Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Agrociencia
On-line version ISSN 2521-9766Print version ISSN 1405-3195
Agrociencia vol.52 n.6 Texcoco Aug./Sep. 2018
Animal Science
GSX-Px activity, selenium concentration and semen quality on rams supplemented with selenium during reproduction estage
1Universidad Autónoma del Estado de México, Facultad de Medicina Veterinaria y Zootecnia.
2Universidad Autónoma Chapingo, Departamento de Zootecnia.
3Centro Universitario UAEM Temascaltepec, Universidad Autónoma del Estado de México.
4División de Químico Biológicas. Facultad de Estudios Superiores Cuautitlán Universidad Nacional Autónoma de México.
There are diagnosed trace minerals deficiencies in grazing sheep in Mexico, but few strategies to correct and evaluate their response are available. Our objective in this study was to evaluate, in rams (10 Hampshire80.6 ± 7.6 kg LW and eight Suffolk 86.3 ± 9.3 kg LW), the effect of a sodium selenite supplement in an extended-release intraruminal bolus (500 mg of Se), on the LW, body condition, scrotal circumference, blood variables, glutathione peroxidase activity, selenium concentration in blood serum and characteristics of the ejaculate. The experimental design was completely randomized with a 2x2x3 factorial arrangement without and with Se (intraruminal bolus), breeds (Hampshire and Suffolk) and three months during the breeding season (November, December and January), where each ram was the experimental unit. Blood samples were obtained in weeks one (before treatment), two and three in July 2005, and then, in the fourth week of each month (August 2005 to February 2006). The ejaculate of the rams were sampled every week from November to January, using an artificial vagina. Every day (08:00 h), before grazing, the rams received a concentrated feed (1 % PV), grazed on Pennisetum clandestinum pastures during the day, and were confined at night. The supplied Se increased (p ≤ 0.05) GSH-Px activity, mobility and number of live and normal sperm. The ejaculate quality was higher (p ≤ 0.05) in volume, density, concentration and viability on the Suffolk rams. In conclusion, a Se supplement in intraruminal boluses increased GSH-Px activity, mobility and viability of the ejaculate in Hampshire and Suffolk rams during their breeding season.
Key words: rams; sodium selenite; intraruminal bolus; semen; glutathione peroxidase
En México se han diagnosticado deficiencias de minerales traza en ovinos en pastoreo, pero hay pocas estrategias para corregirlas y evaluar su respuesta. El objetivo de este estudio fue evaluar, en sementales ovinos (10 Hampshire con PV 80.6±7.6 kg y 8 Suffolk con PV 86.3±9.3 kg), el efecto de un suplemento de selenito de sodio en un bolo intraruminal de liberación prolongada (500 mg de Se), sobre el PV, condición corporal, circunferencia escrotal, variables hemáticas, actividad de glutatión peroxidasa, concentración de selenio en suero sanguíneo y características del eyaculado. El diseño experimental fue completamente al azar con arreglo factorial 2x2x3 sin y con Se (bolo intraruminal), razas (Hampshire y Suffolk) y tres meses en la época reproductiva (noviembre, diciembre y enero), donde cada semental fue la unidad experimental. Las muestras de sangre se obtuvieron en las semanas uno (previo al tratamiento), dos y tres de julio de 2005, y después, en la cuarta semana de cada mes (de agosto de 2005 a febrero de 2006). De noviembre a enero, mediante vagina artificial, cada semana se extrajo el eyaculado de los sementales. Cada día (08:00 h), previo al pastoreo, los sementales comieron un alimento concentrado (1 % PV) y pastaron durante el día praderas de Pennisetum clandestinum, mientras que por la noche estuvieron en confinamiento. El Se suministrado aumentó (p ≤ 0.05) la actividad de GSH-Px, la movilidad y número de los espermatozoides vivos y normales. La calidad del eyaculado fue mayor (p ≤ 0.05) en volumen, densidad, concentración y viabilidad para sementales Suffolk. En conclusión, un suplemento de Se en bolos intraruminales aumenta la actividad de GSH-Px, la movilidad y viabilidad del eyaculado de sementales Hampshire y Suffolk en la época reproductiva.
Palabras clave: carneros; selenito de sodio; bolo intraruminal; semen; glutatión peroxidasa
Introduction
Selenium (Se) is a trace mineral element with antioxidant activity that together with vitamin E performs essential functions to prevent cellular damage (Mahmoud et al., 2013). Therefore, both are part of the body's antioxidant system against oxidative stress, which is a condition that results from the imbalance between reactive oxygen species (free radicals and antioxidants in the body, which can lead to sperm damage). Therefore, both are part of the body's antioxidant system against oxidative stress, which is a condition due to imbalance between reactive oxygen species (free radicals and antioxidants in the body, which can lead to sperm damage, deformities and male infertility (Bansal and Bilaspuri, 2011; Agarwal et al., 2014). The main function of Se in the organism is as a component of several selenoprotein enzymes (Silva et al., 2000). The enzyme glutathione peroxidase (GSH-Px) contains Se in its chemical structure, and one of its functions is to prevent free radicals formation, thus preventing damage by tissue oxidation (Holben and Smith, 1999). There is a selenoprotein with relevant protective functions in the mitochondrial capsule of the sperms (Ahsan et al., 2014). In stallionsrams, Se and vitamin E improved semen quality, associated with a higher testosterone concentration and increased activity of the GSH-Px enzyme in blood serum (Mahmoud et al., 2013). In male goats, organic Se improved the antioxidant status, testosterone and T3 levels in seminal plasma and blood serum, which gives greater protection to sperms against oxidative damage, an important aspect for production of good quality semen ( Kumar et al., 2013). In buffaloes, organic and inorganic Se improved semen quality and increased testosterone and Se content in blood serum (El-Sharawy et al., 2017). Boar spermatozoa with SE Se deficiency have a low fecundating capacity due to their limited mobility associated with abnormal development of their flagellum (Ahsan et al., 2014). In dairy goats, the Se and vitamin E deficiency reduced the immune response and compromises the health and productive behavior of the female and her offspring (Ramírez-Bribiesca et al., 2005).
The inclusion of Se in the diet, or by parenteral application, improved weight gain in lambs, the fertility of the sheep and the immune response in both (Vázquez-Armijo et al., 2011); the above is conditioned to the achievement of the required Se concentrations in tissues and body fluids (Shamberger, 1983). The intraruminal Se supply in boluses increased the Se concentration in blood, improved mobility and sperm viability, as well as the response to the integrity of the sperm's cell membrane (Kendall et al., 2000). The Se and Zn supply in humans has shown intrinsic functions in spermatogenesis and in the general oxidative state, which suggests that both microminerals have important functions in stallion ram reproduction (Irvine, 1996; Vézima et al. , 1996; Oguntibeju et al., 2009; Kumar et al., 2013).
The Se deficiency affected the health and production in sheep, in lambs caused high mortality during lactation and growth, reduced the weight gain of developing sheep and caused reproductive problems such as retained placenta in sheep and a lower quality of the ejaculate in stallions rams (Andrews et al., 1975; Allen et al., 1986; Kendall et al., 2000). At the center most areas in Mexico sheep showed microminerals imbalances, with severe deficiencies of I, Se, Zn and Cu. Still, there are no mineral supply programs established to correct these deficiencies, and evaluate sheep response (Vázquez- Armijo et al., 2011). Ramírez et al. (2004) diagnosed Se deficiencies in ovine flocks at Tlaxcala and Puebla, Mexico. The lack of Cu is associated to the low content of Se in the soil and therefore forages, the low pH in these soils, and to excess Fe in the forages and soils, which affects its absorption and serum content in sheep (Domínguez-Vara and Huerta-Bravo, 2008). Goiter problems in sheep are related to a natural I deficiency, which probably involves the lack of Se and pollutants presentce in the environmental that cause endocrine disorders (Domínguez-Vara et al., 2017). Therefore, these mineral imbalances are factors that affect the health, growth and reproduction in ruminants (Minson, 1990). There is a close relationship between the testicles size and semen production; thus, stallions rams with small testicles may not produce enough sperm during the breeding period to achieve a good fertility rate (Mahmoud, 2013). During the breeding season, increaserise of sexual activity in rams increased their nutritional requirements for semen production in a short period (breeding season), which can induce se Se deficiency and cause greater oxidative stress, with lower semen production and quality (Ahsan et al., 2014; Zubair et al., 2015). In small ruminants, Se supply to supplement the contribution of the forages, includes adding premixes to their food, subcutaneous or buccal application in solutions and boluses or intraruminal Se tablets. The last method allows Se to be complemented in a practical, safe and effective way at a low cost (Langlands et al., 1994, Ramírez-Bribiesca et al., 2004; Revilla-Vázquez et al., 2008).
This study aimede aim of this study was to evaluate the effects of the supplementing Se in intraruminal bolus on ovine stallions rams of the Hampshire and Suffolk breeds on their blood variables, glutathione peroxidase enzyme activity, serum Se concentration and characteristics of the ejaculate during the reproductive season. We hypothesizedOur hypothesis was that Se delivered in prolonged release intraruminal bolus increases the concentration of that mineral, the activity of the enzyme GSH-Px in the blood, and improves the quality of the semen during the breeding season of the rams.
Materials and Methods
Location and sampling period of the experiment
The study was carried out in the ovine production unit (UP) at the town Mayorazgo de León, municipality of Almoloya de Juárez, State ofEstado de MexicoMéxico, located between 19° 14' S and 19° 34' N, 99 ° 42 ' E and 99 ° 58 ' W, at 2500 masl. The climate is temperate sub-humid with rains in summer, higher humidity (86.59 %); sub -humid semi semi-cold, lower humidity (13.41 %), with temperatures ranges ranging between 15 and 22 °C and 6 and 14 ° C, respectively, and annual rainfall of 700 to 1500 mm (INEGI, 2009). The UP is a semi-intensive type, 10 ha with a fixed fence, a ship with three pens equipped with drinking fountains and mobile feeders, housing 250 adult sheep and 20 stallionsrams.
Animals and feeding
The study used 18 stallions rams 2.8 ± 0.3 years old, Hampshire (n = 10, PV LW = 80.6 ± 7.6 kg) and Suffolk (n = 8, PV LW = 86. ± 9.3 kg) breeds. The treatments were: 1) without Se supplement of S (n = 10, five stallions rams of each breed), and 2) with Se supplement (n = 8, four stallions rams of each breed) via intra-ruminal bolus. The boluses weighed 7.3 g, 5 cm long by 1.5 cm wide and 1 cm thick, rounded tips and smooth surface. The Se source was sodium selenite (Na2SeO3), equivalent to 500 mg of elemental Se per bolus, sufficient to cover its daily requirement (0.1 to 0.3 mg kg-1 MS d-1) in adult sheep for more than a year (NRC, 2007). The boluses manufacturing took place at the FESC-UNAM® by melt granulation (thermoplastic granulation). Bolus formulation and composition of the included sodium selenite as elemental Se; the lipid excipient was a hydrogenated vegetable oil; a densifying agent was used to achieve a minimum density of 2.36 g cm-3, which prevents the device regurgitation and facilitates its permanence in the rumen. According to Gyurik (1988), maintaining a density of 2.25 to 3.50 g cm-3 prevents bolus regurgitation in grazing ruminants. The bolus has an effectiveness period of several months, which slowly releases the mineral to maintain an adequate level of 0.025 to 0.150 μg of Se mL-1 of plasma in adult sheep (Puls, 1994). Judson et al. (1991) indicated that Se elemental bolus filled with Fe is sufficiently dense and remains in the rumen and in contact with the ruminal fluid slowly releasing the mineral for a period of approximately one year. The supply of the boluses was via the mouth, with doses of one bolus per stallionram, applied on the first day of the experiment.
During the day, the stallions rams grazed on kikuyu grass pastures (Pennisetum clandestinum), and then confined for 16 h. Prior to grazing, at 08:00, a balanced feed (14 % PCP and 2.8 Mcal of MS ME kg-1 DM), which included a premix of vitamins and minerals without Se and vitamin E, was fed to rams (1 % of the PVLW) in order to cover requirement for essential minerals (NRC, 2007).
Experimental development
During the eight months of the study blood samples were obtained via jugular puncture (From from July 2005 to February 2006). The first blood sample was collected one week before the stallions rams received Se through the intra-ruminal bolus; blood samples were then collected in the subsequent two weeks (2nd and 3rd weeks of July), and then in the last week of each month during the experiment. Besides, each week semen was taken from each stallion ram between 09:00 and 10:00 h, on an immobilized female sheep and an artificial vagina. The semen evaluation was carried out during the rams breeding season, from November to January. The collected semen was tempered at 37° C in graduated polypropylene tubes. Before the semen extraction, stallions rams were weighed on an electronic scale, and their body condition evaluated using the scale described by Russell (1991). The scrotal circumference was measured using a tape measure.
Laboratory analysis
The laboratory analysizes were carried out at the Department of Animal Nutrition and Animal Reproduction, School of Veterinary Medicine and Animal Science, Universidad Autónoma del Estado de México, Campus El Cerrillo, Toluca, México. This study was carried out between July 2005 and and February 2006.
The hematocrit and hemoglobin determinations were performed according to the procedures described by Coffin (1987).
The hemolysate blood sample used to determine the glutathione peroxidase enzymatic activity was processed following the methods by Beutler (1976), and the preparation of the solutions to determine the glutathione peroxidase enzymatic activity were prepared according to the method of Blanchflower et al. (1986). The hemolysate was kept refrigerated at 4 °C until analysis by optical spectrophotometry at 340 nm.
Selenium content in blood serum determination
The serum concentration of Se was determined via spectrofluorometry scanning (AOAC, 2012) in an LS-30 model equipment, series 34371, Perkin Elmer, London, U.K.
Sperm evaluations
The ejaculate was evaluated on a scale of 1 to 5 in terms of its volume (mL), color and mass mobility on a 10X clear field microscope (Evans and Maxwell, 1990, Baril et al., 1993 ), and the sperm cells concentration (x106) was determined in a Neubauer chamber. To evaluate sperm morphology abnormalities, smears of the ejaculate were prepared, dried and stained with eosin-nigrosine and counted in a microscope with a 40X lens. In each semen sample, 200 sperm cells and their abnormalities (total, rolled tail, without tail, two tails, others) were evaluated following the technique described by Baril et al. (1993).
The experimental design was completely randomized with a 2x2x3 factorial arrangement: 1) two treatments, with and without Se (intraruminal bolus), 2) two races breeds (Hampshire and Suffolk), and 3) three months during their reproductive season (November, December and January). Each stallion ram was considered as an experimental unit. The data were analyzed with PROC MIXED (SAS, 2009) using the Tukey test (Steel et al., 1997) for means comparison (p ≤ 0.05). Before the analysis, the values obtained as percentages, were transformed via the arcsine transformation.
Results and Discussion
There were no effects due to the interactions on the analyzed productive and blood variables (p > 0.05). At the end of the experiment Suffolk stallions rams that received the Se supplement had higher LW (p ≤ 0.05) and GSH-Px activity (p ≤ 0.05), compared to those whithout the Se bolus. The supplement with Se or the race breed did not change the scrotal circumference, body condition, hematocrit, hemoglobin or serum Se content (p > 0.05) (Table 1). Mahmoud et al. (2013) did not observed no effect of the Se combined with vitamin E in the LW, weight gain or scrotal circumference on stallionsrams. The hematocrit, hemoglobin and Se serum content were adequate as described by Wheatley and Beck (1988) and Puls (1994). In contrast, GSH-Px activity was deficient in both breeds with and without the Se bolus supply, since the normal activity value for sheep is 130 U g-1 Hb (Ceballos and Wittwer, 1996). The Se bolus was increased, consistently and sustained, the activity of the GSH-Px enzyme in the rams treated during the study period; however, although the Se concentration in blood serum was adequate for both groups of stallions rams with and without Se, the GSH-Px enzyme activity was deficient. The significant increase of the Se level in blood serum of rams treated with the Se bolus is consistent with the results from Kumar et al. (2013) in goat stallions males treated with organic Se and Zn, as well as with the results from El-Sharawy et al. (2017) in buffaloes treated with organic Se (Se yeast) and inorganic Se (sodium selenite). However, in contrast to our results, sodium selenite combined with vitamin E produced adequate activity of the GSH-Px enzyme in Ossimi breed stallions rams of the Ossimi breed (Mahmoud et al., 2013).
Table 1 Live weight averages, scrotal circumference, body condition, hematocrit values, hemoglobin, glutathione peroxidase activity and blood serum selenium concentration in Hampshire and Suffolkraces rams, without or with selenium in an intra-ruminal bolus.
| Variable | Selenio | Raza | Efectos de¶ | ||||
|---|---|---|---|---|---|---|---|
| Testigo | Bolo c/Se | Hampshire | Suffolk | EEM† | Se | Raza | |
| Peso vivo, kg | 84.88a | 83.14a | 80.83b | 88.64a | 6.5362 | ns | ** |
| Condición corporal, escala 1-5 | 3.66a | 3.68a | 3.72a | 3.60a | 0.29907 | ns | ns |
| Circunferencia escrotal, cm | 32.98a | 33.17a | 32.94a | 33.27a | 1.4597 | ns | ns |
| Hematocrito, % | 33.85a | 35.92a | 33.54a | 35.21a | 4.0970 | ns | ns |
| Hemoglobina3, mg dL-1 | 11.28a | 11.97a | 11.18a | 11.63a | 1.3060 | ns | ns |
| Actividad de GSH-Px§ (U g-1 Hb) | 97.16b | 105.07a | 85.60a | 85.68a | 39.070 | * | ns |
| Concentración Se§ (µg mL-1) | 0.10a | 0.16a | 0.15a | 0.16a | 0.0350 | ns | ns |
abValues in a row, for the same factor, with different letter are statistically different (p ≤ 0.05).
†EEM: standard error of the mean.
¶NS: Not significant (p > 0.05; * p ≤ 0.05; ** p ≤ 0.01).
§ Normal value: GSH-Px activity, deficient < 130 U g-1 Hb (Ceballos and Wittwer, 1996); hematocrit, 29-38 %; hemoglobin, 8-16 g dL-1; Se, adequate = 0.08-0.5, marginal = 0.03-0.07, deficient = 0.006-0.03 μg mL-1 (Wheatley and Beck, 1988; Puls, 1994).
During the study, and between the months the LW mean values for PV, scrotal circumference and body condition from the stallions rams were similar (p > 0.05) (Table 2); In in contrast, the hematocrit mean values, hemoglobin, GSH-Px activity and Se content in blood serum were different (p ≤ 0.05) (Figure 1 and Table 2). The hematocrit value was lower in July and higher in October, but with adequate levels throughout the period (29 to 38 %). The hemoglobin content was adequate (8 to 16 mg dL-1) (Puls, 1994). The GSH-Px enzymatic activity was lower during July and higher in January; however, according to the optimum range indicated by Ceballos and Wittwer (1996), the GSH-Px enzymatic activity was low, and only during January it had values higher than 130 U g-1 Hb. The Se level in blood serum was adequate (0.08 to 0.5 μg mL-1) for the Se supply, according to the range proposed by Wheatley and Beck (1988) and Puls (1994). Kendall et al. (2001a, b) observed effects on the Se serum content and increased GSH-Px activity of the enzyme when giving Se in intraruminal boluses. However, the GSH-Px activity, of both stallions rams groups, was less than 60 U g-1 Hb in the basal samplings from July ton September and October (Figure 1). Although the GSH-Px activity was higher in the sires rams treated with Se, only in January it was higher than 130 U g-1 Hb. Kendall et al. (1997a, b) found similar Se serum levels in Se treated lambs, with Co and Zn in a soluble crystal bolus, although serum Se was adequate in the control and treated group. Similar results were found with intraruminal boluses with Se, Co and Zn in sheep (Kendall et al., 1997a, b, ; Kendall et al., 1999, 2000). Therefore, the intraruminal application of boluses with Se is an adequate, practical, safe and economical method to provide a sustained supplement with trace minerals, such as Se, to improve semen quality during the reproductive period
Table 2 Monthly PV averages of PVLW, scrotal circumference, body condition, hematocrit, hemoglobin, GSH-Px activity and blood selenium concentration in Hampshire and Suffolk races rams with or without selenium in an intra-ruminal bolus.
| Mes de muestreo | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Jul. S1¶ | Jul. S2 | Jul. S3 | Ago§. | Sep. | Oct | Nov. | Dic. | Ene. | Feb. | EEM† |
| Peso vivo (kg) | 83.3a | 84.2a | 84.5a | 85.1a | 85.0a | 84.2a | 83.1a | 85.6a | 83.2a | 79.0a | 2.9230 |
| Condición corporal (escala 1-5) | 3.4a | 3.4a | 3.5a | 3.5a | 3.4a | 3.6a | 3.6a | 3.7a | 3.7a | 3.5a | 0.1299 |
| Circunferencia escrotal (cm) | 32.9a | 33.1a | 33.3a | 32.5a | 33.1a | 32.5a | 34.2a | 32.6a | 32.1a | 33.0a | 0.6528 |
| Hematocrito (%) | 34.6b | 35.7ab | 31.7b | 33.4b | 35.4ab | 39.1a | 33.7b | 33.9b | 31.8b | 33.6b | 1.8325 |
| Hemoglobina (g dL-1) | 11.5bc | 11.9ab | 10.5bc | 11.1bc | 11.8abc | 13.0a | 11.2bc | 11.1bc | 10.3c | 11.0bc | 0.5844 |
| Actividad GSH-Px (U g-1 Hb) | 30.1d | 91.7bc | 89.4bc | 96.1bc | 92.0bc | 93.0bc | 113.5ab | 118.1ab | 143.9a | 119.4ab | 17.471 |
| Contenido de Se (µg mL-1) | 0.04d | 0.09d | 0.11d | 0.13cd | 0.17ab | 0.18a | 0.16abc | 0.15bcd | 0.12d | 0.17ab | 0.0160 |
abcdValues in a row with different letterss are statistically different (p ≤ 0.05).
†EEM: standard error of the mean.
¶Blood sampling weeks 1, 2 and 3 in July, §4 to 10 each month from August 2005 to February 2006.
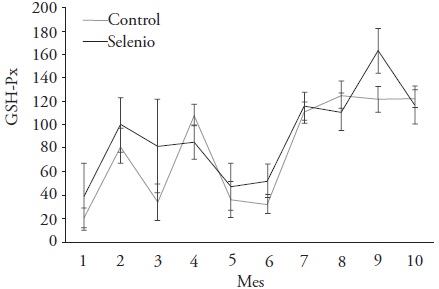
Figure 1 GSH-Px enzyme activity (U g-1 Hb) in blood from Hampshire and Suffolk rams with or without selenium in an intra-ruminal bolus. Samples 1 to 3 correspond to weeks 1, 2 and 3 of July; samples 4 to 10 correspond to August 2005 to February 2006.
There was no interaction effect for semen quality variables (Table 3). Greater motility of the sperm was induced (p≤0.05), also the number of live (p≤0.05) and normal (p ≤ 0.01) sperm in the semen increased. Suffolk stallions rams showed higher semen quality in terms of their volume (p ≤ 0.01), color (p ≤ 0.05), density (p≤0.01) and concentration (p≤0.05). The extracted ejaculates from January showed higher values in the mobility, density (p≤0.01) and concentration variables (p ≤ 0.05) from sperm cells.
Table 3 Averages of ejaculate quality variables from Hampshire and Suffolk rams with and without selenium in intra-ruminal bolus.
| Variable | Selenio | Raza | Mes | Efectos de¶ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Testigo | Bolo c/Se | Hampshire | Suffolk | Nov. | Dic. | Ene. | EEM† | Se | Raza | Mes | |
| Volumen (mL) | 0.91a | 0.87a | 0.80b | 1.03a | 0.86b | 0.92a | 0.86b | 0.298 | ns | ** | ns |
| Color (escala 1-5) | 2.89a | 2.98a | 2.88b | 3.02a | 2.83a | 2.96a | 3.02 | 0.416 | ns | * | ns |
| Movilidad masal (escala 1-5) | 3.20b | 3.47a | 3.20a | 3.40a | 3.04b | 3.33b | 3.69a | 0.723 | * | ns | ** |
| Densidad | 3.07a | 2.98a | 2.98b | 3.37a | 2.88b | 3.10b | 3.68a | 0.687 | ns | ** | ** |
| Concentración (millones/mL-1) | 1,850a | 1,829a | 1,729b | 1,850a | 1,723b | 1,742b | 1,892a | 256.4 | ns | * | * |
| Células vivas (%) | 92.18b | 93.94a | 92.72a | 93.55a | 93.50a | 92.80a | 92.70a | 2.661 | * | ns | ns |
| Células normales (%) | 91.98b | 94.33a | 92.80a | 93.04a | 93.59a | 92.61a | 92.34a | 2.562 | ** | ns | ns |
abValues with different letter in a row are statistically different (p ≤ 0.05).
†EEM = standard error of the mean.
¶NS = not significant (p > 0.05); ), * p ≤ 0.05;, ** p ≤ 0.01).
The significant increase in the mobility variables, concentration, live and normal sperm in the ejaculated semen of the sires rams treated with Se is consistent with the results reported by (Mahmoud et al., (2013) for Ossimi breed stallions rams of the Ossimi race treated with Se and Vitamin vitamin E and, besides, besides, with the results in buffaloes (El-Sharaw et al., 2017)treated with Se yeast and sodium selenite (El-Sharaw et al., 2017). Similarly, Kumar et al. (2013) indicated that goat stallions males treated with organic Se and Zn significantly improved their antioxidant status, the sperm wasere better protected from oxidative damage and the sperm quality was a better.
The increase in the sperm cells mobility, as well as the percentage of live and normal sperm from sires rams supplemented with Se, is probably related to the increased activity of the GSH-Px enzyme promoted by the Intraintra-bolus ruminal bolus. This coincides with findings from Kendall et al. (2000) in sires rams treated with soluble crystal boluses containing Zn, Co and Se. The additional Se supplied with intra-ruminal bolus increased GSH-Px enzyme activity through its antioxidant function, providing greater protection against spontaneous lipid peroxidation of the plasma membrane in sperm cells, creating a barrier with selective permeability, supplying enzymes and cytoplasmic substrates that increase mobility and survival of the spermatozoid (Álvarez and Storey, 1984; Zubair et al., 2015). Therefore, in sires rams that received the Se bolus, semen quality was probably improved by the formation of selenoproteins in the testicular tissue, with greater spermatogenesis stimulation (Ahsan et al., 2014). It directly affects directly the interstitial cells of the testes, thus improving testicular function and semen quality (Underwood, 1977), and indirectly, via the effect on hormones secretion from the anterior pituitary gland (Yousef et al., 1990). The supply of Is Se combined with vitamin E increases the testosterone serum concentration, the activity of the GSH-Px enzyme, and altogether a greater manifestation of male secondary sex characteristics (Bearden and Fuquay, 1997, Mahmoud et al., 2013). BesidesIn addition, Se is necessary for germ cells development in the testes during spermatozoa formation and has positive effects on the total germ cells in adults (Liu et al., 1982). In the field, the improvement in variables indicated in our study, can favorably influence the quality of the ejaculate and, therefore, in the semen fertility of the stallionsrams; however, in sheep, these results should be confirmed with new studies.
Literatura Citada
Ahsan, U., Z. Kamran, I. Raza, S. Ahmad, W. Babar, M. H. Riaz, and Z. Iqbal. 2014. Role of selenium in male reproduction-A review. Anim. Reprod. Sci. 146: 55-62. [ Links ]
Allen, J. G., P. Steele, H. G. Nasters, and N. F. Dantouono. 1986. A study of nutritional myopathy in weaner sheep. Aust. Vet. J. 68: 8-13. [ Links ]
Alvarez, J. G., and B. T. Storey. 1984. Assessment of cell damage caused by spontaneous lipid peroxidation in rabbit spermatozoa. Biol. Reprod. 30: 323-331. [ Links ]
Andrews, E. D., R. G. Hogan, and A. D. Sheppard. 1975. Selenium in soil, pastures and animal tissues in relation to the growth of young sheep on a marginally selenium deficient area. N. Z. Vet. J. 24: 111-116. [ Links ]
AOAC. Association of Official Analytical Chemists. 2012. Official Methods of Analysis. 19th ed. AOAC: International, USA. pp: 34-36. [ Links ]
Agarwal, A., G. Virk, C. Ong, and S. S. du Plessis. 2014. Effect of oxidative stress on male reproduction. The World J. Men's Health. 32: 1-17. [ Links ]
Bansal, A. K., and G. S. Bilaspuri. 2011. Impacts of oxidative stress and antioxidants on semen functions. Vet. Medicine Int. 2011: 686137. [ Links ]
Baril, G., P. Chemineau, Y. Cognie, Y. Guérin, B. Leboeuf, P. Orgeur, J. C. et Vallet. 1993. Manuel de formation pour l'insémination artificielle chez les ovins et les caprins. Monnaie. Organisation des Nations Unies pour L'alimentation et L'agriculture. 111 p. [ Links ]
Bearden, H. J., and J. W. Fuquay. 1997. Applied Animal Reproduction. 4th ed. Prentice Hall, Upper Saddle River, NJ, USA. 351 p. [ Links ]
Beutler, G. E. 1976. Red Cell Metabolism: A Manual of Biochemical Methods. 2nd Ed. Grune and Streaton, N.Y., USA. 160 p. [ Links ]
Blanchflower, W. J., D. C. Rice, and W. B. Davidson. 1986. Blood glutathione peroxidase. A method for measurement and the influence of storage, cyanide and Drabkin´s reagent on enzyme activity. Biolog. Trace Element Res. 11: 89-100. [ Links ]
Ceballos M., A., y F. Wittwer G. 1996. Metabolismo del selenio en rumiantes. Arch. Med. Vet. 2: 5-18. [ Links ]
Coffin L., D. 1987. Laboratorio Clínico en Medicina Veterinaria. Ediciones Científicas la Prensa Médica Mexicana. pp: 125-162. [ Links ]
Domínguez-Vara I., A., y M. Huerta-Bravo. 2008. Concentración e interrelación mineral en suelo, forraje y suero de ovinos durante dos épocas en el Valle de Toluca, México. Agrociencia 42: 173-183. [ Links ]
Domínguez-Vara I., A., F. Salazar-García, R. Montes de Oca-Jiménez, I. Medina-Torres, J. G. Vicente-Martínez, and J. Pinos-Rodríguez M. 2017. Sheep fetal goiter: study case in Mexico. Trop. and Subtrop. Agroecosyst. 20: 307-313. [ Links ]
El‐Sharawy, M., E. Eid, S. Darwish, I. Abdel‐Razek, M. Islam, K. Kubota, and I. El‐Shamaa. 2017. Effect of organic and inorganic selenium supplementation on semen quality and blood enzymes in buffalo bulls. Anim. Sci. J. 88: 999-1005. [ Links ]
Evans G., y C. Maxwell W. 1990. Conservación de semen congelado. In: Inseminación Artificial en Ovejas y Cabras. Evans, G., y C. W. Maxwell. (eds). Edit. Acribia, Zaragoza, España. pp: 123- 142. [ Links ]
Gyurik, R. J. 1988. Rumen Retention Devices. In: Tyle, P. (ed). Drug Delivery Devices. Marcel Dekker, Ney York. pp: 549-561. [ Links ]
Holben, D. H., and A. M. Smith. 1999. The diverse role of selenium within selenoproteins: A review. J. Am. Dietetic Assoc. 99: 836-843. [ Links ]
INEGI. 2009. Prontuario de información geográfica municipal de los Estados Unidos Mexicanos Almoloya de Juárez, México, Clave geoestadística 15005; 2009. http://www.inegi.org.mx/sistemas/mexicocifras/datos-geograficos/15/15005.pdf . (Consulta: julio 2016). [ Links ]
Irvine, D. S. 1996. Glutation as a treatment for male infertility. Rev. Reprod. 1: 12. [ Links ]
Judson, G. H., N. F. Ellis, B. R. Kempe, and M. Shallow. 1991. Long-acting selenium treatment for sheep. Aust. Vet. J. 68: 263-265. [ Links ]
Kendall, N. R., A. M. Mc Kenzie, and S. B. Telfer. 1997a. The use a soluble glass bolus to prevent zinc deficiency in sheep. In: Fisher, P. W. F., M. R. Abbe, K. A. Cockell, and R. S. Gibson (eds). Trace Element in Men and Animals-9: Proc. Ninth Int. Symp. Trace Elements in Man and Animals. NRC Research Press, Otawa, Canada. pp: 303-305. [ Links ]
Kendall, N. R., A. M. Mc Kenzie, and S. B. Telfer. 1997b. Effect a soluble cobalt, selenium and zinc glass bolus on humoral immune response and trace elements status in lambs. In: Fisher, P. W. F., M. R. Abbe, K. A. Cockell, and R. S. Gibson (eds). Trace Element in Men and Animals-9: Proc. Ninth Int. Symp. Trace Elements in Man and Animals . NRC Research Press, Otawa Canada. pp: 442-444. [ Links ]
Kendall, N. R., N. C. Farrar, D. V. Illingworth, D. W. Jackson, and S. B. Telfer. 1999. The use of a soluble glass copper, cobalt and selenium bolus to supply selenium to sheep. Proc. Brit. Soc. Anim. Sci. 99 p. [ Links ]
Kendall, N. R., S. McMullen, A. Green, and R. G. Rodway. 2000. The effect of a zinc, cobalt and selenium soluble glass bolus on trace element status and semen quality of ram lambs. Anim. Reprod. Sci. 62: 277-283. [ Links ]
Kendall, N. R., A. M. Mackenzie, and S. B. Telfer. 2001a. The effect of a copper, cobalt and selenium soluble glass bolus given to grazing sheep. Liv. Prod. Sci. 68: 31-39. [ Links ]
Kendall, N. R., D. W. Jackson, A. M. Mackenzie, D. V. Illingworth, I. M. Gill, and S. B. Telfer. 2001b. The effect of a zinc, cobalt and selenium soluble glass bolus on the trace element status of extensively grazed sheep over winter. Anim. Sci. 73: 163-169. [ Links ]
Kumar, P., B. Yadav, and S. Yadav. 2013. Effect of zinc and selenium supplementation on antioxidative status of seminal plasma and testosterone, T4 and T3 level in goat blood serum. J. Appl. Anim. Res. 41: 382-386. [ Links ]
Langlands, J. P., G. E. Donald, J. E. Bowles, and A. J. Smith. 1994. Selenium supplements for grazing sheep 4. The use of intraruminal pellets containing elevated quantities of selenium. Anim. Feed Sci. Technol. 46: 109-118. [ Links ]
Liu, C. H., Y. M. Chen, J. Z. Zhang, M. Y. Huang, Q. Su, Z. H. Lu, R. X. Yin, G. Z. Shao, D. Feng, P. L. Zheng. 1982. Preliminary studies on influence of selenium deficiency to the developments of genital organs and spermatogenesis of infancy boars. Acta Vet. Zootech. Sin. 13: 73-77. [ Links ]
Mahmoud, G. B. 2013. Sexual behaviour, testosterone concentration, semen characteristics and testes size of Ossimi rams as affected by age and scrotal circumference. Egyptian J. Anim. Prod. 50: 53-58. [ Links ]
Mahmoud, G. B., S. M. Abdel-Raheem, and H. A. Hussein. 2013. Effect of combination of vitamin E and selenium injections on reproductive performance and blood parameters of Ossimi rams. Small Rum. Res. 113: 103-108. [ Links ]
Minson, D. J. 1990. Forages in Ruminant Nutrition. Academic Press. USA. 463 p. [ Links ]
NRC. 2007. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids. Animal Nutrition Series. National Research Council. National Academy Press U.S.A. 362 p. [ Links ]
Oguntibeju, O. O., J. S. Esterhuyse, and E. J. Truter. 2009. Selenium: its potential role in male infertility. Pak. J. Med. Sci. 25: 332-337. [ Links ]
Puls, R. 1994. Minerals Levels in Animal Health. Diagnostic Data. Sherpa International. Clarbrook, Canada. pp: 83-109. [ Links ]
Ramírez-Bribiesca J., E., J. Tórtora L, M. Huerta B, L. Hernández M, R. López, y M. Crosby M. 2005. Effect of selenium-vitamin E injection in selenium-deficient dairy goats and kids on the Mexican plateau. Arq. Bras. Med. Vet. Zoot. 57: 77-84. [ Links ]
Ramírez B., E., C., Hernández E, C. Hernández L, y J. Tórtora P. 2004. Efecto de un suplemento parenteral con selenito de sodio en la mortalidad de corderos y los valores hemáticos de selenio. Agrociencia 38: 43-51. [ Links ]
Revilla-Vázquez A., E. Ramírez-Bribiesca, R. López-Arellano, L. Hernández-Calva, J. Tórtora-Pérez, E. García-García, y M. Cruz R. G. 2008. Suplemento de selenio con bolos intrarruminales de selenito de sodio en ovinos. Agrociencia 42: 629-635. [ Links ]
Russel, A. 1991. Body condition scoring of sheep. In: E. Boden, editor. Sheep and Goat Practice. Philadelphia, USA: Bailliere Tindal. pp: 156-162. [ Links ]
SAS. 2009. SAS/STAT® 9.2 User's Guide, 2nd Ed. SAS Institute Inc, Cary, N. C., U.S.A. [ Links ]
Shamberger, R. J. 1983. Biochemistry of Selenium. Plenum Press. USA. 334 p. [ Links ]
Silva J., H., M. Quiroga A, y N. Auza J. 2000. Selenio en el rumiante. Relaciones suelo, planta, animal. Med. Vet. 17: 229-246. [ Links ]
Steel, R. G. D., J. H. Torrie, and D. A. Dickey. 1997. Principles and Procedures of Statistics: A Biometrical Approach. 3rd ed. McGraw-Hill Series in Probability and Statistics. USA. 622 p. [ Links ]
Vázquez-Armijo J., F., R. Rojo R, D. López, J. Tinoco L, A. González, N. Pescador S, and I. Domínguez-Vara A. 2011. Trace elements in sheep and goats reproduction: a review. Trop. Subtrop. Agroecosyst. 14: 1-13. [ Links ]
Vézina, D., F. Mauffette, K. D. Robert, and G. Bleau. 1996. Selenium vitamin E supplementation in infertile men effect on semen parameters and micronutrient levels and distribution. Biol. Trace Element. Res. 53: 55-83. [ Links ]
Underwood, E. J. 1977. Trace Elements in Human and Animal Nutrition, 3rd. ed. Academic Press, New York, USA. 560 p. [ Links ]
Wheatley, L. E., and N. F. G. Beck. 1988. The influence of season and husbandry on the selenium status of sheep in a deficient area. Brit. Etol. J. 144: 246-251. [ Links ]
Yousef, H. M., A. Abul-Ela, E. R. Farag, Y. L. Awad, F. E. El-Keraby, H. A. Hassanin, 1990. Effect of pre-partum selenium injection on reproductive and lactational performance and post-partum hormone profile in dairy cows. In: Proceedings of 4th Scientific Congress Faculty of Veterinary Medicine Assiut, University Assiut, Egypt, pp: 445-454. [ Links ]
Zubair, M., M. Ali, M. Ahmad, S. M. Sajid, I. Ahmad, and S. T. Gul. 2015. Effect of selenium and vitamin E on cryopreservation of semen and reproductive performance of animals (a review). J. Entomol. Zool. Studies. 3: 82-86. [ Links ]
Received: March 2017; Accepted: January 2018











 text in
text in 


