Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.52 no.5 Texcoco Jul./Ago. 2018
Crop science
Indeterminate and determinate growth habit in Amaranthus hypochondriacus: ontogeny of shoot apical meristem and its effect on seed weight
1 Instituto Tecnológico de Celaya, Avenida García Cubas Pte. # 600, Esquina Avenida Tecnológico, Colonia Alfredo V. Bonfil, 38010 (lorenzogo@yahoo.com). Celaya, Guanajuato, México.
2INIFAP-Campo Experimental Valle de México, km 13.5 Carretera Los Reyes-Texcoco, 56250. Coatlinchán, Texcoco, Estado de México.
3INIFAP-Campo Experimental Bajío, km 6.5 Carretera Celaya-San Miguel de Allende, 38110. Celaya, Guanajuato, México.
4LANGEBIO, Libramiento Norte Carretera León km 9.6, 36821. Irapuato, Guanajuato, México.
5CINVESTAV-Guanajuato, Libramiento Norte Carretera León km 9.6, 36821. Irapuato, Guanajuato, México.
Amaranth (Amaranthus hypochondriacus L.) is important for its high protein content, as the nutritional value of its grain is due partly to the amount of protein higher than that of other cereals. Grains form in the inflorescences and their architecture depend on whether growth is determinate or indeterminate. By means of scanning electron microscopy and thinning techniques, we obtained images of the morphological changes of the apical meristem during the life cycle of the plant. That tissue remained undifferentiated in the indeterminate phenotype and the inflorescence was spiked, the apical meristem stopped its activity with the formation of each terminal flower, originating a synchronized development. The development of the inflorescence in determinate growth did not present phenological overlap. The branches of this last phenotype were shorter. The seeds of plants with determinate growth were heavier than those of indeterminate growth. The study of apical meristem morphology could be the basis for the analysis of morphogenetic aspects of development, which at the same time are support for the genetic improvement and agronomic management of A. hypochondriacus.
Key words: Amaranthus hypochondriacus L.; indeterminate growth; determinate growth; apical meristem; seed
El amaranto (Amaranthus hypochondriacus L.) es importante por su contenido proteínico alto, pues el valor nutricional de su grano se debe, en parte, a la cantidad de proteínas superior a la de otros cereales. Los granos se forman en las inflorescencias y su arquitectura depende de que el crecimiento sea determinado o indeterminado. Mediante microscopia electrónica de barrido y técnicas de clareo se obtuvieron imágenes de los cambios morfológicos del meristemo apical durante el ciclo de vida de la planta. Ese tejido permaneció indiferenciado en el fenotipo indeterminado y la inflorescencia fue espigada, el meristemo apical detuvo su actividad con la formación de cada flor terminal, lo que originó un desarrollo sincronizado. El desarrollo de la inflorescencia en el crecimiento determinado no presentó traslape fenológico. Las ramificaciones de este último fenotipo fueron más cortas. Las semillas de plantas con crecimiento determinado fueron más pesadas que las de crecimiento indeterminado. El estudio de la morfología del meristemo apical podría ser base para el análisis de aspectos morfogenéticos del desarrollo, que a la vez, sean sustento para el mejoramiento genético y manejo agronómico de A. hypochondriacus.
Palabras clave: Amaranthus hypochondriacus L.; crecimiento indeterminado; crecimiento determinado; meristemo apical; semilla
Introduction
The knowledge of ontogeny of the apical meristem in plants, in the transition to the reproductive phase is useful to understand the morphogenetic aspects of development and is the basis for physiological improvement studies; in addition, it can support decision-making in the agronomic management of crops. The cultivation of amaranth (A. cruentus, A. caudatus and A. hypochondriacus) is of great interest for the worldwide consumption of its grain (Ayala et al., 2012). This can be attributed to its nutritional value, due to the quantity and high quality of its protein, which surpasses wheat (Triticum aestivum L.), rice (Oryza sativa L.), oats (Avena sativa L.) and corn (Zea mays L.); in addition, the amaranth protein contains more lysine, deficient in other cereals (Venskutonis and Kraujalis, 2013). Among the nutraceutical properties identified in amaranth are its gluten-free protein, which stimulates the immune system, and other nutraceutical compounds with hypercholesterolemic, anticancer and antioxidant effects (Venskutonis and Kraujalis, 2013).
The shape of the inflorescence has a high influence on floral production and crop yield (Ju et al., 2012). Inflorescences develop from small groups of pluripotent cells known as apical meristem, which first produces leaves and then changes during the transition to an inflorescent meristem, which originates lateral meristems that produce or differentiate to floral meristems.
There are several studies about the ontogeny of the apical meristem, with the subsequent appearance of a terminal flower in many species and is known as determinate growth (Coen and Nugent, 1994, Bradley et al., 1996, 1997, Cremer et al., 2001; Ezhova and Penin, 2001; Penin et al., 2004). Plants of determinate growth may be of interest for genetic improvement in order to uniformize maturity, facilitate mechanized harvesting and standardize seed size (Espitia et al., 2012).
The objective of our research was to study the morphology of the apical meristem in plants of determinate and indeterminate growth in the A. hypochondriacus species and its influence on the size of the seed. The hypothesis was that the morphology of the apical meristem is related to the type of growth in A. hypochondriacus, which has an effect on the size of the seed.
Materials and methods
Biological material
We sowed the seeds of a heterozygous plant of A. hypochondriacus to produce the inflorescences of plants with determinate and indeterminate growth. We moved them forward towards homozygosis by the single-seed method (Fehr, 1993), selected determinate and indeterminate plants and made a generational progress until obtaining a homogeneous population of each phenotype. We did sowings in trays of 200 cavities, in a commercial substrate (Peat Moss Sunshine Mix 3). Plants were irrigated every third day and we planted the fifth generation seed for studying apical meristems.
Sample preparation
We conducted plant samplings from emergence and until flowering. The apical meristem was disclosed with a dissection forceps and we observed it with a stereoscopic microscope (S8 APO, Leica, Germany) to determine its phenological status. The morphology of the apical meristem was observed with a variable pressure scanning electron microscope (EVO LS40, Zeiss, Germany), coupled to a peltier stage with a temperature range of -25 to 50 ° C, at 50 Pa, at room temperature of 17 ° C (Deßen EVO® XVP® Coolstage, United Kingdom), and the sample was fixed to the stage by a copper conductive tape. In all the experiments, we maintained the following operating conditions: high voltage (EHT) 20 kV, Fil I Target 2.508 A, working distance 22 mm and Spot Size of 600 ± 5. The micrographs were captured at 164X, 200X, 800X and 1.00KX magnification with a resolution of 1024x768 pixels in gray scale. In this format, we set a gray scale with 0 for black and 255 for white. Afterwards we took new samples and placed them in Herr's solution (phenol: chloral hydrate: 85% lactic acid: xylene: clove oil [1: 1: 1: 0.5: 1]) (Acosta-García and Vielle-Calzada, 2004), for 24 h and observed it with a compound microscope (Axio Scope, A1, Zeiss, Germany) to analyze the anatomical structure of the apical meristem.
To analyze the morphology of the determinate and indeterminate phenotypes, we collected five plants of each growth type in a mature phenological stage, that is, at the end of the growth cycle. To determine the effect of the type of growth on the yield, we took 25 F5 plants of each growth type. Each inflorescence was divided into three strata (basal, middle and apical), and in each stratum the branches were divided into base and apex. We separated seeds from each fragment of the inflorescence, and counted at random in triplicate 1000 seeds of each of the sections of each plant and weighed them on an analytical balance. The experimental design consisted of randomized complete blocks; we analyzed data with the SAS GLM procedure and compared the means with the Tukey test (p ≤ 0.01).
Results and Discussion
Types of inflorescences and their effect on seed size
The growth of the inflorescence in A. hypochondriacus is usually indeterminate. In the Arabidopsis and Antirrhinum model plants the determinate inflorescences are characterized by the appearance of a terminal flower at the apex of the branches (Coen and Nugent 1994, Bradley et al., 1996, Cremer et al., 2001, Ezhova and Penin, 2001; Penin et al., 2004). However, the determinate inflorescences in the Amaranthus genus are reported only for A. caudatus (Kulakow and Jain, 1987) and A. edulis (Sauer, 1950). Espitia (1994) described the presence of determinate growth in A. hypochondriacus Aztec race (Figure 1).
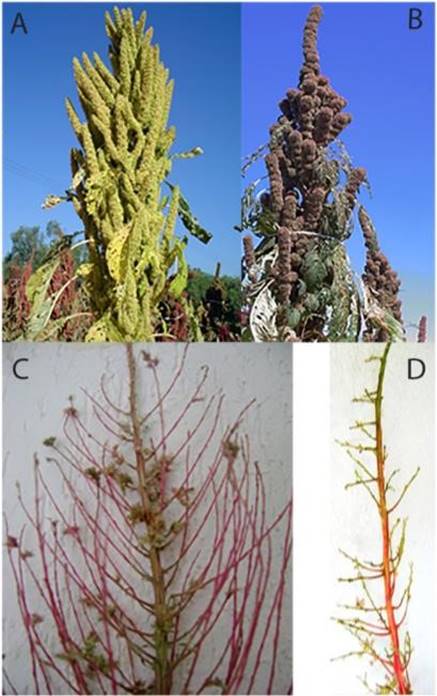
Figure 1 Plant of Amaranthus hypochondriacus A) Inflorescence of indeterminate growth, B) inflorescence of determinate growth, C) branch in indeterminate inflorescence, and D) branch in determinate growth.
During the development of the determinate and indeterminate inflorescences in A. hypochondriacus the phenological phases of both phenotypes were equal during the vegetative phase and the transition to the reproduction phase. However, in the determinate growth after the establishment of a variable number of primordia, the apex emerged as a terminal flower.
To analyze whether the type of growth of the inflorescence affected the yield, we analyzed the seed weight in both genotypes. Among the types of growth, the weight of 1 000 seeds was different (p ≤ 0.01): 0.676 g for the plants of determinate growth (Figure 2A); those with indeterminate growth (Figure 2B) presented greater weight in the middle strata, whereas the apical and basal ones had the lowest weight. In the amaranths of determinate growth (Figure 2D) the highest seed weight was also found in the middle stratum, while the apical stratum and the basal recorded the lowest weight. This makes sense as flowering begins in the middle part of the inflorescence, from where it moves towards the apical meristem, and flowering in the basal part of the inflorescence will most probably happen at the same time than the flowering in the apical part. That is, there is competition between the flowers of the two strata, the movement of photoassimilates is greater towards the apical part (Bertin, 1995) and this could be the reason that the lower weight of the seed is in the basal layer, both in amaranth of indeterminate and determinate growth (Marcelis, 1993). It is likely that at the beginning of flowering in the middle stratum there is no competition for seed filling by the basal and apical strata; therefore, the highest weight of seeds is in the middle stratum.
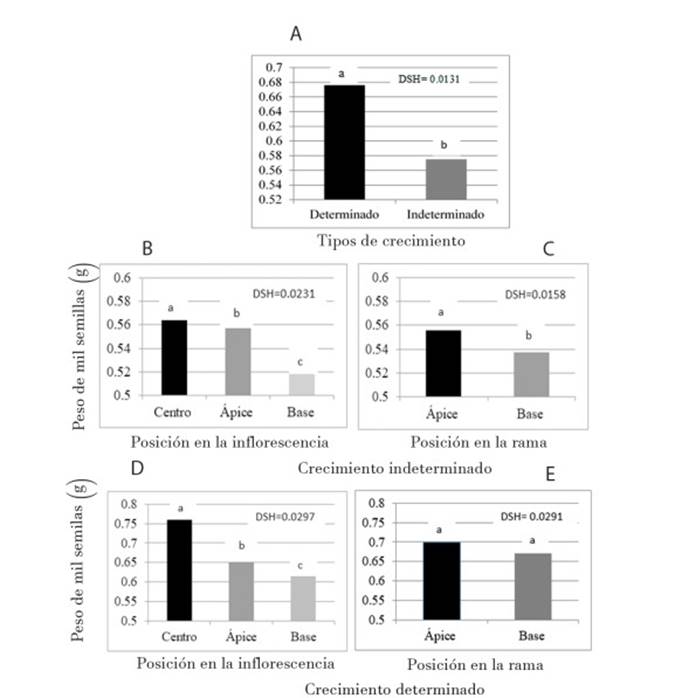
Figure 2 Seed weight of amaranth plants (Amaranthus hypochondriacus) of the two growth types (A), inflorescence strata (B and D) and position in the branches (C and E).
These results agree with those reported by Ramírez et al. (2012) regarding the greater growth of the seed in the middle and apical strata. In relation to the position in the branch in indeterminate growth (Figure 2C), there were significant differences and the apical position presented greater seed weight. In the determinate growth (Figure 2E) there were no differences between the three strata, suggesting a homogeneous growth during inflorescence.
The determinate and indeterminate inflorescences of A. hypochondriacus can be simple or compound, depending on the region in which they are cultivated, and thus shows an important phenotypic plasticity. In determined inflorescences we observed the terminal flower placed at the top of the main axis and in the flower branches.
In the indeterminate inflorescences primary branches were longer, they were 4 to 15 cm long and the distance between nodes was smaller, 0.2 cm; therefore, they were inflorescences with a greater number of floral branches. Thus, there were inflorescences from first to fifth order, a characteristic not present in determinate inflorescences. In this last phenotype the primary branches were on average 10 cm long and the distance between nodes varied from 1 to 2 cm.
In both phenotypes, branches showed an organized basipetal growth in glomeruli. Determinate growth had more complex or agglomerated glomeruli (Figure 3I), unlike indeterminate growth because the primary branches derived from the main axis do not branch and, therefore, the production of flowers occurs at the glomerulus level and not at branch (Figure 3H).

Figure 3 Different stages of female and male flowers of Amaranthus hypochondriacus: A) female flower in early development and next to it the ovule of the contiguous flower can be observed, B) female flower composed of three stigmas, three internal tepals and two external tepals , C) female flower in stage of maturation of the seed, D) seed filling, E) different degrees of flower maturation, F) male flower accompanied by two female flowers, G) secondary branches in indeterminate inflorescences, H) determinate secondary branching, I) determinate glomerulus, and J) indeterminate glomerulus.
Determinate growth presented greater seed weight, which confers agronomic advantages to the crop, like better seed and industrialization quality. This can result in greater use of determinate growth varieties that, in addition to not presenting phenological overlap, facilitates mechanized harvesting by having greater uniformity in maturity (Espitia et al., 2012).
Flowering system in A. hypochondriacus
The structure of the inflorescence in plants of A. hypochondriacus was composed of an ordered set of floral branches and these emerged in the most distal region of the axillary buds located between the petiole of the leaf and the main axis.
During the stage of maturation and elongation of the plant, the main axis was composed of a repetitive set of knots that appeared along the main axis and from which caulinar leaves grew, supported by a petiole; this occurred during the vegetative phase of the plant and the floral branches during the reproductive phase. However, we found interspersed leaves in the inflorescence. The distance between the knots shortened in the area near the apex.
The helical growth of knots and floral stems along the stem was similar in both types of growth; however, the difference was in the position of the flowers and branching of the lateral shoots. In the determinate inflorescences, terminal flowers appeared adjacent to the stem in the axilla of the bract and at the end of the lateral branch. In the indeterminate inflorescences the floral production was indefinite during the life cycle of the plant and branching was observed in lateral shoots (Figure 3G).
Floral biology
Amaranthus hyponchondriacus is a monoecious plant (Espitia et al., 2010). The process of floral transition in A. hypochondriacus occurred gradually in the basipetal direction; the meristem of the inflorescence is responsible for the establishment of a series of floral meristems (Figure 3A), which will give shape to floral branching or differentiate in the organs of flowers (Figure 3B).
Flowering in both types of growth (determinate and indeterminate) occurred gradually and appeared organized in dichasic tops which as a whole formed glomeruli (Figure 3E). The first floral structures that developed were the organs of the male flowers (Figure 3F); firstly the external tepals, internal tepals and finally the stamens. There was a variable number of flowers in each glomerulus.
The staminated flowers were found in the bifurcations of the floral structure. In determinate inflorescences a terminal flower grew in the axilla, directly from the stem or at the top of the floral branches and top of the main axis. In indeterminate inflorescences an initial staminated flower was followed by an indefinite number of pistillate flowers, and all of them gave rise to the glomeruli; this reiterative growth continued during the life cycle of the plant.
A terminal flower appeared on the main axis and secondary axes of determinate inflorescences while, at the glomerulus level, the production of flowers continued. The floral development ceased gradually and was evident as there were no anthers. Thus, it is likely that at the glomerulus level there is also determinate growth. In contrast, in indeterminate inflorescences the glomeruli continued to flower, though they were less dense than the determinate (Figure 3J).
Development and floral growth emerged simultaneously in each type of inflorescence. The ovules presented different degrees of maturity, the largest corresponded to the most developed flowers and so on until finding flowers in development. This was due to the fact that the primordia adjacent to the growing flowers originated the floral organs of the contiguous flower from the last whorl (Flores et al., 2008). This caused the female flowers to present different stages of development, which indicated the existence of phenological overlap. According to Kaur et al. (2015), indeterminate growth tends to accentuate competition in the plant for resources within the stem leaving seeds unfilled.
Floral structure
The inflorescence of A. hypochondriacus is composed of male and female flowers. At the base of the male flowers, sessile female flowers grew (Figure 3F), and this type of growth allowed the formation of dichasic tops since new flowers emerged with this same pattern of development (Figure 3A). There were female flowers composed of one bract, five tepals and the gynoecium, and the bract was subtending the new flower (Figure 3C). The gynoecium when releasing the seed had a horizontal abscission in the middle of it (Figure 3D). Male flowers were composed of two bracts and five tepals that cover the stamens, which coincides with that reported by Townsend (1993).
In determinate growth plants, we found terminal flowers with five, seven, eight, nine and six stamens, while in indeterminate plants there were only pentameric flowers (Figure 4). Terminal flowers are pedicelled, male or hermaphroditic and can appear in the node of the main axis, top of the main axis and top of the floral branches in determinate growth plants.
Meristematic development
Phase I: Vegetative. The meristem appeared immersed in the stem, completely covered with leaf primordia, these in turn had subsequent axillary primordia, and the leaves grew along the stem helically. The meristem in this stage produced the stem, branches and leaves (Figure 5 A-B).

Figure 5 Development of the apical meristem of Amaranthus hypochondriacus: A) vegetative phase, meristem immersed in the main axis, B) transition to reproductive phase, C) and D) elongation of the apical meristem and appearance of floral primordia, E) increase in number of bracts and growth of floral primordia. MA, apical meristem; BR, bract and PF, floral primordium.
Phase II: Reproductive. During floral induction, the apical meristem lengthened producing an inflorescent meristem (Figure 5 C-D). In this stage, the meristem gave rise to a combination of branches, bracts and flowers. The floral transition process began with the production of bract primordia which were observed in the form of a lobe and, when developed, they completely covered the apical meristem, preventing their ongenetic changes from being observed. However, we could observe the moment of transition because the number of bracts increased with respect to the number of leaves (Figure 5 E).
Each one of the bracts had a floral meristem in its axilla, a characteristic also described in Cladium jamaicence (Richards, 2002) and Chenopodium quinoa (Bertero et al., 1996, Bull-Hereñu and Claben-Bockhoff, 2013). The axillary meristems were differentiated into a branch or a floral bud, and this growth process repeated along the floral branching. Thus, thickening was observed at the apex of the plant covered with a set of bracts growing around the apical meristem at different levels, and this was possible due to a longitudinal cut of the early growth of the inflorescence (Figure 6). According to Preston (2010), during the floral induction the levels of cell division increase, which causes the apical meristem to lengthen, producing an inflorescent meristem. The floral transition process occurred in a basipetal direction along the axis of the inflorescence.

Figure 6 Development of the inflorescence in Amaranthus hypochondriacus: A-D) increase of floral primordia, E) male flower in development. BR, bract; FL, flower; MA, apical meristem; PF, floral primordium; T, tepal.
Phase III. Differentiation of the apical meristem. The time of appearance of the terminal flower occurred after the establishment of floral meristems. Bull-Hereñu and Claben-Bockhoff (2013) noted that the size of the apical meristem predetermines the number of nodes it can produce before transforming into a terminal flower. Before the appearance of the terminal flower, we observed that the meristem flattens and then the floral organs, sepals, stamens and carpel developed. In simple terminal inflorescences, the first to mature was the terminal flower.
A basipetal floral development is described for A. hypochondriacus, but Endress and Peter (2010) mention that if in a determinate inflorescence the flower opens before the lateral flowers it is incorrect to describe flowering as basipetal because the terminal flower belongs to a lower axis in the order of appearance of the flowers.
Ontogeny of the apical meristem
The information leading to knowing about the operation of the plants allows the manipulation of mechanisms involved in their growth and development. The study of the apical meristem morphology could be the basis for the analysis of morphogenetic aspects of development, which at the same time may be the foundation for the genetic improvement and agronomic management of A. hypochondriacus.
The statistical studies described in our research indicate that the determinate growth had a larger seed size, which implies an improvement in crop yield. Currently there are no improved amaranth varieties of determinate growth type. Therefore, these studies serve as an instrument for the selection of plants in a certain phenological stage and with a certain phenotype during the breeding program (Table 1).
Table 1 Stages of apical meristem development of indeterminate and determinate growth plants of Amaranthus hypochondriacus.
| Crecimiento indeterminado | Crecimiento determinado | ||
|---|---|---|---|
| A | Vegetativa | A | Vegetativa |
| B | Transición a reproductivo | B | Transición a reproductivo |
| C | Inflorescente | C | Inflorescente |
| D | Inflorescente | D | Diferenciando a flor terminal |
| E | Maduro - indiferenciado | E | Flor terminal en formación |
| F | Flor terminal adulta | ||
By means of a scanning electron microscopy, we recorded morphological changes in the apical meristem. Figure 7 shows micrographs of different stages of development of the indeterminate plant. During the vegetative phase the meristem is flat and generates leaves (Figure 7A). Figure 7B corresponds to the transition stage, time in which the first bracts that hold the first primordia of flowers develop. The apical meristem continues undifferentiated as a dome during the early reproductive stages (Figure 7C-D), floral production and seed filling (Figure 7E).
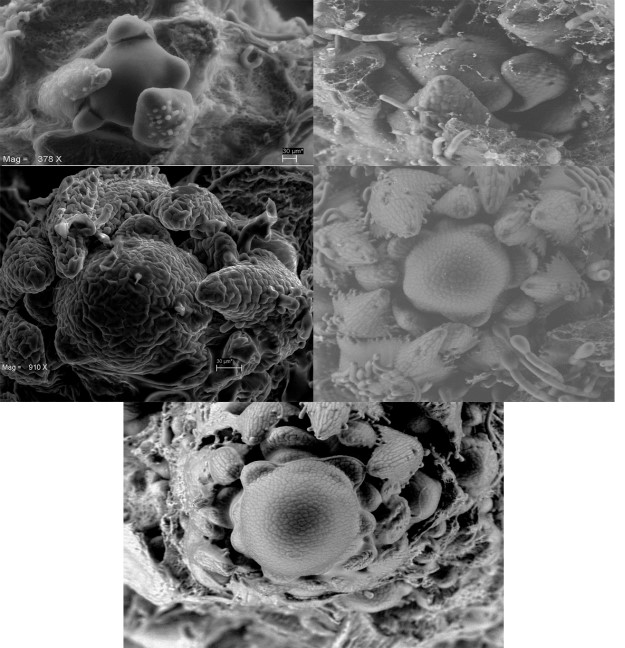
Figure 7 Development of the apical meristem of plants with indeterminate inflorescences. A) Apical meristem, B) formation of bracts, C) dome-shaped inflorescent meristem, D) and E) mature apical meristem.
In the apical meristem of the determinate plants, there were outstanding changes at the beginning of the flower formation, in the vegetative stage corresponding to the formation of leaves (Figure 8A), in the transition stage in which the first bracts were formed (Figure 8B), and when the meristem appeared flat (Figure 8C) and gave rise to the terminal flower (Figure 8 D-F).
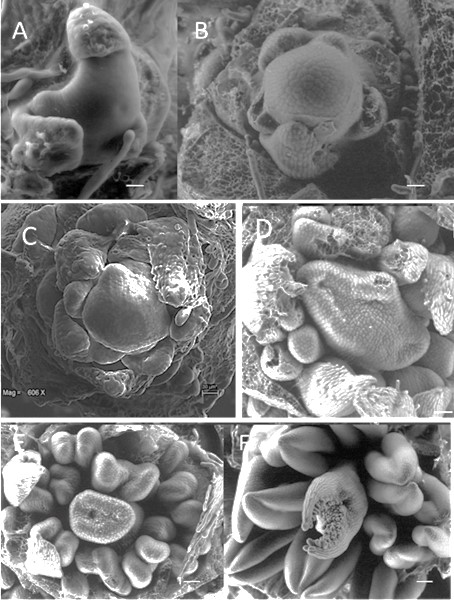
Figure 8 Development of the apical meristem of plants with determinate inflorescences: A) apical meristem in the vegetative phase, B) formation of the first floral organs, the bracts, C) young inflorescent meristem, D) appearance of the terminal flower, E) terminal flower in development, and F) mature terminal flower.
The formation of the terminal flower is the morphological characteristic that indicates the determinate growth at the meristem level. A flat flower of the apical meristem is the characteristic of ontogeny that makes it possible to differentiate the determinate growth from the indeterminate. This coincides with ontogenetic studies related to determinate and indeterminate growth in Daucus carota, in which the apical meristem flattens before the transformation of the terminal flower (Bull-Hereñu and Claben-Bockhoff, 2010).
Conclusions
In this study, when analyzing the morphology of the inflorescences, as well as the ontogeny of the apical meristem in determinate and indeterminate growths, we found outstanding differences. Both phenotypes showed a paniculate inflorescence and the determinate growth had shorter branches and larger seed size. Determinate growth occurs in the main axis, secondary branches and glomerulus. In contrast, indeterminate growth continues with the production of flowers during the life cycle of the plant, which intensifies the phenological overlap in the main branch, secondary branches and glomerulus. The development of the apical meristem during the establishment of the inflorescence evidenced the transformation of the apical meristem into a flower in determinate plants.
Acknowledements
The authors thank CONACYT for the partial financing of the present research within the project: Problemas Nacionales 248415, and for the doctoral scholarship granted to MJAD.
REFERENCES
Acosta-García, G., and J. P. Vielle- Calzada. 2004. A classical arabinogalactan protein is essential for the initiation of female gametogenesis in Arabidopsis. Plant Cell. 16: 2614-2628. [ Links ]
Ayala G, A L., D. Escobedo L., E. Cortés L., y E. Espitia R. 2012. El cultivo de amaranto en México, descripción de la cadena, implicaciones y retos. In: Espitia R., E. (ed). Amaranto: Ciencia y Tecnología. Libro Científico No. 2. INIFAP/SINAREFI. México. pp: 315-330. [ Links ]
Bertero, D., D. Medan, and A. Hall J. 1996. Changes in apical morphology during floral initiation and reproductive development in quinoa (Chenopodium quinoa Willd). Ann. Bot. 78: 317-324. [ Links ]
Bertin N. 1995. Competition for assimilates and fruit position affect fruit set in indeterminate greenhouse tomato. Ann. Bot. 75: 55-65. [ Links ]
Bradley, D., R. Carpenter., L. Copsey., C. Vincent., S. Rothstein, and E. Coen. 1996. Control of inflorescence architecture in Antirrhinum. Nature 379: 791-797. [ Links ]
Bull-Hereñu, K., and R. Claben-Bockhoff. 2010. Developmental conditions for terminal flower production in apioid umbellets. Plant Divers. Evol. 128: 221-232. [ Links ]
Bull-Hereñu, K., and R. Claben-Bockhoff. 2013. Testing the ontogenic base for the transient model of inflorescence development. Ann. Bot. 112: 1543-1551 [ Links ]
Coen, E. S., and J. M. Nugent. 1994. Evolution of flowers and inflorescences. Development 120: 107-116. [ Links ]
Cremer, F., L. Wolf-Ekkehard., H. Saedler, and P. Huijser. 2001. The delayed terminal flower phenotype is caused by a conditional mutation in the centroradialis gene of snap dragon. Plant Physiol. 126: 1031-1041. [ Links ]
Endress K., P. 2010. Disentangling confusions in inflorescence morphology: Patterns and diversity of reproductive shoot ramification in angiosperms. J. System. Evol. 48: 225-239 [ Links ]
Espitia E., R. 1994. Breeding of grain amaranth: A comprehensive review. In: Paredes-López, O. Amaranth, Chemistry and Technology. CRC Press, Boca Raton FL. pp: 23-38. [ Links ]
Espitia R., E., D. Escobedo L., y M. Aguilar D. 2012. Estrategia y metodología para el mejoramiento genético del amaranto. In: Espitia R., E. (ed). Libro Científico No. 2. INIFAP/SINAREFI. México. pp: 227-247. [ Links ]
Espitia E., R., D. Escobedo L., S. Mapes C., M. De la O C., M. Aguilar D., J. Hernández C., A. Ayala G., P. Rivas V., G. Martínez T., M. Ramírez V., y S. Morán B. 2012. Conservación de los recursos genéticos de amaranto (Amaranthus spp.) en México. In: Espitia Rangel, E. (ed). Amaranto: Ciencia y Tecnología. Libro Científico No. 2. INIFAP/SINAREFI. México. pp: 147-163. [ Links ]
Ezhova, T. A., and A. A. Penin. 2001. A new BRACTEA (BRA) gene controlling the formation of an indeterminate bractless inflorescence in Arabidopsis thaliana. Russ. J. Genet. 37: 772-775. [ Links ]
Fehr, W. 1993. Principles of Cultivar Development. Theory and Technique. 3th ed. Iowa State University. 535 p. [ Links ]
Flores, O. H., E. Smets, and A. Vrijodaghs. 2008. Floral and Inflorescence Morphology and Ontogeny in Beta vulgaris with special emphasis on the ovary position. Ann. Bot. 102: 643-651. [ Links ]
Ju, P. S., K. Jiang., M. Schatz, and Z. B. Lippman. 2012. Rate of meristem maturation determines inflorescence architecture in tomato. Proceedings of the National Academy of Sciences. PNAS. 109: 639-644. [ Links ]
Kaur, H., and S. S. Banga. 2015. Discovery and mapping of Brassica juncea Adt, gene associated with determinate plant growth habit. Theor. Appl. Genet. 128: 235-245. [ Links ]
Kulakow, P., A. and S. Jain. 1987. Genetics of grain amaranths. Variation and early response to selection in Amaranthus cruentus L. Theor. Appl. Genet. 74: 113-120. [ Links ]
Marcelis, L. F. M. 1993. Effect of assimilate supply on the growth of individual cucumber fruits. Physiol. Plant. 87: 313-320. [ Links ]
Penin, A., V. Choob, and T. Ezhova. 2004. Basic principles of terminal flower formation. Russian J. Develop. Biol. 36: 65-69. [ Links ]
Preston, J. C. 2010. Evolutionary genetics of core eudictot inflorescence and flower development. Int. J. Dev. Biol. 4: 17-29. [ Links ]
Ramírez V., M. L., E. Espitia R., A. Robledo P., C. Molina R., y R. Bernal M. 2012. Hábito de crecimiento de la inflorescencia y su relación con la calidad física y fisiológica de la semilla de amaranto (Amaranthus hypochondriacus L.). In: Espitia Rangel, E. (ed). Amaranto: Ciencia y Tecnología. Libro Científico No. 2. INIFAP/SINAREFI. México. pp: 257-265. [ Links ]
Richards, J. H. 2002. Flower and spikelet morphology in sawgrass, Cladium jamaicense Crantz (Cyperaceae). Ann. Bot. 90: 361-367. [ Links ]
Sauer, J. D. 1950. The grain Amaranthus. A survey of their history and classification. Ann. Mo. Bot. Gard. USA. 37: 561-632. [ Links ]
Townsen C., C. 1993. Families and genera of vascular plants. In: Kubtzki, K. (ed). Springer-Verlag, Berlín. pp: 70-91. [ Links ]
Venskutonis, P. R., and P. Kraujalis. 2013. Nutritional components of amaranth seeds and vegetables: A review on composition, properties, and uses. Comprehensive reviews in Food Science and Food Safety 12: 381-412. [ Links ]
Received: May 2017; Accepted: October 2017











 texto em
texto em 



