Introduction
The melon (Cucumis melo L.) belongs to the family of cucurbits, and it is an important crop at a worldwide level (FAO, 2016). This crop is cultivated in tropical and subtropical regions of the world, although it is also cultivated in temperate climate regions under protected agriculture (Gómez-Guillamón et al., 1997; Robinson and Decker-Walters, 1997). Seven important varieties of the C. melo species are recognized: cantaloupensis Naud., reticulatus Ser., saccharinus Naud., inodorus Naud., flexuosus Naud., conomon Mak., and dudaim Naud. (Kirkbride, 1993). Of them, cantaloupensis, reticulatus, and inodorus are the ones with the most cultivars in the world. In Mexico, the melon-planted area for 2014 was 18 454 ha. The total production of fresh fruit was 526 991 t, with an average yield of 28.79 t ha-1 and an economical value of 181.79 million United States dollars (SIAP, 2016), placing Mexico as an important exporter of melons. For instance, in 2013, 145 688 t were exported, with a value of over 105 million USA dollars (FAOSTAT, 2017).
However, regardless of the previously stated facts, the melon crop in Mexico is facing diverse problems in agronomical management (Ayala and Carrera, 2012), with attacks from pests, diseases, and issues in nutrition being the most occurring problems. All these problems are due to the market tendencies of soliciting fruit that is free of agrochemical residues. In regards to this issue, diverse microorganisms have beneficial effects when they are present in the rhizosphere of the crops. Among the beneficial bacteria with greatest potential in the cultivation of vegetables, the following can be found: Pseudomonas, Serratia, Streptomices, Calothrix, Aureobasidium, and Bacillus (Choudhary and Johri, 2009; Singh et al., 2011). The species of said genera release a wide range of peptides with microbicide activity that includes small bacterocines and antifungal defense, produced through the ribosomal synthesis, and peptaibols, cyclopeptides, and pseudo-peptides, which are non-peptide secondary metabolites (Montesinos, 2007). Moreover, B. subtilis is a Plant Growth-Promoting Rhizobacteria (PGPR), belongs to this group, and can colonize the plant roots or their vicinity. The inoculation of PGPR in different crops can increase their nutritional status, and generate changes in substances like phytohormones, causing variations in plant physiology, such as increased root development, induction of systemic resistance and tolerance to certain environmental conditions (Beneduzi et al., 2012). Likewise, some strains inhibit the development of pathogenic organisms through siderophores and antibiotics. Similarly, fruit quality can be improved, such as in tomato (Mena-Violante and Olalde-Portugal, 2007).
Among these bacterial genera, Bacillus is notorious for its ability to generate many secondary metabolites, enzymes, and plant growth regulators (Cazorla et al., 2006; Ongena and Jacques, 2008). Likewise, the species of B. subtilis, a Gram-positive bacterium, is one of the best characterized microorganisms, genetically as well as biochemically (Ongena and Jacques, 2008). Many strains are known and they have an excellent capacity of colonization and a great versatility to protect diverse plants from pathogens (Kloepper et al., 2004). Also, B. subtilis possesses a superior ability to sporulate, which ensures its permanence in the environment (Schallmey et al., 2004).
Bacillus subtilis has shown positive effects in the quality and development of fruits other than melons, such as tomatoes (Mena-Violante and Olalde-Portugal, 2007; Mena-Violante et al., 2009). Likewise, B. subtilis-soil inoculation, at laboratory testing, showed an increased C. melo plant growth (Zhao et al., 2011). Some of characteristics improved by the application of B. subtilis related to fruit quality include: the rise in fruit size and weight, increased yield, higher resistance of fruit to degrading organism attacks, increased firmness of fruit due to the reduced ethylene production, which means a higher shelf life and increasing total soluble solids (Mena-Violante and Olalde-Portugal, 2007; Mena-Violante et al., 2009).
Then, the use of Mexican native strains of B. subtilis in melon cultivation could have positive effects by avoiding the incidence of diseases and increasing nutrition to the plant. With this, a higher fruit quality will be attained, and the possibility to obtain a better market price. At the same time, the product will be attractive to the exportation market for its food safety traits. This focus is an attractive alternative from the environmental, economic, market, human health, and scientific points of view. Therefore, the objective of this study was to evaluate the effects of four strains of B. subtilis (BEB-23, LAL-36, BEB-22, and BEB-13) native to Mexico on the development of melon plants (fruit yield and quality) grown under greenhouse conditions. Thus, the application of Mexican native strains of B. subtilis on greenhouse-grown melon plants could induce a significant improvement on plant development and fruit quality.
Materials and Methods
Plant materials and crop growth conditions
The seed variety used was Top Mark (Nunhem Seeds), a melon reticulated type. The experiment was developed in a greenhouse during the Spring-Summer season in 2014. The experimental site was a greenhouse at the Life Sciences Division, Irapuato-Salamanca Campus, of the University of Guanajuato, Mexico, with an area of 90 m2 (20° 44’ 32.18’’ N and 101° 19’ 52.22’’ W, at 1757 m of altitude). The greenhouse has a control system to maintain temperature at 22-30 °C.
The melon seed germination was conducted in laboratory conditions. Ninety melon seeds were placed in the germination chamber (Thempath Junior TE 8J, USA) at a constant temperature of 30 °C for 24 h. All germinated seeds, bearing the emerged root, were planted into a moist peat moss (Sun Shine mix # 3) substrate on a polypropylene germination tray, and kept there for 10 d at 23-25 °C. When the melon seedlings had a height of 10 cm, on average, and had two true leaves, they were transplanted to 10 L plastic black bags, which contained zeolite (clinoptilotite natural type with a 5 mm granulometry). This substrate was kindly donated by Zeolitech S. de R. L. de C. V. (Mexico). The melon plants were kept in the greenhouse until the conclusion of the experiment. The irrigation system, nutritive solution, and the frequency of irrigation were accomplished following the protocols reported by Nuñez-Palenius et al. (2007).
Evaluated treatments
The five treatments were four bacterial strains and one control (without bacteria): 1) control, 2) BEB-23 strain, 3) LAL-36 strain, treatment 4) BEB-22 strain, and 5) BEB-13 strain. These strains were kindly donated by Dr. Olalde-Portugal (CINVESTAV-IPN, Irapuato Unit). The application of the bacterial strains was carried out immediately after the transplant, and following the protocol reported by Mena Violante et al. (2009). An 8-cm deep perforation was placed next to each plant, and afterwards a bacterial solution of 3 mL (1 X 107 UFC mL-1) was applied using a syringe without a needle. Fourteen days after the first application of the bacterial strains, a second one was applied with the final objective of ensuring the bacteria’s establishment in the plant rhizosphere. Hermaphrodite flowers were self-pollinated by hand and tagged for date of pollination. Only three fruits were kept on each plant, with only one used for the quality evaluations. The pest and disease control was completed using the recommendations of Fu and Ramírez (1999) and Pinales and Arellano (2001).
The fruits produced were held to the greenhouse structure with a commercial netting and string, meaning that the mature fruits in each harvest fell in the netting, avoiding contact with the ground.
Evaluated variables
Plant development, fruit yield and quality were evaluated. Concerning the plant development, the height (cm) was evaluated weekly from 45 plants (nine per treatment) during 11 weeks, from the base to the plant apex. Likewise, foliar area data was taken by tracing the leaf edge (five leaves, from the tip to the bottom) from each of the 45 plants (from the ones that the height variable was taken) over a paper sheet that was scanned, producing an image that was run through the Image J software (Figure 1). The shoot fresh and dry weights and the root fresh and dry weights were determined at the end of the experiment. Briefly, all 45 plants whose previous variables were determined, were cut (at the neck of the plant) and the root was separated from the shoot, which was weighed and the fresh weight was scored. Afterwards, shoots were placed in a drying stove (Thermo Scientific™, model Precision™, USA) (60 °C) for 72 h, and then were weighed again to determine shoot dry weight. To obtain the root fresh weight after cutting the plant shoot, the roots were tap washed and cleaned of any substrate residue, then weighed and immediately placed in a drying stove (60 °C) for 72 h; afterwards which they were weighed to obtain the root dry weight.
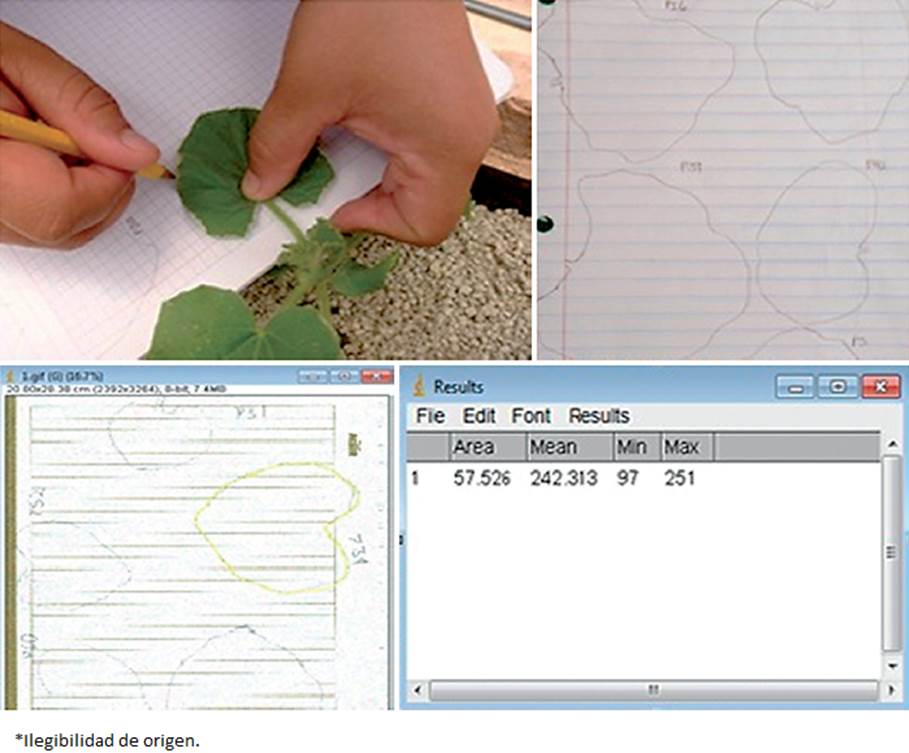
Figure 1 Foliar area of melon plants on paper and their conversion to cm2 using the software Image J.
The fruit yield per ha was calculated with the fruit fresh weight data per harvest, considering three total harvests during the plant cycle and 20 000 plants ha-1.
Melon fruit quality was determined in 45 fruits (one from each plant), by: 1) fresh weight was measured with a digital scale (Mettler PJ6000, Spain); 2) polar and equatorial diameter; 3) area of the seed cavity was measured with the system reported by Nuñez-Palenius et al. (2007); 4) external rind color (exocarp) was determined using a Hunter Lab cromatometer (Minolta model CM-508d, USA), calibrated with a white standard tile to obtain the L*, b*, and a* parameters; 5) fruit firmness was measured as follows: whole unpeeled fruit were tested with a penetration system using the conic probe (speed=1 mm s-1) attached to a TA-XT2 device (Stable Micro Systems, Inc. USA); the max required force to penetrate the fruit was registered at a specified distance of 15 mm at two equidistant points on the equatorial zone of each fruit, obtaining the average force of both measurements per fruit; 6) total soluble solids (TSS), titratable acidity (TA) and the pH of the juice obtained from the mesocarp were also evaluated; for this, the mesocarp was macerated and placed in a centrifuge (28 000 x g) (Thermo Scientific™ Sorvall™ Legend™ XT/XF, USA) and immediately afterwards filtered through Whatman paper No. 1. The obtained juice was analyzed for TSS, TA, and pH, in triplicate, using a digital refractometer (Hanna Instruments HI 96801, Mexico), a Fisher-395 dispenser connected to an electrometer (Fisher 380, USA), and a digital pH meter (Hanna Instruments, Mexico), respectively; 7) another variable of fruit quality was the maturity index, obtained as the result of the ratio of TSS/TA.
Experimental design and statistical analysis
A completely randomized design was used, with nine repetitions per treatment, to set up the experiment. Data were subjected to ANOVA of one factor. In case of significant differences, a comparison of media was carried out using the multiple comparison Tukey’s test (p≤0.05). The analysis was performed with SAS (SAS Institute, Cary, NC).
Results and Discussion
Root fresh and dry weight were the variables with statistically significant difference between the treatments. The strains BEB-22 (128.6±sd or SE) and LAL-36 (64.55±sd or SE) induced the greatest average values compared to the other strains and the control treatment. In contrast, shoot fresh and dry weights, height of the plant, and foliar area did not show significant differences (Table 1).
Table 1 Plant development characteristics of melon (Cucumis melo L.) inoculated with Mexican strains of Bacillus subtilis under greenhouse conditions at Irapuato, Guanajuato, Mexico.
| Treatment (Strain) | Shoot fresh weight (g) | Shoot dry weight (g) | Root fresh weight (g) | Root dry weight (g) | Plant height (cm) | Foliar area (cm2) |
| Control | 933.33 | 133.86 | 79.23 ab | 38.12 a | 458.33 | 375.60 |
| BEB-23 | 972.22 | 132.98 | 72.44 a | 37.88 a | 482.22 | 398.41 |
| LAL-36 | 1194.44 | 164.76 | 124.60 ab | 64.55 b | 446.50 | 355.81 |
| BEB-22 | 1186.21 | 162.14 | 128.60 b | 61.63 ab | 450.22 | 384.67 |
| BEB-13 | 888.88 | 132.83 | 97.01 ab | 43.61 ab | 412.44 | 355.81 |
| Significance | NS | NS | * | * | NS | NS |
NS: Not significant differences. (*) Different letters indicate significant differences between treatments (p≤0.05).
The length and weight showed significant differences among treatments (F0.05 (1) 4,30=2.86, p≤0.05; F0.05 (2) 4,30=2.74, p≤0.05). According to Tukey’s test, the strain LAL-36 promoted the greatest average values in fruit length and weight compared to other treatments. The same was observed for the yield that presented significant differences among treatments, the LAL-36 strain produced the utmost average yield (Table 2).
Table 2 Fruit diameter, weight, and yield of melon plants (Cucumis melo L.) inoculated with Mexican strains of Bacillus subtilis under greenhouse conditions at Irapuato, Guanajuato, Mexico.
| Treatment (Strain) | Polar (cm) | Equatorial (cm) | Fruit weight (g) | Calculated yield (t ha-1) three fruits 20 000 plants |
| Control | 15.83c | 13.27 | 1351.21b | 81.07b |
| BEB-23 | 17.0ab | 13.86 | 1547.20ab | 92.83ab |
| LAL-36 | 17.20a | 14.04 | 1629.46a | 97.76a |
| BEB-22 b | 16.59 abc | 13.91 | 1505.29ab | 90.31a |
| BEB-13 | 15.96bc | 13.80 | 1418.91b | 85.13b |
| Significance | * | NS | * | * |
NS: Not Significant differences. (*) Different letters indicate significant differences between treatments (p≤0.05).
The results for the external rind color were different (p≤0.05) among treatments (Table 3). The control treatment had the lowest values in all of the categories, while the BEB-22 strain had higher averages in comparison to LAL-36 and BEB-23 in the L*, a* and b* parameters of the rind. The BEB-13 strain induced the greatest average values in the a* and b* parameters (Table 3). Regarding the fruit quality, control treatment had the greatest TA value, and for TSS the BEB-22 and BEB-13 strains induced the greatest average values (Table 3).
Table 3 Postharvest fruit characteristics of melon plants (Cucumis melo L.) inoculated with Mexican strains of Bacillus subtilis under greenhouse conditions at Irapuato, Guanajuato, Mexico.
| Treatment (Strain) | External rind color | pH | TA (mL) | TSS (°Brix) | ||
| L | a* | b* | ||||
| Control | 59.66b | 1.73c | 19.07 b | 7.05 | 2.66a | 10.53b |
| BEB-23 | 62.35ab | 3.23ab | 21.12ab | 7.03 | 2.14c | 11.43ab |
| LAL-36 | 61.14ab | 2.46bc | 21.13ab | 7.00 | 2.47ba | 10.97ab |
| BEB-22 | 63.65a | 3.38ab | 21.32ab | 6.90 | 2.23c | 11.64ab |
| BEB-13 | 60.85b | 4.31a | 23.10a | 6.97 | 2.27cb | 11.93a |
| Significance | * | * | * | NS | * | * |
NS: Not Significant differences. (*) Different letters indicate significant differences between treatments (p≤0.05). TA: Titratable Acidity; TSS: Total Soluble Solids
The ANOVA’s results indicated significant differences in fruit firmness (F0.05 (1)4, 30=8.31, p≤0.0001), maturity index (F0.05 (1)4, 30=9.26, p≤0.0001), and ¼ of the seed cavity (F0.05 (1)4, 30=3.45, p≤0.05). Treatment that generated these differences was BEB-23 in firmness; the maturity index formed two groups, one with BEB-23, BEB-22 and BEB-13 having the greater average values, and control and LAL-36 strain showing the lower average data. On the contrary, for ¼ of the seed cavity variable, the greater average data was found for control and LAL-36 strain treatments (Table 4). As it can be expected a greater fleshy mesocarp, a smaller seed cavity size is observed, and vice versa.
Table 4 Postharvest fruit quality of melon plants (Cucumis melo L.) inoculated with Mexican strains of Bacillus subtilis under greenhouse conditions at Irapuato, Guanajuato, Mexico.
| Treatment (Strain) | Firmness (N) | Maturity index (TSS/TA) | 1/4 of the seed cavity (cm2) |
| Control | 75.59c | 3.95b | 15a |
| BEB-23 | 122.92a | 5.34a | 11.81b |
| LAL-36 | 83.70bc | 4.44b | 13.74ab |
| BEB-22 | 98.55b | 5.21a | 12.88b |
| BEB-13 | 97.73b | 5.25a | 12.11b |
| Significance | * | * | * |
NS: Not Significant differences. (*) Different letters indicate significant differences between treatments (p≤0.05).
According to ANOVA’s results, yield as well as the fruit characteristics were improved with some B. subtilis strains, which is in agreement with what Mena-Violante and Olalde-Portugal (2007) reported, that PGPRs improved the tomato fruit size. Melon root fresh and dry weights presented the highest values with LAL-36 and BEB-22 strains, in contrast with the control and strain BEB-23 that had the lowest values in both variables. This indicated that melon plants treated with BEB-22 and LAL-36 strains developed a stronger and more functional radicle system (Alves et al., 2011). A well-developed root system is positively correlated with greater plant vigor (Andrade et al., 2000).
For the fruit weight and size, the LAL-36 strain similarly showed the highest average value compared to other treatments. These results reflect the relationship between the root development and the increased productive capacity of the melon plant. Besides, they illustrates that the strain LAL-36 can be the one that produces the most important effects among the B. subtilis strains evaluated. Similar results were reported with different strains of B. subtilis. For instance, Karlidag et al. (2007) found a strain from Bacillus genus, named M3 that, by itself or combined with other strains, had a strong positive effect on plant growth and yield. Additionally, B. megaterium increased the size of the root and shoot length in plants of rosemary (Rosmarinus officinalis), which was tested to evaluate the controlling effects of these bacteria against vascular wilting produced by Fusarium spp. (Corrales et al., 2010).
The strain LAL-36 consistently was the one that produced the greatest value with 97 760 kg ha-1, which is related to the results obtained in the root and the fresh weight of the fruit, both of which are variables that are associated with yield. Comparable results were obtained on different crops, such as peanuts (Turner and Backman, 1991) and cherries (Esitken et al., 2006). The previous results are consistent with those reported by Mena-Violante and Olalde-Portugal (2007), where tomato roots were inoculated with B. subtilis, later to find higher fruit fresh and dry weights of treated plants compared to those not inoculated. Species of the same genus Bacillus appear to have a positive effect over related variables with melon yield. For example, Rodríguez et al. (2013) found that the B. cerus inoculation increases the plant height up to 50 %, compared to other rhizobacteria species. This significant and positive effect on the morphological analyzed variables (weight, height, and radicular development), was observed not only under laboratory conditions, but also on open-field crops (Nihorimbere et al., 2010). Due to the fact that Bacillus activities, and particularly B. subtilis, concentrate in the plant radicular zone (O’Brien and Kenney, 2000), the positive results are reflected on fruit yield, since roots are fundamental for the water absorption and nutrient uptake necessary for plant development (Balaguera et al., 2008).
Significant differences were found on external rind color, TA, TSS, firmness, maturity index, and ¼ of the seed cavity characteristics among the different B. subtilis treatments. It was shown that the LAL-36 strain induced the greatest fruit size and yield compared to the rest of the evaluated strains, but its fruit firmness was similar to the control treatment, being the lowest value scored of all. BEB-23 strain produced the greatest fruit firmness compared to the other treatments. Similar results were described by Mena-Violante et al. (2009), who showed that BEB-13, -22 and -23 strains induced higher tomato fruit firmness and maturity index than the control. The application of some PGPR increased the fruit quality, and gave a better presentation, since the color components (L*, a* and b*) and sugar content were improved as well. In our study, strains BEB-13, -22 and -23 had higher statistical values in TSS compared to the control treatment, and at the same time, were highly superior to those reported for commercial varieties of melon (Nava-Camberos and Cano-Ríos, 2000). Taking into account the previously stated and that a value above 8 °Brix values were found acceptable for consumption (Tapia et al., 1998), the values reported here demonstrated that evaluated B. subtilis strains improved organoleptic properties of melon fruits adequate for quality commercialization.











 texto en
texto en 


