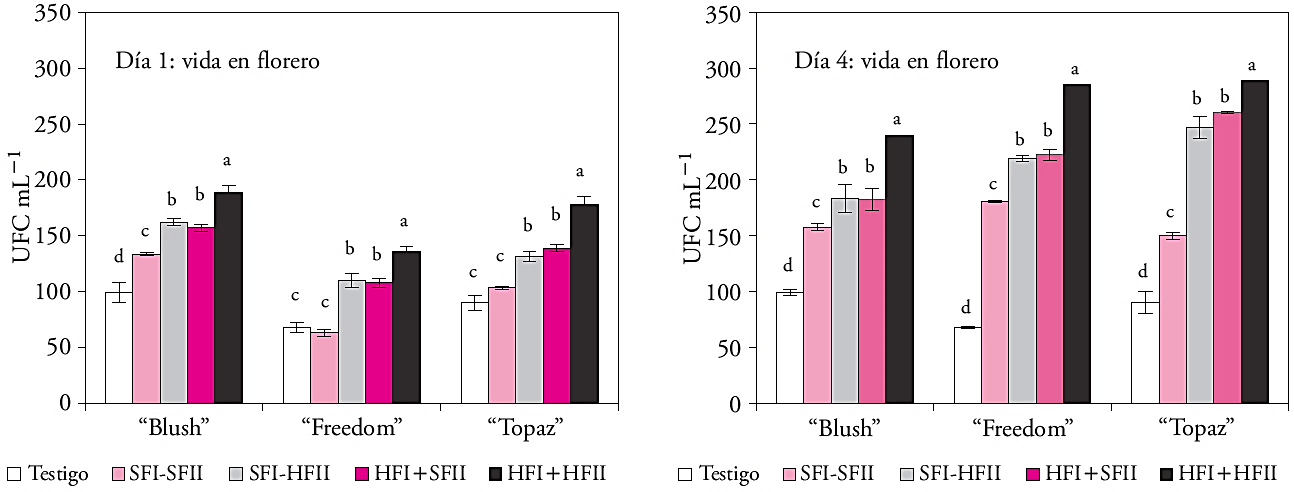
Figure 2 Colony forming units (CFU mL-1 ± standard error; n=2) in three roses (Rosa hybrida) cultivars handled dry and/or wet before (phase I) and during storage (phase II). Different letters on the columns indicate statistical differences in each cultivar (Tukey, p≤0.05).
The number of CFU in the vase solution is a limiting factor in the water absorption at the base of the stems. In this regard, the rose has been identified as one of the most susceptible to microorganism species presence that obstructs water entry and reduces VF (Bleeksma and van Doorn, 2003, Arévalo-Galarza et al., 2012). Concentrations of 107 UFC mL-1 decreased the water conductivity (Kh) and lifespan in ‘Sonia’ roses in vase, while the occlusion of water flow occurred when at the basal section of the flowers stem the number of bacteria was equal to or greater than 108 UFC mL-1 (de Witte and van Doorn, 1988). In the present study the vase solutions presented average values lower than 107 UFC mL-1; and confirmed the disadvantage of wet management, since, if the initial amount of bacteria at the base of the stems is significant and have poor hygiene during handling, bacteria occlusion risks is increased.
Number of stomata and opening of the stomatal pore
Topaz cultivar leaves showed 73.8 stomata mm-2, this value was significantly higher than those of the Blush and Freedom cultivars (54 mm-2 stomata). These values are close to those documented in the leaves of the Vega and Grand Gala of rose cultivars (57.6 and 60.4 stomata mm-2) (Hernández-Hernández et al., 2009). Stomata density depends on genetic characteristics of species, their cultivar and the growth conditions; however, the opening area of the stomata pore can rapidly vary due to treatment effect. The first day in vase, cultivars with dry handling at the first phase (SFI-SFII and SFI-HFII) had smaller stomatal pore aperture compared with those in wet management (HFI-SFII and HFI-HFII). This indicates that dry handling in the first phase determined stomatal opening during the post-harvest period and resulted in a larger vase lifespan. Thus, stomatal opening of the Freedom cultivar was the largest (89 to 102 µm2), with which it’s life in vase was the shortest; in contrast, the Topaz cultivar, which showed the smallest stomatal opening (22.7 to 32 µm2), had the longest VF (Figure 3). These results indicate that the effect of the management type modified stomatal opening during the postharvest stage.
Incidence of Botrytis sp.
The incidence of Botrytis sp. in petals of the three cultivars was lower when the flower stems were handled in dry conditions in both phases (SFI-SFII). Wet handling during phase II (SFI-HFII or HFI-HFII) increased the pathogen incidence in the Freedom cultivar by 50 % and 75 % in the Blush and Topaz ones. In contrast, when the flower stems were handled in wet conditions during the first phase and dry during the second (HFI-SFII) Botrytis sp. incidence was moderate (Figure 4).
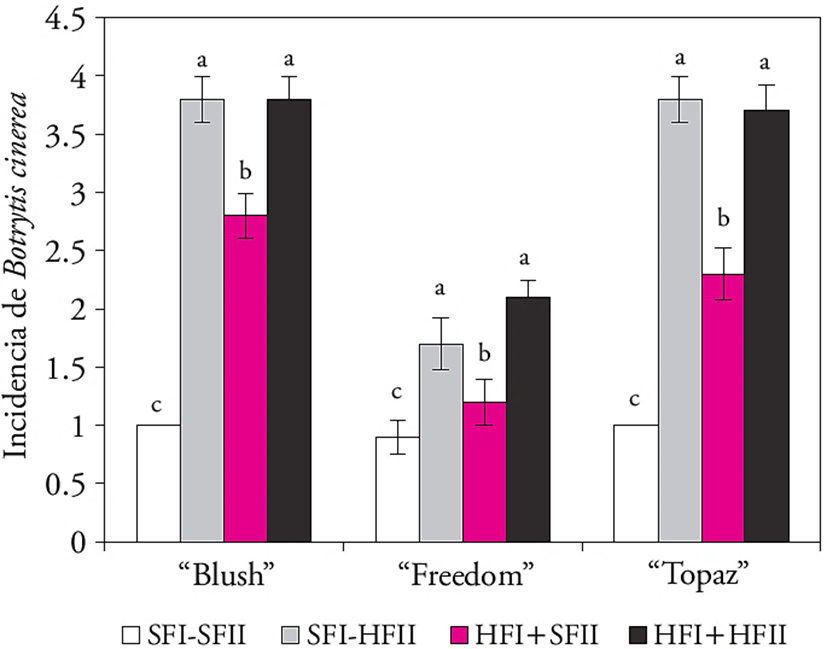
Figure 4 Botrytis sp. incidence (± standard error; n=10) in three rose (Rosa hybrida) cultivars handled dry and/ or wet before (phase I) and during storage (phase II). The values presented as the mean ± standard error (n=10). Different letters on the columns indicate statistical differences in each cultivar (Tukey, p≤0.05).
In this regard, B. cinerea conidia germination depends on the handling and temperature changes caused by condensed water on the petals surface and the cultivar (Harkema et al., 2013). So, excess humidity and the refrigeration temperature in stage II in our study led to an increase in the condensed water on the petals and symptoms development thereon.
Conclusions
Management (wet or dry) during post-harvesting first phase has less effect on the life span of rose flower stems than storage. During storage at low temperatures, floral stems maintained, without water increase, their longevity and opening of flower buds, and maintain an attractive appearance due to decreased incidence of Botrytis sp. The storage of roses in dry conditions seems a good option for areas where potable water availability is scarce.
The response to handling in wet or dry conditions in the vase lifespan of the rose stems partially depends on its cultivar.
Literatura citada
Ahmad I., J. M. Dole, A. Amjad, and S. Ahmad. 2012. Dry storage effects on postharvest performance of selected cut flowers. HorTechnology 22: 463-469. [ Links ]
Arévalo-Galarza, L., C. García-Osorio, and G. H. Rosas-Saito. 2012. Factors affecting the vase life in cut flowers. Agroproductividad 5: 28-35. [ Links ]
Barnett, H. L. and Hunter, B. B. 1998. Illustrated Genera of Imperfect Fungi. Fourth edition. St. Paul, Minn., APS Press. 218 p. [ Links ]
Bleeksma, H. C., and W. G. Van Doorn. 2003. Embolism in rose stems as a result of vascular occlusion by bacteria. Postharvest Biol. Technol. 29: 334-340. [ Links ]
De La Cruz- Guzmán, G. H., M. L. Arévalo-Galarza, C. Peña-Valdivia, A. Castillo-González, M.T. Colinas-León, y M. Mandujano-Piña. 2015. Influencia del índice de cosecha en la vida de florero de siete cultivares de Rosa hybrida. Agroproductividad 8: 3-11. [ Links ]
De Witte, Y., and W. G. Van Doorn. 1988. Identification of bacteria in the vase water of roses, and the effect of the isolated strains on water uptake. Sci. Hort. 35: 285-291. [ Links ]
Evans, R. Y., and M. S. Reid. 1988. Changes in carbohydrates and osmotic potential during rhythmic expansion of petals. Am. Soc. Hort. Sci. 113: 884-888. [ Links ]
Harkema, H., M. G., J. Mensink, D. P. M. Somhorst, R. P. Pedreschi, and E. H. Westra. 2013. Reduction of Botrytis cinerea incidence in cut roses (Rosa hybrida L.) during long term transport in dry conditions. Posth. Biol. Technol. 76: 135-138. [ Links ]
Hernández-Hernández, F., M. L. Arévalo-Galarza, M. T. Colinas-León, H. A. Zavaleta-Mancera, and J. Valdez-Carrasco. 2009. Anatomical differences and pulse solutions in two rose cultivars (Rosa sp.). Rev. Chapingo Serie Hort. 15: 11-16. [ Links ]
Hussen, S., and Y. H. Hassen. 2013. Review on the impact of different vase solutions on the postharvest life of rose flower. Intern. J. Agric. Res. Review. 1: 13-17. [ Links ]
Ichimura, K., and H. Shimizuko-Yumoto. 2007. Extension of the vase life of cut roses by treatment with sucrose before and during simulated transport. Bull. Natl. Inst. Flor. Sci. 7: 17-27. [ Links ]
Jarvis, W. R. 1977. Botryotinia and Botrytis Species. Taxonomy, Physiology and Pathogenicity; a Guide Literature. Department of Agriculture. Ottawa, Canada. pp: 77-78. [ Links ]
Macnish, A. J., D. D. Theije, M. Reid, and C. Z. Jian. 2009. An alternative postharvest handling strategy for cut flowers dry handling after harvest. Acta Hort. 847: 215-222. [ Links ]
Mosqueda-Lazcares, G., M. L. Arévalo-Galarza, G. Valdovinos-Ponce, J. E. Rodríguez-Pérez, and M. T. Colinas-León. 2011. Harvest season and handling of eight cultivars in cut roses. Rev. Mex. Cienc. Agríc. 3: 591-602. [ Links ]
Nowak, J., and R. M. Rudnicki. 1990. Postharvest Handling and Storage of Cut Flowers, Florist Greens and Potted Plant. Portland: Timber Press. 210 p. [ Links ]
Reddy, B. S., and K. Singh. 1996. Effects of selected chemicals on vase life of tuberose cut flowers. J. Maharashtra Agric. Univ. 21: 201-203. [ Links ]
Rezvanypour, S., and M. Osfoori. 2011. Effect of chemical treatments and sucrose on vase life of three cut rose cultivars. J. Res. Agric. Sci. 7: 133-139. [ Links ]
Rudnicki, R. M., D. Goszcynska, and J. Nowak. 1986. Storage of cut flowers. Acta Hort. 181: 285-296. [ Links ]
SIAP, 2017. Servicio de Información Agroalimentaria y Pesquera. Anuario Estadístico de la Producción Agrícola. (Consulta: julio 2017). [ Links ]
Willcox, D., B. Dove, D. Mcdavid, and D. Greer. 2002. Image Tool for Windows ver. 3.0. The University of Texas Health Science Center in San Antonio U.S.A. 275 p. [ Links ]
Received: June 2017; Accepted: November 2017











 texto em
texto em 




