Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.52 no.4 Texcoco may./jun. 2018
Crop Science
Effectiveness of biofertilizers and brassinosteroids in Stevia rebaudiana Bert.
1 Facultad de Ciencias Agrícolas. Universidad Autónoma de Chiapas. Entronque carretera costera y Estación Huehuetán. 30660. Huehuetán, Chiapas, México. (juanf56@prodigy.net.mx); (chico_apodaca@hotmail.com).
2 Postgrado en Innovación en Manejo de Recursos Naturales, Campus San Luis Potosí, Colegio de Postgraduados. 78620. Iturbide No. 73, Col. Centro, Salinas Hidalgo, SLP. (jocadena@colpos.mx)
3 Postgrado en Botánica, Campus Montecillo, Colegio de Postgraduados. 56230. km 36.5 carretera México-Texcoco, Montecillo, Estado de México. México. (msoto@colpos.mx).
The demand for low-calorie products has increased. One of them is a natural product extracted from stevia (Stevia rebaudiana Bert.) whose diterpene glucosides generate outstanding sweetening power. The organic cultivation of this species is increasing in the southern region of Chiapas, México, but its asexual establishment through cuttings presents agronomic limitations. This can be improved with the incorporation of biofertilizers. It is the case of the efficiency in N fixation by Azospirillum brasilense and the endomycorrhizae Rhizophagus intraradices, which improve the transport of nutrients and water to the host plant. These microorganisms have been used in biofertilizer induction programs in annual and perennial crops in México. The objective of this study was to evaluate the effect of R. intraradices (Schenck & Sm.) Walker & Schüßler and A. brasilense Tarrand, Krieg & Döbereiner in interaction with brassinosteroids in S. rebaudiana Bert. plants through morphological, physiological and biochemical changes. The cv Morita 2 was established in a greenhouse, in plastic bags with 6 kg of fluvisol-euthric soil plus washed river sand (1:1) and 4 g of each inoculant at the moment of transplant. The concentration of A. brasilense was 9X106 bacteria g-1 and that of R. intraradices 40 spores g-1 of soil with 95 % colonization of the root system. The brassinosteroid (CIDEF-4, Natura del Desierto, S. A. de C.V., steroid content 80 % with 10 % i.a.) (2 mg L-1 of water) was sprayed on the foliage after 28 d of sowing, plus three later applications with frequency of 14 d. The experimental design was completely random with seven repetitions; the treatments were the microorganisms and the brassinosteroid applied individually and combined, and the control without application or with inoculum. The experimental unit was one pot. The variables recorded were yield, percentage of mycorrhizae root colonization, content of sweetener in leaves, and of N and P in shoots 90 d after sowing. The inoculated plants and treated with brassinosteroid in different modality increased the leaf area (1728 cm2 per plant), content of stevioside (35.8 mg g-1), rebaudioside (29.5 mg g-1) and steviol (5 mg g-1) compared to the control (p≤0.05). The content of N and P increased with the application of R. intraradices alone and combined with brassinoesteroid.
Keywords: Rhizophagus intraradices; Azospirillum brasilense; brassinosteroid; steviosides; nitrogen and phosphorus
La demanda de productos bajos en calorías ha aumentado. Uno de ellos es un producto natural extraído de la stevia (Stevia rebaudiana Bert.) cuyos glucósidos diterpénicos generan poder edulcorante sobresaliente. El cultivo orgánico de esta especie tiende a incrementarse en la región sur de Chiapas, México, pero su establecimiento asexual mediante esquejes presenta limitantes agronómicas. Esto puede mejorarse con la incorporación de biofertilizantes. Es el caso de la eficiencia en la fijación de N por Azospirillum brasilense y el hongo endomicorrízico Rhizophagus intraradices que mejoran el transporte de nutrimentos y agua a la planta hospedante. Estos microorganismos se han utilizados en programas de inducción de los biofertilizantes en cultivos anuales y perennes en México. El objetivo de este estudio fue evaluar el efecto de R. intraradices (Schenck & Sm.) Walker & Schüßler y A. brasilense Tarrand, Krieg & Döbereiner en interacción con brasinoesteroide en plantas de S. rebaudiana Bert. mediante cambios morfológicos, fisiológicos y bioquímicos. El cv Morita 2 se estableció en un vivero, en bolsas de plástico con 6 kg de suelo fluvisol-eútrico más arena de río lavada (1:1) y 4 g de cada inoculante al momento del trasplante. La concentración de A. brasilense fue 9X106 bacterias g-1 y la de R. intraradices 40 esporas g-1 de suelo con 95 % de colonización al sistema radical. El brasinoesteroide (CIDEF-4, Natura del Desierto, S. A. de C.V., contenido esteroidal 80 % con 10 % i.a.) (2 mg L-1 de agua) se asperjó al follaje a los 28 d de la siembra, más tres aplicaciones posteriores con frecuencia de 14 d. El diseño experimental fue completamente al azar con siete repeticiones; los tratamientos fueron los microorganismos y el brasinoesteroide aplicados individualmente y combinados, y el testigo sin aplicación o con inoculo. La unidad experimental fue una maceta. Las variables registradas fueron rendimiento, porcentaje de colonización radical micorrízica, contenido de edulcorantes en hojas y de N y P en vástago 90 d después de la siembra. Las plantas inoculadas y tratadas con brasinoesteroide en diferente modalidad incrementaron el área foliar (1728 cm2 por planta), contenido de esteviósido (35.8 mg g-1), rebaudiósido (29.5 mg g-1) y esteviol (5 mg g-1) con respecto al testigo (p≤0.05). El contenido de N y P aumentó con la aplicación de R. intraradices solo y combinado con brasinoesteroide.
Palabras claves: Rhizophagus intraradices; Azospirillum brasilense; brasinoesteroide; esteviósidos; nitrógeno y fósforo
Introduction
Stevia rebaudiana is a plant from the subtropical forest of high Paraná, native of the mountainous hills of Paraguay, where the inhabitants have used it as sweetener and in herbal medicine (Dacome et al., 2005; Prakash et al., 2008; Anton et al., 2010). The intense sweet taste in the leaves of this species is due to the presence of diterpene glucosides, of which stevioside and rebaudioside A, are in greater proportion. The sweetening power of these compounds is 150 to 300 times higher than sucrose (Midmore and Rank, 2002; Liu et al., 2010) and without caloric content (Anton et al., 2010). Therefore, its demand to sweeten foods and drinks has increased (Brandle and Telmer, 2007; Durán et al., 2012). In México there are environmental conditions for the cultivation of S. rebaudiana (Ramírez-Jaramillo et al., 2011) and it can be profitable.
Research about mineral nutrition of stevia is scarce (Das et al., 2007); the studies have evaluated chemical fertilization (Jaitak et al., 2008; Jarma et al., 2010). The substrates with high content of organic matter and inoculation by microorganisms increase secondary metabolites (Brandt and Møgaard, 2001) and improve plant growth. The increase in use of biofertilizers (mainly with microorganisms) has allowed decreasing the use of up to 69 589 t of chemical fertilizers, which allowed reducing 22.7 mil t of CO2 between 2014 and 2015 (SAGARPA, 2016).
The stevia leaf is the organ with highest concentration of steviosides (Gardana et al., 2010) and its content is influenced primarily by the light hours, phenological stage, and cultivar (Madan et al., 2010). Certain agricultural practices, such as fertilization (Das et al., 2007) and application of endomycorrhizae microorganisms (Portugal et al., 2006), increase nutrient absorption, mainly of P, improve the synthesis of secondary metabolites (Riipi et al., 2002), and can impact the concentration of flavonoids and isoflavonoids (Das et al., 2008; Hanan et al., 2008).
The environmental conditions of the coast of Chiapas, México, differ from those of the place of origin of Stevia and its persistence as a crop tends to decrease. Thus, the possibility of applying exogenous brassinosteroid emerges, which is a hormone that induces diverse responses in the plants, such as favoring the polarization of the cell membrane and increasing the resistance to biotic and abiotic stress (Singh and Shono, 2005; Reyes et al., 2008).
This study had the objective of identifying the influence of Rhizophagus intraradices and Azospirillum brasilense combined with brassinosteriod on the morphological, physiological and biochemical changes of Stevia rebaudiana Bert.
Materials and Methods
Experiment description
The study was carried out in a nursery during the spring of 2015, in the Experimental Field of the Agricultural Sciences School, Campus IV, Huehuetán, Chiapas, located in the junction of the coastal road and Estación Huehuetán, municipality of Huehuetán, Chiapas (15° 00’ and 15° 30’ N, 94° 30’ and 95° 00’ W, altitude of 44 m and minimum and maximum temperature of 15 and 38 °C). The soil used, from the group of the euthric fluvisols, was mixed with washed river sand (1:1). The texture of the substrate was sandy-loam (Bouyoucos, 1962), with 80.7 % sand, 13.3 % loam, 5.8 % clay, 2.6 % organic matter (Walkley-Black, 1934), electric conductivity of 0.05 ds m-1, 5 Meq 100g-1 of cationic exchange capacity, pH 5.7, 0.13 % of N (quantified with Micro-Kjeldahl; Irigoyen et al., 1992), P 14.2 mg kg-1 (evaluated by colorimetry), K++ 64.2 mg kg-1, Ca++ 474 mg kg-1 (determined through atomic spectrophotometry, Thermo Fisher Scientific, model 400 ¼), Mg++ 58.0 mg kg-1 and Na++ 102.5 mg kg-1.
Cuttings of S. rebaudiana Bert. of the Morita 2 variety, were obtained from a plantation, 10 km from the experimental site, from vigorous plants without apparent damage by insects or disease. The cuttings with longitude of 10±2 cm and 60 d of age were obtained from the upper third of the plant and once obtained they were transported in sterile water for their sowing. Six kg of the soil mixture, without sterilization, was placed in black plastic bags (caliber 700) with perforations on the base for drainage. The treatments were: 1) A. brasilense, 2) R. intraradices, 3) A. brasilense plus R. intraradices, 4) Brasinoesteroide, 5) R. intraradices plus brassinosteroid, 6) A. brasilense plus brassinosteroid, 7) A. brasilense, R. intraradices plus brassinosteroid and 8) control (only soil). Four g of the inoculum from each microorganism, depending on the treatment, were deposited in the cavity where the cutting would be placed at the time of transplant. The brassinosteroid (2 mg L-1) was applied 28 d after sowing and then every 14 d.
The host plant of R. intraradices was Brachiaria decumbens Stapf in sterile soil, the colonization reached 95 % in roots and the soil. This substrate was used as inoculum; when it was applied it contained 40 spores g-1 of soil plus propagules. The inoculum of A. brasilense was obtained from the Soil Microbiology Laboratory of the Benemérita Universidad Autónoma de Puebla (México), at the time of the inoculation presented 9X106 cells g-1, and this was impregnated in the peat as substrate. The soluble brassinosteroid CIDEF-4 (Natura del Desierto, S. A. de C. V. in México) had 80 % of the steroidal content and 10 % i.a., is not toxic and is compatible with fertilizers, insecticides and fungicides.
The experimental design was completely random with seven repetitions per treatment. The experimental unit was a pot with one plant. The analysis of N, P, rebaudioside A, and stevioside was determined in dehydrated leaves with four repetitions. The irrigation events were carried out with distilled water. Ninety days after sowing, the leaves were harvested and their biomass and content of N, P and sweeteners were determined.
Number of leaves, number of branches, biomass and leaf area
The total number of leaves and branches was counted; the dry biomass of the plant structures was obtained in an analytical scale (0.1 mg; Ohaus, NJ, USA), after being dehydrated in a stove, with circulating air, at 75-80 °C. The foliar area was recorded in cm2 with an integrator of foliar area (LI-COR, LI 3100, USA).
Content of sweeteners in leaf
The steviosides, rebaudioside A and steviol were extracted from dehydrated leaves at 60 °C and crushed in an electric mill. The compounds were determined by HPLC (Agilent Technologies 1200 Series, California, USA), in an analytical Zorbax CDB C-18 column of 4.6X150 mm and 5 (m of particle size, according to the method described by Hashimoto and Moriyasu (1978), in the Plant Chemistry Laboratory at Colegio de Postgraduados, Montecillo, México.
Content of N and P
The content of N and P was determined in the shoot, the N with the Microkjeldahl method and the P with the Olsen et al. (1954) method in a spectrophotometer (Thermo Fisher Scientific Model 400 ¼), at the Soil, Water and Plant Laboratory of the Agricultural Sciences School in Universidad Autónoma de Chiapas, Huehuetán Chiapas, México.
Mycorrhizae colonization in root
The colonization was quantified solely in the treatments with R. intraradices, with the Phillips and Hayman (1970) technique in 100 segments of root, with 1.5-1.6 cm length, and in optic microscope with immersion objective (100 X).
Results and Discussion
The highest number of leaves (p≤0.05) was found with the mixture of R. intraradices and brassinosteroid. The inoculation of the two microorganisms alone and co-inoculated plus brassinosteroid increased between 5 and 13 % the number of leaves compared to the control. The microorganisms and the brassinosteroid separated did not modify the number of leaves with regard to the control (Table 1).
Table 1 Development characteristics of Stevia rebaudiana Bert. plants inoculated with microorganisms plus brassinosteroid, cultivated in fluvisol-euthric soil from del Soconusco, Chiapas, México.
| Tratamiento | Número | Peso seco (g planta-1) | Área foliar (cm2 planta-1) |
|||
| Hojas | Ramas | Raíz | Tallo y ramas | Lámina foliar | ||
| R. intraradices | 317±7.8 c | 6.71±0.35 bc | 1.57±0.08 bcd | 4.43±0.05 e | 4.76±0.14 cd | 1173±47 cd |
| A. brasilense | 310±10.7 c | 5.86±0.26 bc | 1.75±0.05 abc | 4.98±0.18 cde | 5.33±0.20 bc | 1084±23 d |
| R. intraradices ×A. brasilense | 373±9.9 b | 6.00±0.30 bc | 1.95±0.10 ab | 6.22±0.15 b | 5.97±0.17 ab | 1390±48 b |
| Brasino-esteroide† | 339±9.9 bc | 5.71±0.47 cd | 1.94±0.10 abc | 5.88±0.19 bc | 4.70±0.19 cde | 1179±30 cd |
| Brasino-esteroide ×R. intraradices | 516±9.0 a | 11.29±0.42 a | 2.05±0.08 a | 7.31±0.22 a | 6.21±0.18 a | 1728±58 a |
| Brasino-esteroide ×A. brasilense | 246±6.1 d | 3.86±0.26 d | 1.52±0.12 cd | 4.99±0.11cde | 3.91±0.15 e | 1151±20 d |
| Brasino-esteroide ×R. Intraradices ×A. Brasilense | 344±8.1 bc | 7.71±0.56 b | 1.64±0.10 abc | 5.57±0.30 bcd | 5.00±0.25c | 1356±50 bc |
| Testigo | 328±10.5 c | 6.57±0.57 bc | 1.15±0.07 d | 4.72±0.29 ed | 4.11±0.17 de | 1152±54 d |
| CV¶ | 6.9 | 16.5 | 14.6 | 9.8 | 9.9 | 9.1 |
†Brassinosteroid CIDEF-4. ¶CV: coefficient of variation (%). Means with different letter in a line are statistically different (Tukey, p≤0.05).
The increase in the number of leaves from inoculation with R. intraradices allows suggesting that the absorption of nutrients and water through the radical system increased, since when increasing the growth of the mycelium of these fungi it allows them to act as an extension of the root (Leigh et al., 2009) and to favor changes in their physiology (Barea et al., 2002). The increase in the number of leaves coincided with what was observed in perennial species like Theobroma cacao L. (Aguirre-Medina et al., 2007), Coffea arabica L. (Sánchez et al., 2005), Coffea canephora (Pierre) ex Froehner (Ibarra-Puón et al., 2014) and Tabebuia donnell-smithii Rose (Aguirre-Medina et al., 2014).
The brassinosteroid also favored the growth of the leaf and the number of branches. This seems due to some stress, according to Núñez and Mazorra (2001) who recorded this response in other species in tropical temperatures. In our study the stress could be due to the change in temperature of the austral place of origin of stevia (15 and 30 °C) and of the study site (up to 38 °C). The brassinoesteroid is a growth promotor through the increase in elongation and cell division (Salgado et al., 2008; Clouse, 2011), thermo-tolerance inductor in Bromus inermis (Wilen et al., 1995) and in tomato (Solanum lycopersicum Lam.) (Sing and Shono, 2005).
The mycorrhization of R. intraradices in combination with the brassinosteroid promoted (p≤0.05) the accumulation of biomass with regard to the other treatments; it was 44 and 24 % higher than in the treatment with R. intraradices and with brassinosteroid. The mycorrhization has been related to the transport of nutrients and water to places where the root cannot be explored (Sylvia, 2005). The inoculation with R. intraradices in association with brassinosteroid increased the foliar area (1728±58 cm2 per plant) more than the treatment with R. intraradices plus A. brasilense (1390±48 cm2 per plant) and with brassinosteroid plus R. intraradices plus A. brasilense (1356±50 cm2 per plant); these results contrasted with the values of the control (1152±54 cm2 plant-1). The number of branches and the root weight showed higher coefficients of variation than the other variables, although they did not reach the statistically permissible 20 % in field studies. This indicates that the use of microorganisms and the application of steroids are efficient to modify the physiology of stevia.
Plant growth is increased when combining associative bacteria, such as Azospirillum, and mycorrhizae fungi (Miller and Jastrow 2000). This effect was observed in validation plots of maize and bean in México (Aguirre-Medina, 2006; Trabelsi and Mhamdi, 2013). The control had 55 % lower biomass than that of the best treatment.
The initial increase of biomass in the leaf of S. rebaudiana with R. intraradices influenced the increase in total biomass during its whole development. The leaves are a principal source of photosynthates for other organs of the plant, which is why their increase and persistence favor the net rate of assimilation and relative rate of growth. When the mycorrhizae fungi favor the nutrition of plants, the photosynthetic rate improves (Wright et al., 2005). This fact, according to Milthorpe and Moorby (1982), establishes a positive relationship between the supply of mineral nutrients and rate of photosynthesis, which influences the whole photosynthetic complex.
Content of sweeteners
The S. rebaudiana plants inoculated with R. intraradices in presence of brassinosteroid presented significantly (p≤0.05) more stevioside (35.8±1.76) than the other treatments. The treatment with the two microorganisms plus the brassinosteroid (32.2±1.12), and with A. brasilense without combining (29.7±1.28) were in the same statistical group (Table 2). The arbuscular mycorrhizae fungi increase the absorption of nutrients, primarily P, which is an essential part of molecules like uridine diphosphate glucose which is a glucose donator in the synthesis of diterpene glucosides (Shibata et al., 1995; Richardson et al., 2009).
Table 2 Content of sweeteners in Stevia rebaudiana Bert. leaves inoculated with R. intraradices, A. brasilense and leaf spraying with brassinosteroid.
| Tratamientos | Mg g de peso seco-1 | |||
| Esteviósido | Rebaudiósido A | Esteviol | Total edulcorantes | |
| R. intraradices | 21.5±0.76 d | 17.7±0.90 a | 1.6±0.12 a | 40.9±1.47 b |
| A. brasilense | 29.7±1.28 abc | 26.3±6.66 a | 1.1±0.66 a | 57.2±7.78 ab |
| R. intraradices ×A. brasilense | 25.8±1.56 cd | 17.4±3.19 a | 5.0±0.98 a | 48.2±4.12 ab |
| Brasinoesteroide† | 27.2±1.14 bcd | 21.9±5.72 a | 1.6±0.96 a | 50.8±5.32 ab |
| Brasinoesteroide ×R. intraradices | 35.8±1.76 a | 27.9±2.23 a | 4.5±1.20 a | 68.4±4.84 a |
| Brasinoesteroide ×A. brasilense | 29.4±1.06 bc | 29.5±3.61 a | 4.2±1.18 a | 63.2±5.10 ab |
| Brasinoesteroide ×R. intraradices ×A. brasilense | 32.2±1.12 ab | 35.1±7.84 a | 1.5±0.88 a | 68.8±9.41 a |
| Testigo | 25.0±1.4 cd | 17.8±0.60 a | 1.5±0.90 a | 44.9±2.00 b |
| CV¶ | 2.6 | 37.8 | 68.9 | 20.2 |
†Brassinosteroid CIDEF-4. ¶CV (%): coefficient of variation. Means with different letter in a line are statistically different (Tukey, p≤0.05).
Azospirillum increases the root development of the host plant through the hormones produced and N fixation (Bashan and De Bashan, 2010), something necessary for the normal growth of plants (Jarma et al., 2010).
The plants in presence of endomycorrhizae fungi can modify their content of steviosides and isoflavonoids (Hanan et al., 2008). Portugal et al. (2006) observed a higher concentration of stevioside (72 mg per plant) in stevia plants inoculated with R. intraradices, compared to the control plants (16 mg plant-1) without inoculating. In this case, the highest values of stevioside represented 50 % with R. intraradices plus brassinosteroid.
The coefficient of variation showed high variability of the content of rebaudioside A and steviol, and the difference between the treatments does not suggest a relation in their biosynthesis; that is, the correlation of the content of steviosides and of rebaudiosides is not significant, although the glucosides share a metabolic path with rebaudioside A (Madan et al., 2010). The low contents of steviol showed high CV, although the contents of the plants inoculated with microorganisms and brassinosteroid stood out (5.0±0.98, 4.5±1.2, 4.2±1.18).
Mycorrhizal plants generally absorb higher amounts of micronutrients, such as Mn (Pacovsky et al., 1985), which is a cofactor of enzymes that intervene in the synthesis of ent-kaurenoic acid. This is the precursor of the main sweeteners, such as steviosoid in stevia (Jarma et al., 2010); in addition, S, Cu, Zn and Fe (Habte and Aziz, 1985).
Jarma et al. (2010) pointed out that the stevioside can vary from 3 to 8 % in the dry tissue of stevia leaves. The statistical differences were present only with the stevioside and the highest value was found with the application of brassinosteroid plus R. intraradices (p≤0.05) with the inoculated microorganisms alone, plus the brassinosteroid.
The content of sweeteners, nutrients and biomass increased with the application of brassinosteroid. This indicates the possibility of stevia plants being stressed, possibly from the temperature of the study region. Sing and Shono (2005) treated tomato plants with 24 epibrassinolide and reported higher tolerance at 38 °C and higher photosynthetic efficiency in comparison to the plants treated at 25 °C.
Content of N and P
The plants inoculated with each microorganism or their combination presented a higher content of N compared to the control plants and when brassinosteroid was applied (Figure 1). The content of N increased and was statistically different from the control (p≤0.05) when inoculating A. brasilense plus the brassinosteroid. A. brasilense induces root growth in the host plant as a result of the production of plant hormones (Hungria et al., 2004), such as indole-acetic acid (Dobbelaere et al., 2003; Bashan and de Bashan, 2010), which modifies the morphology and increases the root biomass (Lalitha et al., 2011). Endomycorrhizae fungi transport N through the hyphae from sites of the soil that are inaccessible for the root (Hodge 2003). The inoculation with endomycorrhizae fungi in crops of Phaseolus sp. (Tajini and Drevon, 2012), Coffea arabica L. (Aguirre-Medina et al., 2011) and Cedrela odorata L. (Aguirre-Medina et al., 2014) showed similar results. The highest content of P in the plant tissue was found in the treatment with A. brasilense and when adding brassinosteroid (p≤0.05). Other studies have shown that mycorrhizal plants absorb the P from the soil efficiently in comparison to non-colonized plants (Andrade et al., 2009) from soil regions beyond the zone of exhaustion around the root (Wright et al., 2005).
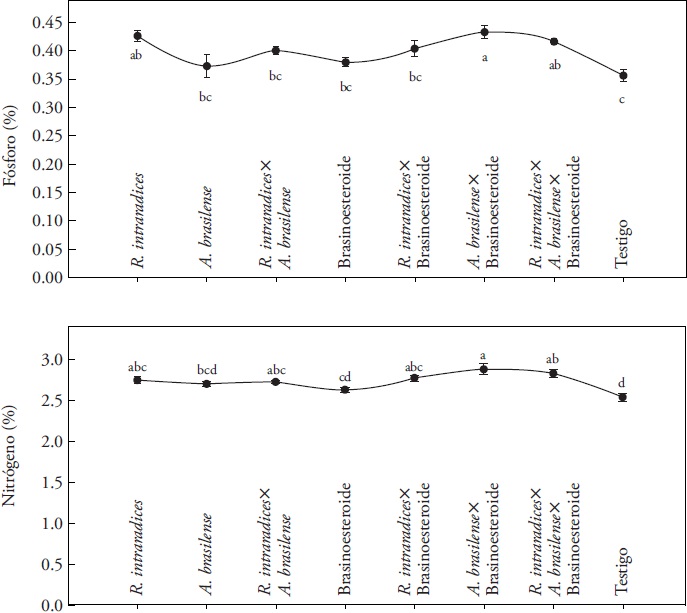
Figure 1 Percentage of phosphorus (P) and nitrogen (N) in S. rebaudiana Bert. leaves biofertilized with R. intraradices, A. brasilense and foliar application of CIDEF-4 (brassinosteroid) under greenhouse conditions (n=4) ± standard error. Different letters in each treatment (N or P) are statistically different (p≤0.05). CV=2.6 % for N and CV=5.8 % for P.
The root colonization of all plants inoculated with R. intraradices was high. In average, the colonization was 83 to 90 %. In the case of the control without application of the endomycorrhizae fungus, the root colonization was 52 %. The root colonization in the control treatment and in the treatment with brassinosteroid or A. brasilense is surely due to native mycorrhizae that are found in the substrate.
Conclusions
S. reabudiana plants inoculated and in presence of brassinosteroid, in different modes of association, showed certain increases in foliar area and in content of stevioside, rebaudioside and steviol. The content of N and P increased with R. intraradices inoculated alone and plus brassinosteroid.
Literatura Citada
Aguirre-Medina, J. F. 2006. Biofertilizantes microbianos: Experiencias agronómicas del programa nacional del INIFAP en México. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias-Centro de Investigaciones Regionales Pacífico Sur-Campo Experimental Rosario Izapa. Primera Edición. México. 206 p. [ Links ]
Aguirre-Medina, J. F., A. Mendoza-López, J. Cadena-Iñiguez, y C. H. Avendaño-Arrazate. 2007. Efecto de la biofertilización en vivero del cacao (Theobroma cacao) con Azospirillum brasilense Tarrand, Krieg & Döbereiner y Glomus intraradices Schenk & Smith. Interciencia 32: 541-546. [ Links ]
Aguirre-Medina, J. F., D. M. Moroyoqui-Ovilla, A. Mendoza-López, J. Cadena-Iñiguez, J., C. H. Avendaño-Arrazate, y J. F. Aguirre-Cadena. 2011. Aplicación de A. brasilense y G. intraradices a Coffea arabica en vivero. Agron. Mesoam. 22: 71-80. [ Links ]
Aguirre-Medina, J. F., F. O. Mina-Briones, J. Cadena-Iñiguez, J. D. Dardón-Zunun, y D. A. Hernández-Sedas. 2014. Crecimiento de Cedrela odorata L. biofertilizada con Rhizophagus intraradices y Azospirillum brasilense en vivero. Rev. Chapingo Ser. Cienc. For. Ambient. 20: 177-186. [ Links ]
Andrade, S. A. L., P. Mazzafera, M.A. Schivinato, and A. P. D. Silveira. 2009. Arbuscular mycorrhizal association in coffee. J. Agric. Sci. 147: 105-115. [ Links ]
Anton, S. D., C. K. Martin, H. Han, S. Coulon, W. T. Cefalu, P. Geiselman, and D. A. Williamson. 2010. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite 55: 37-43. [ Links ]
Barea, J. M., R. Azcón, and C. Azcón-Aguilar. 2002. Mycorrhizosphere interactions to improve plant fitness and soil quality. Anton. Leeuw. Int. J. G. Molecular Microbiol. 81: 343-351. [ Links ]
Bashan Y., and E. de- Bashan Luz. 2010. How the plant growth-promoting bacterium Azospirillum promotes plant growth -A critical assessment. Adv. Agron. 108: 77-136. [ Links ]
Bouyoucos, G. J. 1962. Hydrometer method improved for making particle size analysis. Agron. J. 54: 464-465. [ Links ]
Brandle, J. E., and P. G. Telmer. 2007. Steviol glycoside biosynthesis. Phytochemistry 68: 1855-1863. [ Links ]
Brandt, K., and J. P. Møgaard. 2001. Organic agriculture: does it enhance or reduce the nutritional value of plant foods? J. Sci. Food Agric. 81: 924-931. [ Links ]
Clouse, S. D. 2011. Brassinosteroids. In: The Arabidopsis Book. American Society of Plant Biologists. 28 p. doi: 10.1199/tab.0151 [ Links ]
Dacome, A., D. A. C. Silva, D. A. C. Costa, J. Fontana, J. Adelmann, and D. A. S. Costa. 2005. Sweet diterpenic glycosides balance of a new cultivar of Stevia rebaudiana (Bert.) Bertoni: Isolation and quantitative distribution by chromatographic, spectroscopic, and electrophoretic methods. Process Biochem. 40: 3587-3594. [ Links ]
Das, K., R. Dang, T. N. Shivananda, and N. Sekeroglu. 2007. Influence of biofertilizers on the biomass yield and nutrient content in Stevia (Stevia rebaudiana Bert.) grown in Indian subtropics. J. Med. Plants Res. 1: 5-8. [ Links ]
Das, K., R. Dang, and T. N. Shivananda. 2008. Influence of bio-fertilizers on the availability of nutrients (N, P and K) in soil in relation to growth and yield of Stevia rebaudiana grown in South India. Int. J. Appl. Res. Nat. Prod. 1: 20-24. [ Links ]
Dobbelaere, S., J. Vanderleyden, and Y. Okon, 2003. Plant growth-promoting effects of diazotrophs in the rhizosphere. Crit. Rev. Plant Sci. 22: 107-149. [ Links ]
Durán, A., S. N. M. Rodríguez, K. A. Cordón, y C. J. Record. 2012. Estevia (Stevia rebaudiana), edulcorante natural y no calórico. Rev. Chil. Nutr. 39: 203-206. [ Links ]
Gardana, C., M. Scaglianti, and P. Simonetti. 2010. Evaluation of steviol and its glycosides in Stevia rebaudiana leaves and commercial sweetener by ultra-high performance liquid chromatography-mass spectrometry. J. Chromatogr. 8: 1537-1555. [ Links ]
Habte, M., and T. Aziz. 1985. Response of Sesbania grandiflora to inoculation of soil with vesicular-arbuscular mycorrhizal fungi. Appl. Environ. Microbiol. 5: 701-703. [ Links ]
Hanan, A. A. T., R. El-Mergawi, and S. Radwan. 2008. Isoflavonoids, flavonoids, phenolic acids profiles and antioxidant activity of soybean seeds as affected by organic and bioorganic fertilization. Am.-Eurasian J. Agri. Environ. Sci. 4: 207-213. [ Links ]
Hashimoto, Y., and M. Moriyasu. 1978. Determination of sweet components in Stevia rebaudiana by high performance liquid chromatography ultraviolet detection. Shoyakugaku 32: 209-11. [ Links ]
Hodge, A. 2003. Plant nitrogen capture from organic matter as affected by spatial dispersion, interspecific competition and mycorrhizal colonization. New Phytol. 157: 303-314. [ Links ]
Hungria, M., R. J. Campo, E. M. Souza, and F. O. Pedrosa. 2004. Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil 331: 413-425. [ Links ]
Ibarra-Puón, J.C., J. F. Aguirre-Medina, A. Ley-De Coss, J. Cadena-Iñiguez, A. Zavala-Mata. 2014. Inoculación de Coffea canephora (Pierre) ex Froehner con Rhizophagus intraradices (Schenck et Sm.) Walker et Schuessler y Azospirillum brasilense Tarrand, Krieg et Döbereiner en vivero. Rev. Chapingo Ser. Hortic. 20: 201-213. [ Links ]
Irigoyen, J. J., D. W. Emerich, and M. Sánchez-Díaz. 1992. Water stress induced changes in concentrations of proline and total soluble sugar in nodulate alfalfa (Medicago sativa) plants. Physiol. Plant. 84: 55-60. [ Links ]
Jaitak, V., A. Gupta, V. Kaul y P. Ahuja. 2008. Validated high-performance thin-layer chromatography method for steviol glycosides in Stevia rebaudiana. J. Pharm. Biomed. Anal. 47: 790-794 [ Links ]
Jarma, A., C. E. M. Combatt, y I. J. A. Cleves. 2010. Aspectos nutricionales y metabolismo de Stevia rebaudiana (Bertoni). Una revisión. Agron. Colomb. 28: 199-208. [ Links ]
Lalitha, S., K. Rajeshwaran, P. Senthil Kumar, and S. Deepa. 2011. Role of AM fungi and rhizobial inoculation for reclamation of phosphorus deficient soil. Asian J. Plant Sci. 10: 227-232. [ Links ]
Leigh, J., A. Hodge, and A. H. Fitter. 2009. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol. 181: 199-207. [ Links ]
Liu, J., L. Jin-Wei, and T. Jian. 2010. Ultrasonically assisted extraction of total carbohydrates from Stevia rebaudiana Bertoni and identification of extracts. Food Bioprod. Process. 88: 215-221. [ Links ]
Madan, S., S. Ahmad, G. N. Singh, K. Kohli, Y. Kumar, R. Singh, and M. Garg. 2010. Stevia rebaudiana (Bert.) Bertoni: A Review. Indian J. Nat. Products Res. 1: 267-286. [ Links ]
Midmore, D. J., and A. H. Rank. 2002. A new rural industry Stevia to replace imported chemical sweeteners. A report for the Rural Industries Research and Development Corporation. RIRDC Web Publication No W02/022. Australia. 55 p. [ Links ]
Miller, R., and J. Jastrow. 2000. Mycorrhizal fungi influence soil structure. In: Kapulnik Y., and D. D. Douds (eds). Arbuscular Mycorrhizae: Molecular Biology and Physiology. Kluwer Academic Press. Dordrecht, the Netherlands. pp: 3-18. [ Links ]
Milthorpe, F. L., y J. Moorby. 1982. Introducción a la Fisiología de los Cultivos. Segunda edición. Ed. Hemisferio Sur, Buenos Aires Argentina. 259 p. [ Links ]
Núñez, M., y L. M. Mazorra. 2001. Los brasinoesteroides y la respuesta de las plantas al estrés. Cultivos Trop. 22: 19-26. [ Links ]
Olsen, S. R., Cole, C.V., Watanabe, F. S., y L. A. Dean. 1954. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA circ. 939. [ Links ]
Pacovsky, R. S., E. A. Paul, and G. J. Bethlenfalvay. 1985. Nutrition of sorghum plants fertilized with nitrogen or inoculated with Azospirillum brasilense. Plant Soil 85: 145-148. [ Links ]
Phillips, J. M., and D. J. Hayman. 1970. Improved procedures for clearing and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Brit. Mycol. Soc. 55: 158-161. [ Links ]
Portugal, E. P., G. C. Mercuri Quitério, and S. L. Honório. 2006. Seleção de fungos micorrízicos arbusculares para estévia, Stevia Rebaudiana (Bert.) Bertoni. Multiciencia 7: 1-20. [ Links ]
Prakash, I., G. E. Dubois, J. F. Clos, K. L. Wilkens, and L.E. Fosdick. 2008. Development of rebiana, a natural, non-caloric sweetener. Food Chem. Toxicol. 46: S75-S82. [ Links ]
Ramírez-Jaramillo, G., B. W. Aviles, O. Y. Moguel, G. S. Góngora, y L. C. May. 2011. Estevia (Stevia rebaudiana Bertoni), un cultivo con potencial productivo en México. Primera edición. Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias. Centro de Investigación Regional Sureste. Mérida, Yucatán, México. 88 p. [ Links ]
Reyes, Y., L. M. Mazorra, y M. Núñez. 2008 Aspectos fisiológicos y bioquímicos de la tolerancia del arroz al estrés salino y su relación con los brasinoesteroides. Cult. Trop. 29: 67-75. [ Links ]
Richardson, A. E., J. M. Barea, A. M. Mc Neill y C. Prigent-Combaret. 2009. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305-339. [ Links ]
Riipi, M., V. Ossipova, K. Lempa, J. Haukioja, S. Ossipova, and K. Pihlaja, 2002. Seasonal changes in birch leaf chemistry: are there tradeoffs between leaf growth and accumulation of phenolics. Oecologia 130: 380-390. [ Links ]
SAGARPA (Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación). 2016. Nota de prensa núm. 270. Trabaja SAGARPA para mitigar efectos del cambio climático en México. Fecha de publicación 14 de junio de 2016. https://www.gob.mx/sagarpa/prensa/trabaja-sagarpa-para-mitigar-efectos-del-cambio-climatico-en-mexico (Consulta: octubre 2016). [ Links ]
Salgado G. R., M. A. Cortés R., y R. E. Del Río 2008. Uso de brasinoesteroides y sus análogos en la agricultura. Biológicas 10: 18-27, [ Links ]
Sánchez, L., S. Weidmann, C. Arnould, A. R. Bernard, S. Gianinazzi, S., y V. Gianinazzi-Pearson. 2005. Pseudomonas fluorescens and Glomus mosseae Trigger DMI3-Dependent activation of genes related to a signal transduction pathway in roots of Medicago truncatula. Plant Physiol 139: 1065-1077. [ Links ]
Shibata, H., Y. Sawa, T. Oka, S. Sonoke, K. Kim, y M. Yoshioka. 1995. steviol and steviol-glycoside: glucosyltransferase activities in Stevia rebaudiana Bertono -Purification and partial characterization. Arch. Biochem. Biophys. 321: 390-396. [ Links ]
Singh, I., and M. Shono. 2005. Physiological and molecular effects of 24-epibrassinolide, a brassinosteroid, on thermotolerance of tomato. Plant Growth Regul. 47: 111-119. [ Links ]
Sylvia, M. D. 2005. Mycorrhizal symbioses In: Sylvia, M. D., J. J. Fuhrmann, G. P. Harte, and A. D. Zuberer (eds). Principles and Applications of Soil Microbiology. Second Edition, Pearson Prentice Hall. Upper Saddle River, New Jersey, USA. pp: 263-282. [ Links ]
Tajini, F., and J. J. Drevon. 2012. Phosphorus use efficiency in common bean (Phaseolus vulgaris L.) as related to compatibility of association among arbuscular mycorrhizal fungi and rhizobia. Afr. J. Biotechnol. 11: 12173-12182. [ Links ]
Trabelsi, D., and R. Mhamdi. 2013. Microbial inoculants and their impact on soil microbial communities: a review. BioMed Res. Int. ID 863240: 1-11. [ Links ]
Walkley, A., and I.A. Black, 1934. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 37: 29-38. [ Links ]
Wilen, R.W., M. Sacco, V. G. Lawrence, and P. Krishna. 1995. Effects of 24-epibrassinolide on greening and thermo tolerance of brome grass (Bromus inermis) cell cultures. Physiol Plantarum 95: 195-202. [ Links ]
Wright, D. P., J. D. Scholes, D. J. Read, and S. A. Rolfe. 2005. European and African maize cultivars differ in their physiological and molecular responses to mycorrhizal infection. New Phytol. 167: 881-896. [ Links ]
Received: March 2017; Accepted: February 2018











 texto en
texto en 


