Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.52 no.4 Texcoco may./jun. 2018
Crop Science
Usage of biosurfactants extracted from corn steep liquor to eliminate burned engine oil on sandy soil
1 Unidad Académica Multidisciplinaria Mante, Área de Ingeniería Bioquímica Industrial, Universidad Autónoma de Tamaulipas. Boulevard E. C. González 1201 Poniente. Colonia Jardín, 89840, Ciudad Mante, Tamaulipas, México.
2 Departamento de Ingeniería Química, Escuela de Ingeniería Industrial, Universidad de Vigo, Campus-As Lagoas, Marcosende, 36310 Vigo, Pontevedra, España.
3 Departamento de Ingeniería Química, Escuela de Ingeniería de Barcelona Este (EEBE), Universitat Politècnica de Catalunya (UPC)-Barcelona TECH, C/ Eduard Maristany, 19 Campus Diagonal-Besòs, 08930 Barcelona, España. (gbustos@docentes.uat.edu.mx)
The interest in biosurfactants for the removal of hydrophobic contaminants on contaminated soils is increasing. The objective of this study was to evaluate the decontamination process in sandy soils polluted with burned car engine oil, using a natural detergent, extracted from a residual stream from the corn milling industry named “corn steep liquor”. The emulsifier and interactive capacity of the biosurfactant with the burnt oil was first evaluated. Then, discontinuous extraction experiments were carried out in batch by triplicate on a mixture of 5 g of sandy soil containing 50,000 ppm burned oil, using several formulated cleaner solutions with the biosurfactant, above and below its critical micelle concentration (CMC), using solid/liquid relations between 1/10 and 1/30 (w:v). Results show that the 1/20 solid/liquid ratio (w:v) was the most suitable for the washings. After 6 h in contact, the cleaning process showed good results. The high biodegradability of the biosurfactant extract and the relatively low treatment times allow us to suggest its use in "in-situ" decontamination processes of petroleum-based oils contaminated areas.
Key words: biosurfactant; burnt oil; corn steep liquor; sand
El interés creciente por el uso de biosurfactantes con el propósito de eliminar contaminantes hidrofóbicos en suelos contaminados va en aumento. El objetivo de este estudio fue evaluar el proceso de descontaminación de un suelo arenoso, contaminado con aceite quemado de motor de coche, utilizando un detergente natural, extraído de una corriente residual de licores de lavado de maíz, para su uso como surfactante natural en el tratamiento de vertidos. Primero se evaluó la capacidad emulsionante e interactiva del biosurfactante con el aceite quemado y después se realizaron experimentos de extracción en discontinuo por triplicado con 5 g de suelo arenoso y 50,000 ppm de aceite quemado, utilizando varias disoluciones limpiadores formuladas con el biosurfactante, por encima y por debajo de su concentración micelar crítica (CMC), utilizando relaciones sólido/líquido entre 1/10 y 1/30 (p:v). Los resultados mostraron que la relación sólido/líquido 1/20 (p:v) fue la más adecuada para el lavado; después de 6 h de contacto el proceso de limpieza presentó buenos resultados. La alta biodegradabilidad del extracto de biosurfactante y los tiempos de tratamiento, relativamente bajos, permiten sugerir su uso en procesos de descontaminación in situ de áreas contaminadas con aceites derivados del petróleo.
Palabras clave: biosurfactante; aceite quemado; licores de lavado de maíz; arena
Introduction
Mobilization and solubilization are the mechanisms to eliminate hydrophobic contaminants from soils (Edwards et al., 1991; West and Harwell, 1992; Mulligan, 2005; Vreysen and Maes, 2005). Mobilization depends on the surfactant´s capacity to reduce surface tension and facilitate the washing solution to drag the contaminant (Cameotra and Makkar, 2010). Tenso-active molecules reduce the interfacial tension in oil/water systems and increase the contact angle of the contaminant with the soil, reducing capillary forces that kept them together, and facilitates oils elimination (Moldes et al., 2011). Solubilization takes place above the critical micelle concentration (CMC). At these concentrations, surfactants form micelles, inside of which the contaminant is retained, so that the amount of removed oil will increase when surfactants are above their CMC. Solubilization depends both, on the surfactant and the degree of contamination of the solid matrix, and might not be significant in the remediation process when the pollutants concentration is very high (Abdul et al., 1990; Urum and Pekdemir, 2004).
A lot of industrial waste, such as fuels and synthetic oils, kitchen oils and with heavy metals, from metal containers found in household waste, are the main source of pollution of soils, rivers and lakes, causing habitats destruction in extreme cases (Solans and Gadea 2015; Regulation (UE) No. 1357/2014). For example, soil and groundwater pollution at gas stations due to the nature of lubricants, petrol or diesel should be carefully evaluated because of its magnitude and the negative impact that produces.
Within this waste there is burnt engine oil, which by its impact on the environment, is one of the major residues that undergo strict regulations (Solans and Gadea 2015, Regulation (UE) No. 1357/2014). Most of the hydrocarbons causing pollution are water insoluble and generate hydrophobic unions to certain surfaces, such as rocks, for which their disposal is very difficult, since microorganisms that could degrade them cannot access them because they are not bioavailable. For this reason, bioremediation is trying to find microorganisms that produce biosurfactants substances favoring these hydrocarbons bioavailability (Banat et al., 2000; Ortíz-Hernández et al., 2001; Youssef et al., 2007; Yañez-Ocampo et al., 2009, 2011 and 2013; Vecino et al., 2015).
Biosurfactants are amphiphilic compounds from microbial origin with high tenso-active properties that allow to reduce the environmental impact caused by different pollutants released to the environment as hydrocarbons (Moldes et al., 2011). Compared to surfactants produced by chemical synthesis, biosurfactants are less toxic than their petroleum counterparts (Levison, 2009). Due to their biodegradability and biocompatibility, biosurfactants are studied in several industrial fields (Kourkoutas et al., 2004; Banat et al., 2010; Reis et al., 2013): in the pharmaceutical industry (Rodrigues et al., 2004; Cameotra and Makkar, 2010), cosmetics (Gharaei-Fathabad, 2011; Vecino et al., 2017), in waste water treatment (Perez-Ameneiro et al., 2015), as well as in the bioremediation of soils contaminated with hydrocarbons (Desai y Banat, 1997; Banat et al., 2010; Moldes et al., 2011 and 2013; Damasceno et al., 2012).
Vecino et al. (2014a, b and c and 2015a) evaluated and patented the corn steep liquors as a source of “low-cost” biosurfactants and proposed a method for the extraction and characterization of these surfactant compounds that could be applied in different industrial sectors. The advantage of these biosurfactants, compared with its chemical counter partners, is that they are economically competitive with chemical surfactants because their production does not require a controlled fermentation process and spontaneously occur in liquid residual from corn industry.
The objective of this study was to evaluate the biosurfactant extract, obtained from corn steep liquor, for the removal of burned motor oil from sandy soil. This is the first time that this biosurfactant is evaluated as a cleaning agent of oil on soil and it is expected that the results allow to establish the conditions for their use as natural surfactant of renewable origins on industrial discharges.
Materials and Methods
Soil and contaminant
Sandy soil was obtained from a beach in the Galician coast, Northwestern Spain, which was mixed with car engine burned oil from a local automotive workshop (Vigo, Spain).
Biosurfactant extraction from corn steep liquors
Biosurfactant (BS) in the corn steep liquors (CSL) was extracted with chloroform in three stages (Vecino et al., 2015): 1) a liquid-liquid extraction at 65 °C using a CSL/chloroform ratio of 1:2 (v:v) for 1 h, 2) the mixture remained 24 h in a separating funnel to allow its equilibrium, we obtained aqueous phase and the organic phase containing the biosurfactant, and 3) we obtained a concentrated biosurfactant in chloroform extract by distilling and after diluting in water to obtain the cleaning solution.
Evaluation of the biosurfactant capacity to form emulsions with burnt engine oil
The emulsifying ability of the biosurfactant obtained from corn steep liquors to form emulsions was calculated as a function of two variables, EV (relative emulsion volume, %) (Equation 1) and ES (biosurfactant stability, %, to maintain emulsion when mixed with oil and water) (Equation 2). Variables that measure the emulsifying ability of the biosurfactant (EV and ES) were assessed 30 d with the Neufeld and Zajíc (1984) and the Das et al. (1998) equations. For this purpose, a 2 mL sample of burned oil was a dissolved in BS to the CMC (200 mg L- 1), stirred 2 min in a vortex, stabilized for 72 h and then quantified the EV and ES. The EV calculation is based on the percentage of emulsion formed between the oil phase and the aqueous phase, respect to the total volume. For it, height of emulsion and both phases total height values are taken, as well as the cross section of tube (equation 1). ES is the relationship between EV at a determinate time and EV at time cero (equation 2):
Oil displacement test
The test of the oil displacement by the biosurfactant indicates it ability to solubilize oil. To perform this test, 20 mL of water were placed in a Petri dish, then, 40 µL of the engine burned oil were added and 80 µL of the biosurfactante dissolution were added over the oil, above the CMC. As a control, two other experiments were performed. On them, water or a sodium dodecyl sulphate solution (SDS) was added (detergent used for cleaning oil tanks, obtained by chemical synthesis) at its CMC (2365 mg L-1). The cleaning solution that displaces the largest oil quantity over the water and shows a greater diameter of displacement will have greater capacity as a cleaning solution.
Surface tension evaluation
The surface tension of the biosurfactant solution, as well as the water made control, were assessed before and after the sand washing with a surface tensiometer (KRUSS K6, Spain) equipped with a 1.9 cm platinum ring (Du Noüy ring method). The measurements were carried out by triplicate at room temperature.
Contaminated soil washing test at laboratory scale
For these tests, 5 g of sandy soil we weighed. These were contaminated with burnt car engine oil with 0.25 g (50,000 ppm) oil content. Laboratory scale washing experiments were performed in batch, in 250 mL Erlenmeyer flasks, at room temperature in an orbital shaker at 150 ppm. The solid/liquid ratio established between the contaminated soil and the cleaning solutions was between 1:10 and 1:30 (w:v). Cleaning solutions were prepared with the biosurfactant twice below the CMC (BS/2) or the CMC (BS). Cleaning time was set at 6 h.
After washing the sand, the cleaning solution was separated by filtration, using a porous plate funnel, and then quantifying the extracted oil in the cleaning solution by gravimetric analysis. For this, 1 mL cleaning solution sample remained 48 h in an oven at 100 °C.
The decontaminated and filtered sand then passed to an extraction process in a Soxhlet device using acetone (Nahita, Heating mantles, Mod 655. Navarra-Spain) to quantify the oil that remained in the sand, which must be complementary to the oil extracted in the cleaning solutions.
Process to extract remaining oil in sandy soil
For the extraction of the oil remaining in the sand from the Soxhlet, the treated sand was introduced into a cellulose cartridge, and acetone was placed inside a heating mantle at 65 °C (temperature higher than the acetone´s boiling point, 56 °C), which allowed its evaporation. The Soxhlet device includes a condensation system that drags as it falls the remaining oil in the sand. This device worked for 1 h, then the Soxhlet cartridge was removed, and an acetone sample containing the residual oil from the sand was assessed in a double beam spectrophotometer at 328 nm (Jasco Mod. V-650, Spain). The relationship between the oil concentration in the acetone and the absorbance measured at 328 nm was linear (Figure 1), in this way the equation for oil concentration was obtained in relation to absorbance.
Calculation of the extraction process efficiency
Extraction efficiency (EE) is defined according to equation 3 as the percentage of the oil removed from the sand:
where A0 and A1 represent the initial and final grams of oil present in the sand, respectively.
Results and Discussion
Evaluation of the emulsifying and dispersing capacity of the biosurfactant on burnt engine oil
After the extraction process, a biosurfactant extract with a yield of 8 ± 0.5 g extract kg-1 of CSL with 50 % solids was obtained, although this yield may vary slightly between batches because the corn washing liquors are spontaneously fermented in uncontrolled conditions, if an industrial waste is assumed.
The extract was dissolved in water above its CMC (Figure 2). At the CMC (200 mg L-1) or above it, the biosurfactant can keep the water surface tension constant at values close to 40 mN m-1, this is the value where the biosurfactant begins to form micelles with the capacity to surround those molecules not soluble in water, which facilitates their mobilization and solubilization.

Figure 2 Aspect of the biosurfactant dissolution extracted from corn steep liquors solubilized in water.
Once established the biosurfactant CMC the cleaning solution was prepared to CMC concentration (BS) or to half of the CMC (BS/2) and their ability to stabilize oil/water emulsions was assessed. The biosurfactant to the CMC (BS) was able to emulsify the oil with water as a whole, compared to the water/oil system in absence of the biosurfactant (Figure 3). This emulsion was stable for more than one month, period during which its stability was evaluated.

Figure 3 Water/oil system stabilized and emulsified with a biosurfactant from corn steep liquors (left), compared to an oil/water system without biosurfactant (right).
The biosurfactant from corn steep liquor scattered the oil and displaced it to a greater extent to the edges of the plate compared with the detergent of synthetic origin, SDS, indicating a greater capacity of the biosurfactant to extract and solubilize oil (Figure 4).
Washing tests for sandy soils contaminated with burned car engine oil
The efficiency of the extraction process achieved with the biosurfactant extracted from corn steep liquor was significantly greater than that achieved with water as the control, especially when the cleaning solution was formulated with biosurfactant concentrations around the CMC (Figure 5).
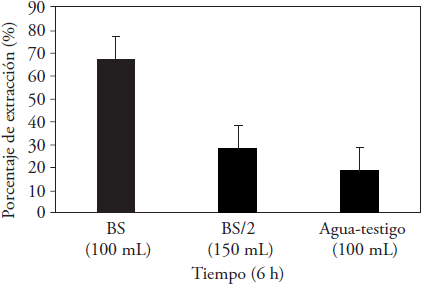
Figure 5 Oil extraction percentages reached with a biosurfactant obtained from corn steep liquors after 6 h of treatment compared with water (control).
Biosurfactant concentrations around the CMC, defined as BS, produced nearby 67 % of oil extraction, while BS/2 set the oil extraction percentage at 28 %, in these conditions although, the cleaning solution volume increased, the results were closer to those obtained with the cleaning solution consisting of the water control.
The results obtained in our study regard the BS are better than those reported by Vreysen and Maes (2005) of 50 % and 20 % extraction of diesel oil using Tergitol NP-10 surfactant. Moldes et al. (2011) used a biosurfactant produced by Lactobacillus pentosus and eliminated 58.6 % octane after 15 d of contact between the BS and contaminated soil.
This allows us to consider that the causes of the less satisfactory results below the CMC in our study could be due the low biosurfactant concentrations, which does not form micelles that surround oil molecules, or promotes mobilization and solubilization of the oil present in the sandy soil.
Figure 6 shows the surface tension values recorded in the different cleaning solutions after the treatment of the contaminated sand. Experiments that used 100 mL cleaning solution had a solid/liquid ratio of 1:20 (w:v), while with 150 mL this ratio was of 1:30 (w:v). The lowest surface tension (37 mN m-1) was observed in the cleaning solution with the highest biosurfactant concentration (200 mg L-1), but the BS/2 cleaning solution with 100 mg L-1 biosurfactant has a higher surface tension (46 mN m-1), which explains its lower cleaning capacity compared with the BS cleaning solution. The approximate surface tension of the water-based cleaning solution (water control) was 60 mN m-1 (Figure 6).
Moya-Ramirez et al. (2014) showed that, during the treatment of a sandy soil with 10 g L-1 of a synthetic surfactant (G600), the increase of temperature makes the extraction process more effective. These authors reported extraction rates under 20 % at room temperature, and up to almost 40 % when the temperature increased up to 65 °C. The use of temperatures around 65 °C makes this process unviable for industrial scale, mainly in “in situ” extraction processes, since the energy cost and equipment for such a process would be high. Compared to natural detergents Moya-Ramirez et al. (2014) used a biosurfactant produced by Bacillus subtillis and obtained higher percentages than those obtained with G600 synthetic surfactant cleaning agent; although, higher biosurfactant concentrations caused lower yields. The extraction with biosurfactant used by these authors was less than 40 %, at 45 °C, and was less than that obtained with the biosurfactant evaluated in our study (67 %) at room temperature (25 °C).
Conclusions
The biosurfactant extract obtained from corn steep liquor could solubilize engine burned oil in water and displaced it to a greater extent than the SDS. Biosurfactant concentrations around the CMC show good properties as contaminated sandy soil cleaning agent. Therefore, this natural detergent could be used on hydrophobic industrial discharges instead of cleaning agents from non-renewable sources.
Literatura Citada
Abdul S., A., T. Gibson, and S. Kia. 1990. Contamination of soil and groundwater by automatic transmission fluid: Site description and problem assessment J. Hydrol. 121: 133-153. [ Links ]
Banat, I., R. Makkar, and S. Cameotra. 2000. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 53: 495-508. [ Links ]
Banat, I. M., A. Franzetti, I. Gandolfi, G. Bestetti, M. G. Martinotti, L. Fracchia, T. J. Smyth and R. Marchant. 2010. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 87: 427-444. [ Links ]
Cameotra, S. S., and R. S. Makkar. 2010. Biosurfactant enhanced bioremediation of hydrophobic pollutants. Pure Appl. Chem. 82: 97-116. [ Links ]
Damasceno, F. R. C., M. C. Cammarota, and D. M. G. Freire. 2012. The combined use of a biosurfactant and an enzyme preparation to treat an effluent with a high fat content. Colloids Surf., B. 95: 241-246. [ Links ]
Das, M., S. K. Das, and R. K. Mukherjee. 1998. Surface active properties of the culture filtrates of a Micrococcus species grown on n-alkenes and sugars. Bioresour. Technol. 63: 231-235. [ Links ]
Desai, J., and I. Banat. 1997. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. 61: 47-64. [ Links ]
Edwards D., A., R. G. Luthy, and L. Zhongbao. 1991. Solubilization of polycyclic aromatic hydrocarbons in micellar nonionic surfactant solutions. Environ. Sci. Technol. 25: 127-133. [ Links ]
Gharaei-Fathabad, E. 2011. Biosurfactants in pharmaceutical industry: A mini-review. Am. J. Drug Discov. Dev. 1: 58-69. [ Links ]
Kourkoutas, Y., and M. Banat, 2004. Biosurfactant production and application. In: Pandey, A. (ed). Concise Encyclopedia of Bioresources Technology. pp: 505-516. [ Links ]
Levison, M. I. 2009. Surfactant production: present realities and future perspectives. In: Zoller, U., and P. Sosis (eds). Handbook of Detergents, Part F. Vol. 142. pp: 1-37. [ Links ]
Moldes, A. B., R. Paradelo, D. Rubinos, R. Debesa-Rey, J. M. Cruz, and M. T. Barral. 2011. Ex Situ treatment of hydrocarbon-contaminated soil using biosurfactants from Lactobacillus pentosus. J. Agric. Food Chem. 59: 9443-9447. [ Links ]
Moldes, A. B., R. Paradelo, X. Vecino, J. M. Cruz, E. Gudiña, L. Rodrigues, J. A. Teixeira, J. M. Domínguez, and M. T. Barral. 2013. Partial characterization of biosurfactant from Lactobacillus pentosus and comparison with sodium dodecyl sulphate for the bioremediation of hydrocarbon contaminated soil. BioMed Research International. Vol. 2013, Article ID 961842, 6 pages doi:10.1155/2013/961842. [ Links ]
Moya-Ramírez, I., M. García-Román, M. Henares-Jiménez, E. Jurado-Alameda, y D. Altmajer-Vaz. 2014. Remediación de suelos contaminados con aceite de motor mediante tensioactivos altamente biodegradables. Av. Cien. Ing. 5: 21-29. [ Links ]
Mulligan N., C. 2005. Environmental applications for biosurfactants. Environ. Pollut. 133: 183-198. [ Links ]
Neufeld, R. J. and J. E. Zajic. 1984. The surface activity of Acinetobacter calcoaceticus sp. 2CA2. Biotechnol. Bioeng. 26: 1108-1113. [ Links ]
Ortíz-Hernández, L., M. Monterrosas-Brisson, G. Yañez-Ocampo, and E. Sánchez-Salinas. 2001. Biodegradation of methyl-parathion by bacteria isolated of agricultural soil. Rev. Int. Contam. Amb. 17: 147-155. [ Links ]
Perez-Ameneiro, M., X. Vecino, J. M. Cruz, and A. B. Moldes. 2015. Wastewater treatment enhancement by applying a lipopeptide biosurfactant to a lignocellulosic biocomposite. Carbohydrate Polymers 131: 186-196. [ Links ]
Reglamento (UE) Nº1357/2014 de la comisión de 18 de diciembre de 2014. Diario oficial de la Unión Europea. L365:89-96. [ Links ]
Reis, R. S., G. J. Pacheco, A. G. Pereira, and D. M. G. Freire. 2013. Biosurfactant: Production and Applications. In: Chamy, R., and F. Rosenkranz (eds). Biodegradation Life of Science. Chapter 2. pp: 31-61. [ Links ]
Rodrigues, L., H. Van der Mei, J. Teixeira, and R. Oliveira. 2004. Influence of biosurfactants from probiotic bacteria on formation of biofilms on voice prosthesis. Appl. Environ. Microbiol. 70: 4408-4410. [ Links ]
Solans, X., y E. Gadea. 2015. Gestión de residuos: clasificación y tratamiento: Instituto Nacional de Seguridad e Higiene en el trabajo (INSHT). Notas Técnicas de Prevención. 1-8. [ Links ]
Urum, K., and T. Pekdemir. 2004. Evaluation of biosurfactants for crude oil contaminated soil washing. Chemosphere 57: 1139-1150. [ Links ]
Vecino, X., L. Barbosa-Pereira, R. Devesa-Rey, J.M. Cruz, and A.B. Moldes. 2014a. Study of the surfactant properties of aqueous stream from the corn milling industry. J. Agric. Food Chem. 62: 5451-5457. [ Links ]
Vecino-Bello, X., R. Devesa-Rey, J. M. Cruz-Freire, y A.B. Moldes-Menduíña, 2014b. Aplicación de licores de lavado de maíz (“corn steep liquor”) como surfactante. ES 2.424.399 (CI. B09C1/00 (2006.01)), 13 Enero 2014. Solicitud 201.200.330, 27 Marzo 2012. 9 p. [ Links ]
Vecino-Bello, X., R. Devesa-Rey, J. M. Cruz-Freire, y A. B. Moldes-Menduiña. 2014c. Procedimiento de separación de los surfactantes presentes de licores de lavado de maíz y usos. ES 2.435.324 (CI. B01F17/00, C02F1/26, C09K3/32, B09C1/00, A23L1/035, A61K8/97, A61K36/899, C02F103/26, C02F103/32 (2006.01)), 14 Abril 2014. Solicitud 201.100.649, 18 Junio 2012. 9 p. [ Links ]
Vecino, X., L. Barbosa-Pereira, R. Devesa-Rey, J. M. Cruz, and A. B. Moldes. 2015a. Optimization of liquid-liquid extraction of biosurfactants from corn steep liquor. Bioprocess Biosyst. Eng. 38: 1629-1637. [ Links ]
Vecino, X., L. Rodríguez-Loṕez, J. M. Cruz, and A. B. Moldes. 2015b. Sewage sludge polycyclic aromatic hydrocarbon (PAH) decontamination technique based on the utilization of a lipopeptide biosurfactant extracted from corn steep liquor. J. Agric. Food Chem. 63: 7143−7150. [ Links ]
Vecino, X., J. M. Cruz, A. B. Moldes, and L. R. Rodrigues. 2017. Biosurfactants in cosmetic formulations: trends and challenges. Crit. Rev. Biotechnol. 2017, DOI: 10.1080/07388551.2016.1269053. [ Links ]
Vreysen, S., and A. Maes. 2005. Remediation of a diesel contaminated, sandy-loam soil using low concentrated surfactant solutions. J. Soils Sediments. 5: 240-244. [ Links ]
West, C. C., and J. H. Harwell. 1992. Surfactants and subsurface remediation. Environ. Sci. Technol. 26: 2324-2330. [ Links ]
Yañez-Ocampo, G., E. Sanchez-Salinas, G. Jimenez-Tobon, M. Penninckx, and M. L. Ortiz-Hernandez. 2009. Removal of two organophosphate pesticides by a bacterial consortium immobilized in alginate or tezontle. J. Haz. Mat.168: 1554-1561. [ Links ]
Yañez-Ocampo, G., E. Sanchez-Salinas, and M. L. Ortíz-Hernandez. 2011. Removal of methyl parathion and tetrachlorvinphos by a bacterial consortium immobilized on tezontle- packed up-flow reactor. Biodegradation 22: 1203-1213. [ Links ]
Yañez-Ocampo, G., y A. Wong-Villarreal. 2013. Biosurfactantes microbianos, producción potencial con residuos agroindustriales de Chiapas. BioTecnología. 17: 12-28. [ Links ]
Youssef, N., D. Simpson, K. Duncan, M. McInerney, M. Folmsbee, T. Fincher, and R. Knapp. 2007. In situ biosurfactant production by Bacillus strains injected into a limestone petroleum reservoir. Appl. Environ. Microbiol. 73: 1239-1247. [ Links ]
Received: March 2017; Accepted: September 2017











 texto en
texto en 





