Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.52 no.6 Texcoco ago./sep. 2018
Animal Science
Germinative energy in guaje (Leucaena leucocephala cv. Cunningham) with different methods of seed scarification
1Campus Veracruz. Colegio de Postgraduados. 91690, Veracruz, México. (adrian.gomez@colpos.mx), (color@colpos.mx).
2Universidad Autónoma Metropolitana. Unidad Iztapalapa. División de Ciencias Biológicas y de la Salud. San Rafael Atlixco 186. Colonia Vicentina, Iztapalapa, Ciudad de México, D.F., México. (jmvr@xanum.uam.mx).
3Campus Montecillo. Colegio de Postgraduados. 56230. Montecillo, Estado de México, 56230, México.
The germination velocity of guaje (Leucaena leucocephala) after planting should be considered in the reforestation of spaces dedicated to grazing in the intertropical zone of hot climates. In the present study scarification treatments were compared in guaje seeds with the objective of evaluating its effect on germinative energy and germination value. The experimental design was completely randomized, with three replications per treatment, and the experimental units were groups of 100 seeds. The treatments were as follows: 1) without scarification; 2) immersion in water at 80 ºC for 3 min (hydrothermal); immersions for 12 h in 3) water at 24 ºC; 4) ethylic alcohol of 70 ºGL; 5) organic thinner diluent, and 6) hydrogen peroxide diluted to 2 %. The seeds were deposited in a clay loam soil substrate and remained exposed to the weather protected by shade mesh. Total germination was higher with hydrothermal scarification (55%) with respect to the other treatments, except for immersion in water (p > 0.05), but with this treatment the germinative energy and viability was the lowest. Immersion in water presented the highest germinative energy (31.66 %), thus it improved germination velocity of the guaje seeds without affecting their germination value and viability. The hydrothermal scarification stimulated total germination and germination value, but it reduced germination velocity with respect to the other treatments, which showed intermediate values of germinative energy. Scarification with organic diluent caused the lowest value of germination (4 %) and total germination (31 %), respectively. The present study makes it possible to conclude that in guaje seeds scarification by immersion in water at 24 ºC for 12 h and in water at 80 ºC for 3 min favored total germination, but immersion in water increased germination velocity.
Key words: Leucaena leucocephala; germination velocity; germination value; silvopastoral grazing systems
La velocidad de germinación del guaje (Leucaena leucocephala) después de la siembra debe tenerse en cuenta en la reforestación de espacios dedicados al pastoreo en la zona intertropical de climas cálidos. En este estudio se compararon tratamientos de escarificación en semillas de guaje con el objetivo de evaluar su efecto sobre la energía germinativa y el valor de germinación. El diseño experimental fue completamente al azar, con tres repeticiones por tratamiento y las unidades experimentales fueron grupos de 100 semillas. Los tratamientos fueron: 1) sin escarificación; 2) inmersión en agua a 80 °C por 3 min (hidrotérmico); inmersiones por 12 h en 3) agua a 24 °C; 4) alcohol etílico de 70 °GL; 5) diluyente orgánico thinner, y 6) peróxido de hidrógeno diluido a 2 %. Las semillas se depositaron en un sustrato de suelo tipo franco arcilloso y permanecieron expuestas a la intemperie protegidas con malla sombra. La germinación total fue mayor con la escarificación hidrotérmica (55 %) respecto a los demás tratamientos excepto para la inmersión en agua (p > 0.05), pero con este tratamiento la energía germinativa y viabilidad fue la más baja. La inmersión en agua presentó la mayor energía germinativa (31.66 %), por lo que mejoró la velocidad de germinación de las semillas de guaje sin afectar su valor de germinación y viabilidad. La escarificación hidrotérmica estimuló la germinación total y valor de germinación, pero redujo la velocidad de germinación con respecto a los otros tratamientos, los cuales mostraron valores intermedios de energía germinativa. La escarificación con diluyente orgánico causó el valor más bajo de germinación (4 %) y de germinación total (31 %), respectivamente. Este estudio permite concluir que en semillas de guaje la escarificación por inmersión en agua a 24 °C por 12 h y en agua a 80 °C por 3 min favorecieron la germinación total, pero la inmersión en agua incrementó la velocidad de germinación.
Palabras clave: Leucaena leucocephala; velocidad de germinación; valor de germinación; sistemas silvopastoriles
Introduction
Grazing based cattle production is co-responsible for the change in the landscape of tropical forests; furthermore, it is an economic activity with environmental and social repercussions (Chará et al., 2011). Leucaena leucocephala (Lam.) de Wit. (guaje) is a sustainable alternative for reforestation in spaces dedicated to livestock production in the intertropical zone of hot climates (Bacab et al., 2013), due to the positive impact in the reestablishment of the arboreal strata that contributes to countering desertification, as well as providing environmental services and increasing the productivity and quality of products of animal origin (Cuartas et al., 2014).
Orthodox guaje seeds (Arriaga et al., 1994) are covered with a light coating of the polysaccharides galactose and mannose (Gutiérrez et al., 2007) which impedes the passage of water and oxygen, decreases germinative vigor and propitiates germination percentages below 20 %, which limits their use (Sánchez-Paz and Ramírez-Villalobos, 2006). The pre-germinative treatments, such as scarification help to increment the germination response of diverse seeds, including those of guaje, interrupting their period of dormancy. The scarification methods are mechanical and chemical, such as the manual removal of the shell, scarification with sandpaper, hydrothermal treatments, immersion in water at room temperature or immersion in sulfuric acid (Teles et al., 2000; Gómez-Merino et al.,2010; González et al., 2012). For the scarification of hard seeds such as those of the genera Prosopis and Acacia, Ffolliott and Thames (1983) recommend ethylic alcohol and for scarification of seeds of Heliconia, organic diluents such as turpentine. Barba-Espín et al. (2012) consider hydrogen peroxide as a metabolic potentiator of germination in other legumes.
Total germination of guaje seeds increased by more than 20 % with scarification with water at 80 ºC with 2 to 10 min immersion (Sánchez-Paz and Ramírez-Villalobos, 2006; González and Mendoza, 2008; González et al., 2009; González et al., 2012). According to Teles et al. (2000), germination was higher than 90 % using scarification with water at 80 ºC for 5 and 10 min. When scarification with hot water is applied, a positive effect occurs in total germination, but in the studies that were performed, germination velocity was not considered. The heliophyllic behavior of guaje, its slow growth and development in the first 90 d after planting, indicate that the seed should be treated to improve its establishment and to have an early germination in under 7 d, because the seedling has difficulties in propagation due to competition with other species in land with dense canopy (Calle et al., 2011).
Germinative energy, defined as the percentage of seeds of a sample that emerge until reaching the moment of maximum germination (Pece et al., 2010b) or a short period of 7 d for forest species, and the germination value, which combines germinative energy and germination velocity (Djavanshir and Pourbeik, 1976), are indicators pertinent to seed vigor during the first days of germination. These indicators are appropriate because the seeds that germinate with speed and vigor under controlled conditions have a higher probability of generating vigorous seedlings in natural plots (Ffolliott and Thames, 1983). Although the hydrothermal treatment of the guaje seed has shown positive effects in total germination, its effect on germination speed is unknown, therefore it is convenient to compare it with other treatments that have shown better effect on the viability and speed of germination and which are used to scarify seeds.
Therefore, the objective of this study was to compare total germination and viability of five scarification methods of the guaje seed, considering the effect on its germinative energy and germination value, as indicators of germination velocity under semi-controlled greenhouse conditions.
Materials and Methods
Location
The study was carried out in the El Huilango plot, municipality of Cotaxtla, Veracruz, Mexico, at 18º 53' N and 96º 15' W and 30 masl during October and November of 2014. Mean annual temperature and rainfall are 25.4 ºC and 1,042 mm, climate Aw0(w)(i')g hot sub-humid, with scant Ganges type thermic oscillation, without heatwave and with rains in summer (García, 1988).
Plant material
The guaje seed used was from the Fall-Winter 2012/2013 production cycle, treated with N-trichloromethylthium-4-cyclohexane-1,2-dicarboxymide 50 % (Captan®) and Deltametrine at a dose of 1.5 L Mg-1 and 60 mL Mg-1. The seeds had been stored for 18 months at the time of the study.
Response variables
The variables were total germination (TG, %), non-germinated viable seeds (VS, %) and viability (VI, %, VI = TG + SV), measured 25 days after planting. Accumulated daily germination (ADG, %) was determined as the sum of the percentage of daily germination on the day of counting, and the daily germination velocity (DGV), seeds d-1) such as ADG d-1, where d corresponds to the day after planting on which the observation was made.
Germinative energy (GE, %) was estimated indirectly, calculating daily germination accumulated at the moment in which most of the treatments reached their maximum daily germination, know as energy period (Pece et al., 2010b), in our study 7 d after the start of the test.
Germination value (GV) was obtained with the equation GV = DGV x GE, proposed by Czabator (1962), where DGVf is the velocity of daily germination at the end of the test, calculated 25 d after planting and GE is germinative energy.
Experimental design
The experimental design was completely randomized, with three replications per treatment and the experimental units were groups of 100 seeds. Treatments were as follows: 1) without scarification; 2) immersion for 3 min in water at 80 ºC (hydrothermal); immersion for 12 h in 3) water at 24 ºC; 4) ethylic alcohol of 70 ºGL; 5) organic diluent thinner (toluene 50 %, methylic alcohol 15 %, acetone 5 %, hexane 5 %, ethylic alcohol 5 %, xylene 5 % and ethyl acetate 15 %); and 6) hydrogen peroxide diluted to 2 %. The seeds, after scarification, were rinsed under running water and were placed for 3 h in absorbent paper in the open air.
All of the seeds were planted in thermoformed plastic trays of 200 cavities and 3.81 cm height with a substrate of clay loam soil (pH 6.8, 2.15 % organic matter and apparent density of 1.1 g cm-3), at a planting depth of 1 cm. The trays were maintained in a nursery covered with shade mesh (50 % filtration of solar radiation) to prevent the rain from uncovering the seeds. The conditions of temperature and rainfall during the experiment are shown in Figure 1; auxiliary irrigations were applied on days 0, 2 and 13 of the test.
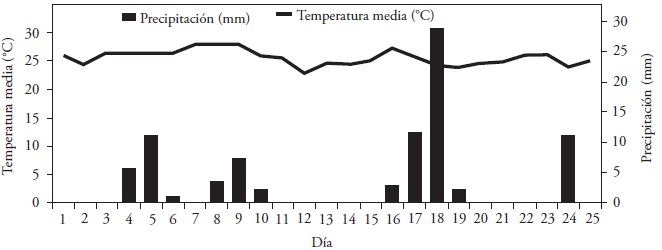
Figure 1 Climogram, rainfall (mm) and mean temperature (ºC) registered in the period 15/Oct/2014 - 08/Nov/2014 (INIFAP, 2014).
The germinated seeds were counted every 48 h from the date of planting during 25 d; the seed was considered germinated when the hypocotyl and the cotyledons emerged to the surface. At day 25 after planting, the trays of un-germinated seeds were extracted and those that showed no evident physical damage were considered un-germinated viable seeds.
Statistical analysis
To analyze the results of total germination, un-germinated viable seeds, viability, germinative energy and germination value, a completely randomized experimental design was used, while the data of accumulated daily germination were analyzed with a mixed model with repeated measurements:
where Yijk = measurement of the i-th treatment of the j-th day of the k-th replication; μ = constant that characterizes the population; Ti = fixed effect of the i-th treatment (Ii = 1, 2, 3, 4, 5, 6); Rk(i) = random effect of the k-th replication nested in the i-th treatment (k = 1, 2, 3), Rk(i) ~ IIDN (0,
The data were processed with the procedures GLM and MIXED of SAS® 9.4 with structure of covariances in integrated autoregressive model of mobile averages (SAS Institute, 2010). The comparison of means of treatments was made with the Tukey test (p ≤ 0.05).
Results and Discussion
The interval of total germination was 45 to 55 % in the hydrothermal and immersion treatments in water (Table 1). The hydrothermal treatment showed the highest total germination with respect to the other treatments, except for immersion in water (p > 0.05). The other treatments did not show differences with respect to the seeds without scarification (p > 0.05), and their percentages of germination were lower than 40 %.
Table 1 Germinative behavior of the guaje seeds (Leucaena leucocephala cv. Cunningham) with different methods of scarification.
| Tratamiento | Variable de respuesta | ||||
|---|---|---|---|---|---|
| GT (%) | SV (%) | VI (%) | EG (%) | VG | |
| SE | 39.0 b | 54.3 a | 93.3 a | 20.3 bc | 5.0 ab |
| IA80 | 55.0 a | 0.7 b | 55.7 c | 10.3 c | 8.1 a |
| IA24 | 45.3 ab | 48.7 a | 94.0 a | 31.7 a | 8.9 a |
| IAE | 36.0 b | 54.0 a | 90.0 a | 26.3 ab | 5.9 ab |
| IDO | 31.0 b | 40.3 a | 71.3 b | 22.0 ab | 4.0 b |
| IPH | 37.0 b | 53.0 a | 90.0 a | 26.0 ab | 5.7 ab |
| Error Estándar ± | 2.6 | 2.9 | 2.2 | 2.6 | 0.8 |
⁎SE: without scarification; IA80: immersion in water at 80 ºC for 3 min; IA24: immersion in water at 24 ºC for 12 h; IAE: immersion in ethylic alcohol of 70 ºGL for 12 h; IDO: immersion in organic thinner diluent for 12 h; IPH: immersion in hydrogen peroxide diluted to 2 % for 12 h. GT: total germination; SV: non-germinated viable seeds; VI: viability (TG + SV); GE: germinative energy; GV: germination value. Means with different literal in each variable are statistically different (Tukey p ≤ 0.05).
The un-germinated viable seeds were more than 40 % in all of the treatments (p≤0.05), except in the hydrothermal treatment, in which it was lower than 1 % and only remains of seeds in a state of decomposition were found at the end of the experiment. With the organic diluent the non-viable seeds had a dry and porous appearance, which was probably due to physical damage from the solvent used.
Viability was higher than 90 %, except in the hydrothermal treatment (55.7 %) and the organic diluent (71.3 %) (p ≤ 0.05).
On day 3 of the test, all of the treatments had an accumulated daily germination of under 5 % (Figure 2). At 5 and 7 d, accumulated daily germination was variable in all of the treatments with difference of 20 % between those of the highest and lowest response 9 (p ≤ 0.05). The hydrothermal treatment showed the lowest accumulated daily germination during the first 7 d, with respect to the other treatments at 9 d (p > 0.05), with tendency higher than the rest starting from day thirteen, and higher than the organic diluent, ethylic alcohol and hydrogen peroxide (p ≤ 0.05).

Figure 2 Daily accumulated germination (DAG) of guaje seeds (Leucaena leucocephala cv. Cunningham) under six scarification methods. SE: without scarification; IA80: immersion in water at 80 ºC for 3 min; IA24: immersion in water at 24 ºC for 12 h; IAE: immersion in ethylic alcohol of 70 ºGL for 12 h; IDO: immersion in organic thinner diluent for 12 h; IPH: immersion in hydrogen peroxide diluted to 2 % for 12 h.
Daily germination velocity reached maximum values at 5 d for the treatments of immersion in water and organic diluent, and at 7 d for the ethylic alcohol and hydrogen peroxide, while the seeds without scarification and the hydrothermal treatment presented the maximum value at 9 d and 11 d, respectively (Figure 3). The energy period considered for the calculation of germinative energy was 7 d, because most of the treatments reached the maximum value of DGV during this period (Ffolliott and Thames, 1983).

Figure 3 Daily germination velocity (DGV) of guaje seeds (Leucaena leucocephala cv. Cunningham) under six scarifcation methods. SE: without scarifcation; IA80: immersion in water at 80 °C for 3 min; IA24: immersion in water at 24 °C for 12 h; IAE: immersion in ethylic alcohol of 70 °GL for 12 h; IDO: immersion in organic thinner diluent for 12 h; IPH: immersion in hydrogen peroxide diluted to 2 % for 12 h.
Daily germination velocity was higher for immersion in water with respect to the other treatments until day nine (p ≤ 0.05), after which the treatments were similar and with negative biases (Figure 3). For germinative energy immersion in water (31.7 %) was higher than the hydrothermal (10.3 %) (p ≤ 0.05; Table 1), although both treatments had a germination value higher than 8 units, in contrast to the organic diluent with 4 units (p ≤ 0.05), Table 1); the other treatments showed values in the interval of 5 to 6 units.
Total germination of 55 % of the guaje seed with the hydrothermal treatment was higher than that of other tropical legumes for forage use such as Neonotonia wightii (Wight & Arn.) Verdc. (forage soybean) 33.5 %, Pueraria phaseoloides (Roxb.) Benth (kudzu) 39 % and Macrotiloma axilare (E. Mey.) Verdc. 18.8 %. A similar effect was observed in the guaje seeds without scarification 39 % and 26, 18 and 24.5 % for the same forage legumes, respectively (Morais, 2014). For guaje seeds from different cultivars and accessions without scarification, the values of total germination oscillated from 5 to 35 % and with the hydrothermal treatment from 45 to 96 % (González et al., 2009).
With cv. Peru guaje seeds stored 18 months to the environment without scarification and immersion in water at 80 ºC for 2 min, total germination was 81.8 and 97.7 % (González and Mendoza, 2008), higher than those of our study. However, González et al. (2012) reported total germinations of 51 % in seeds without scarification and 61.3 % in seeds with hydrothermal treatment, similar to those of our study. The above suggests a high variability in total germination of this species among cultivars and for same times of storage.
The environmental temperature could have affected total germination and during the experiment the mean temperatures 21.5 to 26.6 ºC (Figure 1) could diminish the germination response in the guaje seeds, because a better response is obtained between 25 and 30 ºC (Sánchez et al., 2005). However, a total germination below 60 % in scarified seeds is frequent in scantly domesticated legumes due to the fact that latency acts as an evolutive mechanism to obtain the highest probability of survival of the seed and of the progeny (Jara, 1996).
González et al. (2012) counted rotten seeds (unviable) and reported from 30 to 40 % with hydrothermal treatment plus a partial hydration period of 28 h in guaje seeds stored 18 months, which is similar to the viability in our study. These results suggest that the scarification in water at 80 ºC for 3 min can negatively affect the viability of the seed due to excess moisture. Doria (2010) indicated that seeds in excessive moisture are vulnerable to attack by fungi and to deficiencies in the availability of oxygen for the embryo.
Accumulated daily germination is related to daily germination velocity; a high accumulation of germinated seeds per period indicates a higher germination velocity. A positive slope in the accumulated daily germination curve higher than that of the seed without scarification (Figure 2) suggests the start of the effect of the treatment and an almost constant slope at the end. The lower daily germination during the interval of 3-7 d in the hydrothermal treatment indicated a germination velocity close to zero and delayed 2-4 d the initial rise of the curve with respect to the other treatments.
In our study accumulated daily germination of the hydrothermal treatment at 7 d was 10.3 %. Sánchez-Paz and Ramírez-Villalobos (2006) report 22 % with a hydrothermal treatment of 10 min and an almost constant germination after day 20, which is similar to our study.
Arboreal legumes germinate at a more rapid velocity than non-leguminous species, and when the external protective coating of legume seeds was removed, daily germination increased from 15 to 33 % at 10 d after planting (Vargas et al., 2015). In our study, the guaje seeds scarified with water at 7 days after planting behaved in a manner similar to legume seeds without an external coating, with 31 % of accumulated daily germination, while for the non-scarified seeds and the hydrothermal treatment it was from 20 and 10 % (Figure 2). It is possible to increment germinative energy (Table 1) utilizing a scarification method which suppresses dormancy of seeds in legumes, such as what occurred in the guaje in our study and in other legumes (Vargas et al., 2015).
For Pterogyne nitens (Tul.), arboreal legume of South America, immersion in water for 1.5 h also improved germinative energy by close to 10 %, with respect to the seed without scarification (Pece et al., 2010a), and in our study the increase was 11.4 %. Germinative energy is an indicator of germination during the first days post-planting; with more seeds that germinate during the first days post-planting the risk of affectation by pests and diseases is reduced, which enables the development of seedlings with adaptive mechanisms, such as a higher growth rate under adverse conditions.
The hydrogen peroxide acts as a potentiator of germination during the first days in some legumes such as pea (Barba-Espín et al., 2012). However, in our study the germinative energy was similar to the other treatments and only surpassed the hydrothermal treatment. The hydrogen peroxide acts at the metabolic level at concentrations of 20 to 40 mM and if the external coating of the guaje seed is not penetrated, as it does in other species, its effect is negligible.
Imbibement in water favors the removal of inhibiting substances of the tegument of the seed and accelerates water absorption (Pece et al., 2010a). In our study, all of the treatments of immersion of the guaje seed in a liquid substance during 12 h showed a germinative energy superior to the hydrothermal treatment (p ≤ 0.05). This treatment did not have an imbibement period prior to planting, thus its germinative energy could have been affected due to a delay in water absorption or to the effect of the high temperature of the treatment on the metabolism, which could have induced low viability.
The germination value is the only indicator which fits in a single value the germinative capacity of the seed at the end of a germination test and the velocity with which it germinates, but it is very sensitive to changes in its components (Djavanshir and Pourbeik, 1976). The water immersion and hydrothermal treatments, with similar germination values (p > 0.05) were favorable for their high maximum and final daily germination velocity, respectively. This is similar to the germination value of 1.76 in the seeds of the arboreal legume Enterolobium cyclocarpum (Jacq.) Griseb treated with hydrothermal scarification, due to its low values of maximum germination velocity (Suárez et al., 2014). When using the germination value as criterion for selecting a scarification method in guaje seeds, it is important to consider the planting method, because the water immersion treatment increases germination velocity in the first days. This implies reducing the practices of control of undesired species and more vigorous seedlings when the planting is done in the field, whereas scarification with the hydrothermal treatment favors the percentage of total germination and can be favorable when the planting is done in nurseries and later transplanted to the field.
Conclusions
In seeds of Leucaena leucocephala cv. Cunningham, scarification by immersion in water at 80 ºC for 3 min and at 24 ºC for 12 h favored germination. However, in the former the viability of the seed was reduced and with the latter a higher germination velocity was observed during the first days after planting.
Literatura Citada
Arriaga, M. V., V. Cervantes G., y A. Vargas-Mena. 1994. Manual de Reforestación con Especies Nativas: Colecta y Preservación de Semillas, Propagación y Manejo de Plantas. Instituto Nacional de Ecología. México. 186 p. [ Links ]
Bacab, H. M., N. B. Madera, F. J. Solorio, F. Vera, y D. F. Marrufo. 2013. Los sistemas silvopastoriles intensivos con Leucaena leucocephala: una opción para la ganadería tropical. AIA 17: 67-81. [ Links ]
Barba-Espín, G., J. A. Hernández, y P. Díaz-Vivancos. 2012. Role of H2O2 in pea seed germination. Plant Sign. Behav. 7: 193-195. [ Links ]
Calle, Z., E. Murgueitio, C. Giraldo, S. D. Ospina, A. Zapata, C. H. Molina, E. J. Molina, J. D. Chará, F. Uribe, y K. Reyes. 2011. La Leucaena leucocephala no se comporta como una planta invasora en Colombia. Cta. FEDEGAN 127: 70-80. [ Links ]
Chará, J., E. Murgueitio, A. Zuluaga, y C. Giraldo (eds). 2011. Ganadería Colombiana Sostenible. Fundación CIPAV. Cali, Colombia. 158 p. [ Links ]
Cuartas, C. A., J. F. Naranjo R., A. M. Tarazona M., E. Murgueitio R., J. D. Chará O., J. Ku V., F. J. Solorio S., M. X. Flores E., B. Solorio S., y R. Barahona R. 2014. Contribution of intensive silvopastoral systems to animal performance and to adaptation and mitigation of climate change. Rev. Col. Cienc. Pec. 27: 76-94. [ Links ]
Czabator, F. J. 1962. Germination value: An index combining speed and completeness of pine seed germination. For. Sci. 8: 386-396. [ Links ]
Djavanshir, K., and H. Pourbeik. 1976. Germination value: A new formula. Silvae Gen. 25: 79-83. [ Links ]
Doria, J. 2010. Generalidades sobre las semillas: su producción, conservación y almacenamiento. Cult. Trop. 31: 74-85. [ Links ]
Ffolliott, P. F. and J. L. Thames. 1983. Recolección, manipuleo, almacenaje y pre-tratamiento de las semillas de Prosopisen América Latina. FAO. Roma, Italia. 43 p. [ Links ]
García, E. 1988. Modificaciones al Sistema de Clasificación Climática de Köppen. Instituto de Geografía, UNAM. México. 191 p. [ Links ]
Gómez-Merino, F. C., B. Vidal-Morales, L. Trejo-Téllez, y C. Molinos da Silva. 2010. Escarificación y germinación in vitro de semillas de Heliconias. Univ. Ciencia 26: 293-297. [ Links ]
González, Y., y F. Mendoza. 2008. Efecto del agua caliente en la germinación de las semillas de Leucaena leucocephala cv. Perú. Pas. For. 31: 47-52. [ Links ]
González, Y., J. Reino, y R. Machado. 2009. Dormancia y tratamientos pregerminativos en las semillas de Leucaena spp. cosechadas en suelo ácido. Pas. For. 32: 1-6. [ Links ]
González, Y., J. Reino, J. A. Sánchez, y R. Machado. 2012. Efecto del almacenamiento al ambiente en semillas de Leucaena leucocephala cv. Cunningham sometidas a hidratación parcial. Pas. For. 35: 393-399. [ Links ]
Gutiérrez G. O., O. Añez S., G. León P., D. Abed E., y E. Molina. 2007. Análisis fisicoquímico y estructural del polisacárido de la goma de semilla de Leucaena leucocephala. Ciencia 15: 481-487. [ Links ]
INIFAP. 2014. Red de estaciones del Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias. Estación meteorológica Cotaxtla-Medellín. http://clima.inifap.gob.mx/redinifap/est.aspx?est=35925 (Consulta: noviembre 2014). [ Links ]
Jara, N. L. F. 1996. Biología de semillas forestales. CATIE - Danida Forest Seed Centre. Turrialba, Costa Rica. 32 p. [ Links ]
Morais, F., L., J. C. C. Almeida, B. B. Deminicis, F. T. Pádua, M. J. F. Morenz, J. B. R. Abreu, R. P. Araujo, and D. D. Nepomuceno. 2014. Methods for braking dormancy of seeds of tropical legumes. Am. J. Plant Sci. 5: 1831-1835. [ Links ]
Pece, M. G., C. Gaillard de Benítez, M. Acosta, C. Bruno y S. Saavedra. 2010a. Tratamientos pregerminativos para Tipa colorada (Pterogyne nitens Tul.). Foresta Ver. 12: 17-25. [ Links ]
Pece, M. G., C. Gaillard de Benítez, M. Acosta, C. Bruno, S. Saavedra, y O. Buvenas. 2010b. Germinación de Tipuana tipu (Benth.) O. Kuntze (tipa blanca) en condiciones de laboratorio. Qebracho - Rev. Ciencia Fores. 18: 5-15. [ Links ]
Sánchez, J. A., J. Reino, B. Muñoz, Y. González, L. Montejo, y R. Machado. 2005. Efecto de los tratamientos de hidratación-deshidratación en la germinación, la emergencia y el vigor de las plántulas de Leucaena leucocephala cv. Cunningham. Pas. For. 28: 209-220. [ Links ]
Sánchez-Paz, Y., y M. Ramírez-Villalobos. 2006. Tratamientos pre germinativos en semillas de Leucaena leucocephala (Lam.) de Wit. y Prosopis juliflora (Sw.) DC. Rev. Fac. Agron. 23: 257-272. [ Links ]
SAS Institute. 2010. User’s Guide, version 9.4. Satistical Analysis System Institute. North Caroline, USA. [ Links ]
Suárez, M. S. D., B. González-Rivas y O. G. Mendoza-Sánchez. 2014. Energía y valor de germinación en las especies arbóreas genízaro (Phitecellobium saman (Jacq.) Benth.) y guanacaste negro (Enterolobium cyclocarpum (Jacq.) Griseb.). La Calera. 14: 28-32. [ Links ]
Teles, M. M., A. Azevêdo A., J. C. Gomes de Oliveira, y A. M. Esmeraldo B. 2000. Métodos para quebra da dormência em sementes de Leucaena (Leucaena leucocephala (Lam.) de Wit. Rev. Bras. Zoot. 29: 387-391. [ Links ]
Vargas, G., L. K. Werden, and J. S. Powers. 2015. Explaining legume success in tropical dry forests based on seed germination niches: A new hypothesis. Biotrop. 47: 277-280. [ Links ]
Received: March 2017; Accepted: May 2017











 texto en
texto en 


