Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.52 no.4 Texcoco Mai./Jun. 2018
Animal Science
Inhibitory effect of selenium concentrations on microbial activity during oat hay in vitro rumen fermentation
1 Instituto de Ciencias Agropecuarias, Universidad Autónoma del Estado de Hidalgo. Rancho Universitario. Avenida Universidad Km. 1, Ex-Hacienda de Aquetzalpa, 43600. Tulancingo. Hidalgo. (oscardelrazo@hotmail.com)
2 Ganadería, Campus Montecillo. Colegio de Postgraduados. 56230, Montecillo, Estado de México.
Selenium (Se) has been supplied to ruminants in concentrations higher than that of their established tolerable maximum, but its effects on the ruminal fermentation are scarcely documented. Our objective of this study was to evaluate the effect of Se added to in vitro fermentation of oats hay. The treatments were: control, Se20, Se40, Se60 and Se80 which correspond to 0, 20, 40, 60 and 80 mg Se kg-1 MS sodium selenite in the substrate. Oat hay samples (500 mg) were incubated with 40 mL of culture media (rumen fluid, mineral solutions, buffer solution, resazurin and reducing solution) at 39 °C for 72 h, using the gas production technique. The evaluated variables were: accumulated gas volume (AGV), gas production kinetics (maximum volume (V), gas production rate (S) and delay time (L)), methane (CH4), pH, degradation of dry matter (DMS), volatile fatty acids (VFA) and ammoniacal nitrogen (N-NH3). The completely randomized experimental design with repeated measures had seven independent repetitions per treatment. The data were analyzed with the MIXED procedure in the SAS® software. Oat hay fermented with Se60 and Se80 had higher pH and lower AGV, V, S, and CH4 compared to the other treatments (p ≤ 0.05). Se addition did not affect DMS, VFA or N-NH3 (p > 0.05), but these variables linearly decreased with the highest microelement concentrations (p ≤ 0.05). The microbial activity during the in vitro fermentation of oat hay kept constant with 20 and 40 mg Se kg-1 MS; the last concentration may be the maximum limit that guarantees the appropriate in vitro fermentation.
Key words: sodium selenite; degradation; gas production; methane; volatile fatty acids; ammonia nitrogen
El selenio (Se) se ha suministrado a rumiantes en concentraciones superiores a la establecida como máxima tolerable, pero su efecto en la fermentación ruminal se ha documentado poco. El objetivo de este estudio fue evaluar el efecto de Se adicionado en la fermentación in vitro de heno de avena. Los tratamientos fueron: testigo, Se20, Se40, Se60 y Se80 correspondientes a 0, 20, 40, 60 y 80 mg Se kg-1 MS en el sustrato, como selenito de sodio. La incubación de 500 mg de heno se realizó con 40 mL de medio de cultivo (líquido ruminal, soluciones minerales, solución amortiguadora, resazurina y solución reductora) a 39 °C por 72 h, con la técnica de producción de gas. Las variables fueron: volumen de gas acumulado (VAG), cinética de producción de gas (volumen máximo (V), tasa de producción de gas (S) y tiempo de retardo (L)), metano (CH4), pH, degradación de la materia seca (DMS), ácidos grasos volátiles (AGV) y nitrógeno amoniacal (N-NH3). El diseño experimental fue completamente al azar con medidas repetidas, con siete repeticiones independientes por tratamiento y los datos se analizaron con el procedimiento MIXED de SAS®. Cuando el heno se fermentó con Se60 y Se80 el pH fue mayor y VAG, V, S y CH4 fueron menores respecto a los otros tratamientos (p ≤ 0.05). El Se adicionado no afectó DMS, AGV y N-NH3 (p > 0.05), pero estas variables disminuyeron linealmente con la concentración mayor del microelemento (p ≤ 0.05). La actividad microbiana durante la fermentación in vitro de heno de avena se mantuvo constante con 20 y 40 mg Se kg-1 MS, y la última concentración puede ser el límite máximo que garantice la fermentación in vitro apropiada.
Palabras clave: selenito de sodio; degradación; producción de gas; metano; ácidos grasos volátiles; nitrógeno amoniacal
Introduction
Selenium (Se) is an essential microelement for animals because it is part of the selenocysteine, an oxide-reducing proteins amino acid (Stock and Rother, 2009). Se deficient consumption alters the metabolism of thyroid hormones and the immune response, causes muscle pathologies and reproductive disorders (Hefnawy and Tórtora-Pérez, 2010). Adding Se in the diet, intraruminal bolus and injectable solutions, are recommended practices in regions with microelement deficiency (Hernández-Calva and Ramírez-Bribiesca, 2006; Revilla-Vázquez et al., 2008).
Ruminants require between 0.1 to 0.3 mg kg-1 MS of Se (NRC, 2001; NASEM, 2016), the requirement varies with the weight and type of diet (NRC, 2007). Oviene and caprine livestock are susceptible to microelement deficiency (Ramírez-Bribiesca et al., 2001; Ramírez-Bribiesca et al., 2004), because ruminal microorganisms reduce the inorganic form to unavailable forms to ruminants (elemental Se and selenide) as a mechanism of energy conservation and to avoid selenosis (Nancharaiah and Lens, 2015), or incorporate it into microbial protein such as selenoaminoacids (Mainville et al., 2009; Panev et al., 2013). The effects of its addition in ruminal fermentation, and the addition of nutrients concentrations close to those required have been variable (Serra et al., 1994; Kim et al., 1997; Del Razo-Rodriguez et al., 2013).
Sheep in different physiological stages have received diets with Se concentrations above their tolerable maximum (5 mg kg-1 MS; NRC, 2005). The supply of 10, 20 and 40 mg Se kg-1 MS, as sodium selenite, reports no selenosis or negative effects on the live weight in sheep (Cristaldi et al., 2005; Davis et al., 2006a, Davis et al., 2006b; Davis et al., 2008). However, information on the effects of high Se concentrations on ruminal fermentation and dry matter degradation (DMD) is limited (Datt et al., 2013; Eun et al., 2013).
Therefore, we hypothesized that the Se added as sodium selenite in concentrations higher than the ruminant´s tolerable maximum affects the in vitro fermentation and the DMD. The aim of this study was to evaluate the effect of Se in the gas production, CH4, pH, DMD, volatile fatty acids (VFA) concentration and ammoniacal nitrogen (N-NH3) during in vitro oat hay fermentation.
Materials and Methods
Location
The study took place at the Animal Nutrition and Special Analysis laboratories of the Institute of Agricultural Sciences of the Universidad Autónoma del Estado de Hidalgo (UAEH), at Tulancingo de Bravo, Hidalgo state (central Mexico).
Treatments
The treatments were: control, Se20, Se40, Se60 and Se80, which correspond 0, 20, 40, 60 and 80 mg Se kg-1 MS of Se added to the substrate. Each treatment had seven independent repetitions, and the experimental unit was a 500 mg substrate jar. The concentration of the microelement in each treatment was obtained from a base solution with 99 mg Se L-1 (220 mg of food grade sodium selenite, 45 % of Se 1000 mL-1); 500 μL of the solutions with 20, 40, 60 and 80 mL graduated to 100 mL were added to the jars according to each treatment, and distilled water for the control.
In vitro fermentation
The in vitro fermentation was carried out following a gas production technique (Theodorou et al., 1994). The culture medium contained 225 mL buffer solution (4 g NH4HCO3 (Meyer) and 35 g of NaHCO3 (Meyer), in 1000 mL), 225 mL macromineral solution (5.7 g Na2HPO4 (Meyer), 6.2 g of KH2PO4 (Meyer) and 0.6 g of MgSO4 • 7H2O (Sigma), in 1000 mL), 100 mL ruminal fluid and 450 mL distilled water, per 1000 mL. In addition to 100 μL of a micromineral solution (13.2 g of CaCl2 • 2H2O (Sigma), 10 g of MnCl • 4H2O (Sigma), 1 g CoCl • 6H2O (Meyer) and 8 g FeCl3 (Meyer), in 100 mL), 1.2 mL 0.1 % resazurin and a reducing solution (0.57 g Na2SO4 and 4 mL NaOH 0.1 N, in 100 mL), on a 2 mL: 60 mL proportion of the media. The media components were mixed with agitation, at 39 °C and constant carbon dioxide (CO2) flow.
Ruminal fluid was obtained from two sheep with cannulas (Suffolk x Rambouillet, 2 years old), filtered through three sky blanket layers and kept at 39 °C until use. The sheep were fed oat hay (free access) and 500 g d-1 commercial feed with 15 % crude protein. The surgical placement of cannulas was authorized by the Institutional Committee of Ethics for Care and Use of Laboratory Animals of the UAEH. The care before and after the surgery was carried out following the Official Mexican Standard NOM-062-ZOO-1999, which establishes the technical specifications for the production, care and use of laboratory animals (DOF, 2001).
The substrate´s fermentation (particle of 1 mm, humidity of 13.5 % and PC of 9.4 %) was carried out in transparent glass jars (120 mL capacity and 20 mm mouth). 40 mL of the culture medium were added to each vial with a constant CO2 flow for 15 s. The bottles hermetically sealed with silicon plugs and aluminum caps placed with a manual crimper (Wheaton, E-Z Crimper, USA). The incubation was carried out at 39 °C in a water bath (Thermo Scientific, 2864, USA) for 72 h. Three bottles without substrate (blanks) were included to measure the gas volume produced by the inoculum digestion; this was subtracted from that produced by the fermentation of the samples.
Gas and CH4 production
Gas partial volume was recorded via water displacement at (Ramirez-Bribiesca et al., 2011) 1, 2, 3, 4, 5, 7, 9, 12, 24, 32, 48 and 72 h after the incubation and used to calculate the accumulated gas volume (AGV; mL g-1 MS). The assessed variables of the gas production kinetics were V: maximum volume (mL g-1 MS), S: production rate (h-1) and L: delay time (h); these were calculated with the Schofield and Pell statistical model (1995) Va = V / (1 + e2-4S(t-L)) with the NLIN procedure in the SAS® statistical procedure (SAS Institute Inc., 2012).
CH4 samples were extracted from two bottles by treatment with disposable syringes (5 mL and 21 G x 32 mm needle) before the assessments at 9, 12, 24, 32 and 48 h of incubation. The samples were stored in clear glass jars (15 mL capacity 20 mm mouth wide) previously filled with a saturated saline solution (6 N, pH 2 adjusted with concentrated HCl and methyl red as indicator), hermetically sealed with a silicone plug and an aluminum cap. The samples were then injected into the bottles through the stopper, allowing the solution to move through the needle. The samples were kept refrigerated (4 °C) with the jars until analysis.
The CH4 concentration was determined from a 200 μL sample injected in a gas chromatograph (Perkin Elmer, Autosystem XL, USA) equipped with a methanizer, flame ionization detector (FID) and capillary column (Elite Plot Q 30 mx 0.53 mm, GC Series, USA). The analysis conditions were: 200 °C in the detector and injector, and 50 °C and in the furnace; during 5 minutes run time. The concentration was calculated using a standard curve constructed with increasing CH4 concentrations (30, 50 and 100 %) and used to calculate the accumulated CH4 volume.
DMD and VFA and N-NH3 analysis
At the end of the fermentation, samples were kept for 2 h at 4 °C to stop microbial activity. The pH was then recorded with a potentiometer (Hanna Instruments, HI 2211, Romania). The DMD was estimated with the non-degraded DM recovered after centrifuging the jars contents at 20 980 x g, for 10 min (Hermle, Z 326 K, Germany) and drying the residues at 65 °C for 72 h in an oven (Terlab, TH-45DM, Mexico). The formula was: DMD = [(PSM - PSMR) / PSM] * 100; where PSM: dry weight of the sample (g) and PSMR: residual material dry weight (g).
VFA concentration in the medium was determined by gas chromatography at 72 h of incubation (Erwin et al., 1961). For this, an 800 μL sample of the liquid content of the jars was mixed with 200 μL 25 % w / v metaphosphoric acid (J.T. Baker). The mixture was then centrifuged at 17,000 x g for 15 min (Thermo Scientific, PICO 17, Germany) and its supernatant stored at 4 °C until analysis. The chromatograph (HP, Agilent 6890, USA) was equipped with a 0.25 mm x 30 m capillary column (HP-FFAP, Agilent, USA). The temperatures for the oven were 140, 250 and 240 °C, detector and injector, each. The carrier gas was N at 10 psi, the air and H2 flow were of 350 and 35 mL min-1. The injected sample was of 1 μL.
N-NH3 concentration was determined by colorimetry (Weatherburn, 1967) on a UV / visible spectrophotometer (Jenway, 6305, United Kingdom). For this, 20 μL of the supernatant used for VFA were mixed with 1 mL phenol (50 mg Na2Fe(CN)5NO•2H2O (Meyer) and 10 g of phenol (Fermont), in 1000 mL) and 1 mL sodium hypochlorite (7.5 g NaOH (Meyer), 21.3 g of anhydrous Na2HPO4 (Meyer) and 15 mL of 5 % sodium hypochlorite, in 1000 mL). The samples were diluted with 5 mL distilled water after 30 min of incubation at 37 °C in a water bath (Thermo Scientific, 2864, USA) and assessed at 630 nm.
Statistical analysis
The experimental design was completely randomized with repeated measures. The data of all the variables were analyzed with the MIXED procedure in the SAS® software (SAS Institute Inc., 2012), considering the treatment as fixed effect in the statistical model (time was also considered as a fixed effect for VFA and CH4) and the experimental unit, repetition and experimental error as random effects. The minimum square means were obtained with the LSMEANS procedure; the means comparison were performed for the variables with significant effect on the treatment using PDIFF. The linear and quadratic behavior was determined via the ESTIMATE procedure. The repeated means analysis was carried out with the TYPE = SP statement (POW). The level of significance was p ≤ 0.05.
Results and Discussion
Gas production occurs as result of carbohydrates degradation by ruminal microorganisms. The resulting products are VFA, CO2 and CH4. Se addition affected AGV at 24 and 72 h of incubation (p ≤ 0.05, Figure 1). Means linearly decreased at 12, 24 and 32 h, and decreased in quadratic way at 48 and 72 h (p ≤ 0.05) with the higher concentration of the microelement (Figure 2). Differences between means were observed from 12 h of incubation, at which time the AGV with Se 60 was lower than that generated in the other treatments (p ≤ 0.05). Then, the differences defined the concentrations that affected gas production; Se60 and Se80 treatments produced less AGV (between 11 and 6.9 %) at 48 and 72 h of incubation (p ≤ 0.05), with no difference between them at 72 h (p > 0.05). Results were similar to those observed by Datt et al. (2013) with sodium selenite additions to a rice straw and concentrate mixture (40:60) during fermentation; they report lower gas production with 60 and 70 mg Se kg-1 MS, at 24 and 48 h of incubation, respect to lower microelement concentrations.
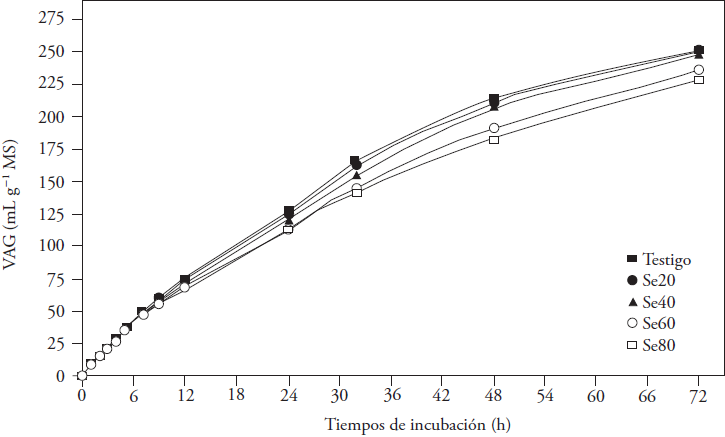
Figure 1 Accumulated gas volume (AGV) generated at in vitro fermentation of oat hay for 72 h with sodium selenite additions (control: 0, Se20: 20, Se40: 40, Se60: 60 and Se80: 80 mg kg-1 MS).
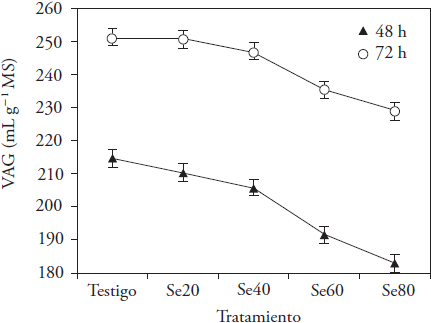
Figure 2 Changes in accumulated gas volume (AGV) generated in oat hay in vitro fermentation at 48 and 72 h of incubation with sodium selenite additions (control: 0, Se20: 20, Se40: 40, Se60: 60 and Se80: 80 mg kg-1 MS).
The treatments effect was also observed in the gas production kinetics, in V and S (p ≤ 0.05), their means decreased linearly with the Se increase (p ≤ 0.05); this did not happen with L (p = 0.07; Table 1). The value of the variables on Se60 and Se80 treatments was similar between each other, but lower than that of other treatments (except Se60 with respect to Se40). These results allow us to infer that Se concentrations above 40 mg kg-1 MS affect in vitro fermentation of oat hay and therefore microbial activity, because gas production kinetics reflects the microbial activity in the culture media (Schofield et al., 1994).
Table 1 Variables in gas production kinetics and accumulated methane volume (CH4) obtained in in vitro oat hay fermentation at 72 h with selenium (Se) addition.
| Variable/tiempo | Tratamientos | EE | ||||
| Testigo | Se20 | Se40 | Se60 | Se80 | ||
| Variables de la cinética de producción de gas | ||||||
| V (mL g-1 MS) †¶ | 244.0a | 243.0a | 240.5a,b | 229.1b,c | 221.2c | 4.3 |
| S (mL h-1) †¶ | 0.0246a | 0.0241a | 0.0235a,b | 0.0227b | 0.0220b | 0.0005 |
| L (h) | 3.00 | 3.11 | 3.32 | 2.93 | 1.53 | 0.51 |
| Volumen acumulado de CH4 (mL g-1 MS) | ||||||
| 32 h ¶ | 6.0 | 5.8 | 5.7 | 4.6 | 3.9 | 0.61 |
| 48 h †¶ | 9.5a | 8.2a | 9.3a | 7.8a,b | 6.4b | 0.61 |
a, b, c: Means with different letter in a row are different (p ≤ 0.05). Control: 0, Se20: 20, Se40: 40, Se60: 60 and Se80: 80 mg Se kg-1 MS; SE: mean standard error; V: maximum volume; S: production rate; L: delay time; DM: dry matter. † Treatment effect (p ≤ 0.05). ¶ Linear trend of the means (p ≤ 0.05).
H4 is produced during the fermentation of carbohydrates in the rumen by the activity of archaea such as Methanobrevibacter ruminantium, M. gottschalkii, Methanosphaera spp., Methanimicrococcus spp. and Methanobacterium spp. (Hill et al., 2016, Yang et al., 2016). The microbial enzymes involved in greenhouse gases production and use contain metallic cofactors (Glass and Orphan, 2012); Se is part of the dehydrogenase format that participates in the formation of CH4 (Stock and Rother, 2009).
The effect of treatments on the accumulated volume was observed at 48 h of incubation (p ≤ 0.05), but not at 32 h (p = 0.06), and means linearly decreased with the increase in Se concentration of in both times (p ≤ 0.05). Se80 produced a CH4 volume similar to that obtained with Se60 (p > 0.05), but lower than that on the other treatments (p ≤ 0.05, Table 1). With 50 mg Se kg-1 MS (sodium selenate) added during in vitro fermentation of ball-leaf grass hay (Dactylis glomerata), in a continuous double-flow culture system, no changes were observed in the CH4 concentration (p = 0.10) (Eun et al., 2013).
This indicates that CH4 in vitro production with high Se concentrations may be associated with the concentration and source of the microelement and the technique used.
The treatments affected the pH of the media 72 h after incubation (p ≤ 0.05, Table 2). With Se40, Se60 and Se80 the pH was similar (p > 0.05), but higher than the Se20 treatment and the control (p ≤ 0.05). The effect of the treatments was not observed in the DMD (p = 0.07) nor in the total concentration VFA (p = 0.10), acetate (p = 0.07), propionate (p = 0.27), butyrate (p = 0.06) and N-NH3 (p = 0.64); but, with a higher concentration of Se the means (except propionate and N-NH3) decreased linearly (p ≤ 0.05). These results show a relationship with the decrease in gas production with the increase in Se concentration; furthermore, they indicate that the AGV in vitro production is positively correlated with DM degradability and gas production (p < 0.05; Getachew et al., 2004).
Table 2 pH, dry matter degradation (DMD) and concentration of volatile fatty acids (VFA) and ammonia nitrogen (N-NH3) obtained by in vitro fermentation of oat hay for 72 h with selenium (Se) addition of selenium.
| Variable | Tratamientos | EE | ||||
| Testigo | Se20 | Se40 | Se60 | Se80 | ||
| pH †¶ | 6.74a | 6.75a | 6.77a,b | 6.81b | 6.82b | 0.02 |
| DMS (%) ¶ | 61.0 | 61.7 | 60.8 | 59.7 | 57.8 | 0.91 |
| AGV total (mMol L-1) ¶ | 46.4 | 47.0 | 46.1 | 44.5 | 39.7 | 1.81 |
| Acetato (mMol L-1) ¶ | 30.4 | 30.6 | 29.9 | 28.8 | 25.8 | 1.12 |
| Propionato (mMol L-1) | 13.0 | 13.4 | 13.3 | 13.0 | 11.5 | 0.64 |
| Butirato (mMol L-1) ¶ | 3.0 | 3.0 | 2.9 | 2.8 | 2.4 | 0.13 |
| N-NH3 | 8.5 | 9.9 | 8.8 | 8.3 | 8.6 | 0.76 |
a, b, c: Means with different letter in a row are different (p ≤ 0.05). Control: 0, Se20: 20, Se40: 40, Se60: 60 and Se80: 80 mg Se kg-1 MS; SE: mean standard error; V: maximum volume; S: production rate; L: delay time; DM: dry matter. † Treatment effect (p ≤ 0.05). ¶ Linear trend of the means (p ≤ 0.05).
The effect of the addition of high Se concentrations in the DMD and the generated products is not completely defined, and could depend on the Se concentration and source, and on the analysis technique used. Kim et al. (1997) added 2 mg Se kg-1 MS in vitro from sodium selenite; they detect no changes in the total VFA concentration, but observed higher butyrate and less acetate proportions. Eun et al. (2013) added 50 mg Se kg-1 MS, using sodium selenate, but found no changes in the pH, VFA concentration and N-NH3. Xun et al. (2012) reported positive DMS changes, total VFA concentration, N-NH3 and acetate:propionate ratio with the addition of 4 mg Se kg-1 MS, from enriched yeast and nanoparticles with Se in a in situ study .
There are bacteria and archaea that use selenate and selenite as final electron acceptors during their anaerobic respiration, and form nanospheres which is an insoluble Se form. This reduction is a mechanism to prevent selenosis (Nancharaiah and Lens, 2015). In this regard, Eun et al. (2013) indicate that ruminal bacteria adapt to high concentrations of the microelement, because they detected growth of selenate-reducing bacteria by adding 50 mg Se kg-1 MS to the medium. This explains why the supply of 40 mg Se kg-1 MS in sheep did caused no selenosis or changes in their live weight (Davis et al., 2008). However, the production of bacterial mass has a negative correlation (p < 0.05) with the Se concentration in ruminal liquid (Kim et al., 1997), so that bacterial activity can also decrease with the increase in the concentration of the microelement. Thus, it is possible to infer that the bacteria adapted to concentrations of 20 and 40 mg Se kg-1 MS, which kept the fermentation constant and the DMD unchanged.
Conclusions
The microbial activity during in vitro fermentation of oat hay was kept constant with 20 and 40 mg Se kg-1 MS, and decreased with 60 and 80 mg Se kg-1 MS. The aggregate did not affect the dry matter degradation, the AGV concentration and N- NH3, although it showed a linear reduction in these variables. It seems that 40 mg Se kg-1 MS is the maximum limit that guarantees microbial activity without affecting the fermentation or in vitro degradation of oat hay dry matter.
Literatura Citada
Cristaldi, L. A., L. R. McDowell, C. D. Buergelt, P. A. Davis, N. S. Wilkinson, and F. G. Martin. 2005. Tolerance of inorganic selenium in wether sheep. Small Ruminant Res. 56: 205-213. [ Links ]
Datt, C., A. Kumar, and S. S. Kundu. 2013. Effect of different levels of added selenium without or with arsenic on rumen fermentation parameters in buffaloes under in vitro conditions. Indian J. Anim Sci. 83: 1203-1206. [ Links ]
Davis, P. A., L. R. McDowell, N. S. Wilkinson, C. D. Buergelt, R. Van Alstyne, R. N. Weldon, and T. T. Marshall. 2006a. Effects of selenium levels in ewe diets on selenium in milk and the plasma and tissue selenium concentrations of lambs. Small Ruminant Res. 65: 14-23. [ Links ]
Davis, P. A., L. R. McDowell, N. S. Wilkinson, C. D. Buergelt, R. Van Alstyne, R. N. Weldon, and T. T. Marshall. 2006b. Tolerance of inorganic selenium by range-type ewes during gestation and lactation. J. Anim. Sci. 84: 660-668. [ Links ]
Davis, P. A., L. R. McDowell, N. S. Wilkinson, C. D. Buergelt, R. Van Alstyne, R. N. Weldon, T. T. Marshall, and E. Y. Matsuda-Fugisaki. 2008. Comparative effects of various dietary levels of Se as sodium selenite or Se yeast on blood, wool, and tissue Se concentrations of wether sheep. Small Ruminant Res. 74: 149-158. [ Links ]
Del Razo-Rodriguez, O. E., J. E. Ramirez-Bribiesca, R. Lopez-Arellano, A. L. Revilla-Vazquez, S. S. Gonzalez-Munoz, M. A. Cobos-Peralta, L. M. Hernandez-Calva, and L. R. McDowell. 2013. Effects of dietary level of selenium and grain on digestive metabolism in lambs. Czech J. Anim. Sci. 58: 253-261. [ Links ]
DOF (Diario Oficial de la Federación). 2001. Norma Oficial Mexicana NOM-062-ZOO-1999, especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. Agosto 22. pp: 107. [ Links ]
Erwin, E. S., G. J. Marco, and E. M. Emery. 1961. Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. J. Dairy Sci. 44: 1768-1771. [ Links ]
Eun, J. S., T. Z. Davis, J. M. Vera, D. N. Miller, K. E. Panter, and D. R. ZoBell. 2013. Addition of high concentration of inorganic selenium in orchardgrass (Dactylis glomerata L.) hay diet does not interfere with microbial fermentation in mixed ruminal microorganisms in continuous cultures. PAS. 29: 39-45. [ Links ]
Getachew, G., P. H. Robinson, E. J. DePeters, and S. J. Taylor. 2004. Relationships between chemical composition, dry matter degradation and in vitro gas production of several ruminant feeds. Anim. Feed Sci. Tech. 111: 57-71. [ Links ]
Glass, J. B., and V. J. Orphan. 2012. Trace metal requirements for microbial enzymes involved in the production and consumption of methane and nitrous oxide. Front. Microbiol. 3: 1-20. [ Links ]
Hefnawy, A. E., and J. L. Tórtora-Pérez. 2010. The importance of selenium and the effects of its deficiency in animal health. Small Ruminant Res. 89: 185-192. [ Links ]
Hernández-Calva, L. M., and J. E. Ramírez-Bribiesca. 2006. Diagnosis of selenium status and sodium selenite injection in fighting cattle on the Mexican plateau. Cuban J. Agr. Sci. 40: 43-46. [ Links ]
Hill, J., C. McSweeney, A. D. G. Wright, G. Bishop-Hurley, and K. Kalantar-zadeh. 2016. Measuring methane production from ruminants. Trends Biotechnol. 34: 26-35. [ Links ]
Kim, J., P. J. Van Soest, and G. F. Combs. 1997. Studies on the effects of selenium on rumen microbial fermentation in vitro. Biol. Trace Elem. Res. 56: 203-213. [ Links ]
Mainville, A. M., N. E. Odongo, W. J. Bettger, B. W. McBride, and V. R. Osborne. 2009. Selenium uptake by ruminal microorganisms from organic and inorganic sources in dairy cows. Can. J. Anim. Sci. 89: 105-110. [ Links ]
Nancharaiah, Y. V., and P. N. L. Lens. 2015. Ecology and biotechnology of selenium-respiring bacteria. Microbiol. Mol. Biol. Rev. 79: 61-80. [ Links ]
NASEM (National Academies of Sciences, Engineering, and Medicine). 2016. Nutrient Requirements of Beef Cattle (8th Ed.). The National Academy Press, Washington, DC. 494 p. [ Links ]
NRC (National Research Council). 2001. Nutrient Requirements of Dairy Cattle (7th Ed.). National Academy Press, Washington, DC. 408 p. [ Links ]
NRC (National Research Council). 2005. Mineral Tolerance of Animals (2nd Ed.). National Academy Press, Washington, DC. 510 p. [ Links ]
NRC (National Research Council). 2007. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids and New World Camelids. National Academy Press, Washington, DC. 384 p. [ Links ]
Panev, A., K. Hauptmanová, L. Pavlata, A. Pechová, J. Filípek, and R. Dvořák. 2013. Effect of supplementation of various selenium forms and doses on selected parameters of ruminal fluid and blood in sheep. Czech J. Anim. Sci. 58: 37-46. [ Links ]
Ramírez-Bribiesca, J. E., J. L. Tórtora, L. M. Hernández, and M. Huerta. 2001. Main causes of mortalities in dairy goat kids from the Mexican plateau. Small Ruminant Res. 41: 77-80. [ Links ]
Ramírez-Bribiesca, E., E. Hernández-Camacho, L. M. Hernández-Calva, y J. L. Tórtora-Pérez . 2004. Efecto de un suplemento parenteral con selenito de sodio en la mortalidad de corderos y los valores hemáticos de selenio. Agrociencia 38: 43-51. [ Links ]
Ramirez-Bribiesca, J. E., Y. Wang, L. Jin, T. Canam, J. R. Town, A. Tsang, T. J. Dumonceaux, and T. A. McAllister. 2011. Chemical characterization and in vitro fermentation of Brassica straw treated with the aerobic fungus, Trametes versicolor. Can. J. Anim. Sci. 91: 695-702. [ Links ]
Revilla-Vázquez, A., E. Ramírez-Bribiesca, R. López-Arellano, L. M. Hernández-Calva, J. Tórtora-Pérez, E. García-García, y R. G. Cruz-Monterrosa. 2008. Suplemento de selenio con bolos intrarruminales de selenito de sodio en ovinos. Agrociencia 42: 629-635. [ Links ]
SAS Institute Inc. 2012. SAS/STAT® 12.1 User's Guide. SAS Institute Inc. Cary, NC, USA. 9030 p. [ Links ]
Schofield, P., R. E. Pitt, and A. N. Pell. 1994. Kinetics of fiber digestion from in vitro gas production. J. Anim. Sci. 72: 2980-2991. [ Links ]
Schofield, P., and A. N. Pell. 1995. Validity of using accumulated gas pressure readings to measure forage digestion in vitro: a comparison involving three forages. J. Dairy Sci. 78: 2230-2238. [ Links ]
Serra, A. B., K. Nakamura, T. Matsui, T. Harumoto, and T. Fujihara. 1994. Inorganic selenium for sheep II. Its influence on rumen bacterial yield, volatile fatty acid production and total tract digestion of timothy hay. Asian-Australas. J. Anim. Sci. 7: 91-96. [ Links ]
Stock, T., and M. Rother. 2009. Selenoproteins in Archaea and Gram-positive bacteria. BBA-Gen. Subjects. 1790: 1520-1532. [ Links ]
Theodorou, M. K., B. A. Williams, M. S. Dhanoa, A. B. McAllan, and J. France. 1994. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Tech. 48: 185-197. [ Links ]
Weatherburn, M. W. 1967. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 39: 971-974. [ Links ]
Xun, W., L. Shi, W. Yue, C. Zhang, Y. Ren, and Q. Liu. 2012. Effect of high-dose nano-selenium and selenium-yeast on feed digestibility, rumen fermentation, and purine derivatives in sheep. Biol. Trace Elem. Res. 150: 130-136. [ Links ]
Yang, C., J. A. Rooke, I. Cabeza, and R. J. Wallace. 2016. Nitrate and inhibition of ruminal methanogenesis: microbial ecology, obstacles, and opportunities for lowering methane emissions from ruminant livestock. Front. Microbiol. 7: 1-14. [ Links ]
Received: December 2016; Accepted: July 2017











 texto em
texto em 


