Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.52 no.1 Texcoco ene./feb. 2018
Crop Science
Variation of phenolic compounds, flavonoids and tannins in Vanilla planifolia jacks. ex andrews in the Huasteca hidalguense, Mexico
1Estrategias para el Desarrollo Agrícola Regional. Campus Puebla. Colegio de Postgraduados. 72760. Cholula, Puebla. (gdlandrade@hotmail.com, behc@colpos.mx, lauracaso2004@yahoo.com).
2Fruticultura. Campus Montecillo. Colegio de Postgraduados. 56230. Montecillo, Estado de México. (larevalo@colpos.mx).
Phytochemicals or secondary metabolites can be bioactive compounds, with a low molecular weight, which generally protect the plant against pests, bacteria, environmental stress, UV radiation, or other factors. In vanilla (Vanilla planifolia Jacks. ex Andrews) compounds are synthesized that show microbial inhibition against phytopathogens; however, the type of phytochemicals in the vegetative structures of this species is scarcely known. Under the hypothesis that the type and concentration of phytochemicals varies according to the plant tissue and the site of plant collection, the objective of the study was to detect the type of phytochemicals and to quantify the concentration of total phenolic compounds (TPC), total tannins (TT), condensed tannins (CT) and flavonoids (Flav) in leaf, stem, flower and cured fruit (pod) from vanilla, from three sites (Huizotlaco, Coacuilco and Contepec) of the Huasteca Hidalguense in México. The different types of metabolites were identified in methanol, chloroform and hexane extracts through thin-layer chromatography (TLC). TPC, TT, CT and Flav were quantified in the methanol extracts of the four vegetative structures through spectrophotometric methods. TLC showed the presence of Flav, saponins and terpenoids. The pod presented the highest concentration of TPC (749.608 mg 100 g-1) and TT (102.141 mg 100 g-1), and the leaf had the highest concentration of Flav (127.023 mg 100 g-1) and condensed tannins (40.992 mg 100 g-1). Among the collection sites, Coacuilco stood out for the highest concentration of Flav and TT, and Contepec for the highest concentration of TPC and CT.
Keywords: Vanilla planifolia; secondary metabolites; plant tissues; phytochemical analysis
Los fitoquímicos o metabolitos secundarios pueden ser compuestos bioactivos, con peso molecular bajo, que generalmente protegen a la planta contra plagas, bacterias, estrés ambiental, radiación UV u otros factores. En vainilla (Vanilla planifolia Jacks. ex Andrews) se sintetizan compuestos que muestran inhibición microbiana contra fitopatógenos; pero, el tipo de fitoquímicos en las estructuras vegetales de esta especie es poco conocido. Bajo la hipótesis de que el tipo y concentración de fitoquímicos varía de acuerdo con el tejido vegetal y el sitio de recolecta de la planta, el objetivo del estudio fue detectar el tipo de fitoquímicos y cuantificar la concentración de compuestos fenólicos totales (CFT), taninos totales (TT), taninos condensados (TC) y flavonoides (Flav) en hoja, tallo, flor y fruto (vaina) beneficiado de vainilla, de tres sitios (Huizotlaco, Coacuilco y Contepec) de la Huasteca Hidalguense de México. Los diferentes tipos de metabolitos se identificaron en los extractos de metanol, cloroformo y hexano mediante cromatografía en capa fina (CCF). CFT, TT, TC y Flav se cuantificaron en los extractos metanólicos de las cuatro estructuras vegetales mediante métodos espectrofotométricos. CCF mostró la presencia de Flav, saponinas y terpenoides. La vaina presentó la concentración mayor de CFT (749.608 mg 100 g-1) y TT (102.141 mg 100 g-1) y la hoja tuvo la concentración mayor de Flav (127.023 mg 100 g-1) y taninos condensados (40.992 mg 100 g-1). Entre los sitios de recolecta, Coacuilco destacó la concentración mayor de Flav y TT y Contepec por la concentración mayor de CFT y TC.
Palabras clave: Vanilla planifolia; metabolitos secundarios; tejidos vegetales; análisis fitoquímico
Introduction
Vanilla (Vanilla planifolia Jacks. ex Andrews) is an orchid native of México and Central America, which stands out because it is the only of its genus with economic importance and the second most expensive aromatic spice in the food industry, after saffron (Anilkumar, 2004). Vanilla is cultivated traditionally in the region of Totonacapan in México, which covers the states of Puebla and Veracruz, and in other regions with agroclimate conditions that are appropriate for its cultivation, in Chiapas, Oaxaca, Tabasco, San Luís Potosí and Hidalgo (Castro-Bobadilla and García-Franco, 2007).
The vegetative structures of plants contain chemical compounds that can carry out functions of protection against pathogens, UV radiation, pests and communication to attract pollinators, or others (Mazid et al., 2011; Pagare et al., 2015). In some orchids with medicinal use, numerous secondary metabolites have been identified that justify its medicinal properties, such as alkaloids, flavonoids, phenanthrenes, anthocyanins, sterols and terpenoids, particularly in the flower extracts and in the leaves (Pérez, 2010; Hossain, 2011). The Aztecs used vanilla in the treatment of hysteria, fever, impotence, and rheumatism (Bruman, 1948).
The mature vanilla fruit has been studied and used as an antioxidant, antimicrobial, anti-inflammatory, and anti-cancerous agent (Sinha et al., 2008; Shanmugavalli et al., 2009). The studies have been performed mostly in the cured vanilla fruit (Pérez-Silva et al., 2006; Sinha et al., 2008), and around 200 metabolites of the aroma have been described, identified structurally as organic acids, ethers, esters, alcohols, phenolic compounds and carbonyls (Klimes and Lamparsky, 1976). Of these compounds almost a third are volatile aromatics, among which four phenolic compounds stand out because of their high concentration and importance of the aroma: p-hydroxybenzoic acid, vanillic acid, p-hydroxybenzaldehyde and vanillin; the latter is the most abundant compound (Pérez-Silva et al., 2006; Sharma et al., 2006). There is information about the volatile metabolites in the extract of the cured fruits, but their type and quantity in other structures and their variation with the development stage of the plant and the environment are scarcely understood (Shanmugavalli et al., 2009). Sun et al. (2001) identified phenolic compounds in ethyl acetate extracts from the leaf and the stem, such as p-ethoxymethylphenol, p-butoxymethylphenol, vanillin and two identified for the first time in this species, p-hydroxy-2-methoxycynnamaldehyde and 3,4-dihydroxyphenylacetic acid, with insecticide properties. Shanmugavalli et al. (2009) mentioned that among the metabolites, particularly in the leaf, some have activity against pathogens such as Pseudomonas aeruginosa and Escherichia coli.
The hypothesis of this study was that the type and the quantity of phytochemicals varies between plant tissues and plant collection sites; the objective was to identify the profile of phytochemicals and to quantify the variation of the total phenolic compounds, total tannins, condensed tannins, and flavonoids in leaf, stem, flower and cured pod from vanilla plants collected in Huizotlaco, Coacuilco and Contepec, in the Huasteca Hidalguense, México.
Materials and Methods
Plant material collection
The plant tissues were collected in plantations in Huizotlaco, Coacuilco and Contepec in Hidalgo, México (Figure 1, Table 1). Collecting of the leaves, stems and flowers was done during the flowering stage in May 2014. The fruits of the plants from each site were collected 32 weeks after pollination and cured with the traditional process (Beneficio Primero de Mayo, Papantla, Veracruz) by the curing master Veremundo Rodríguez.
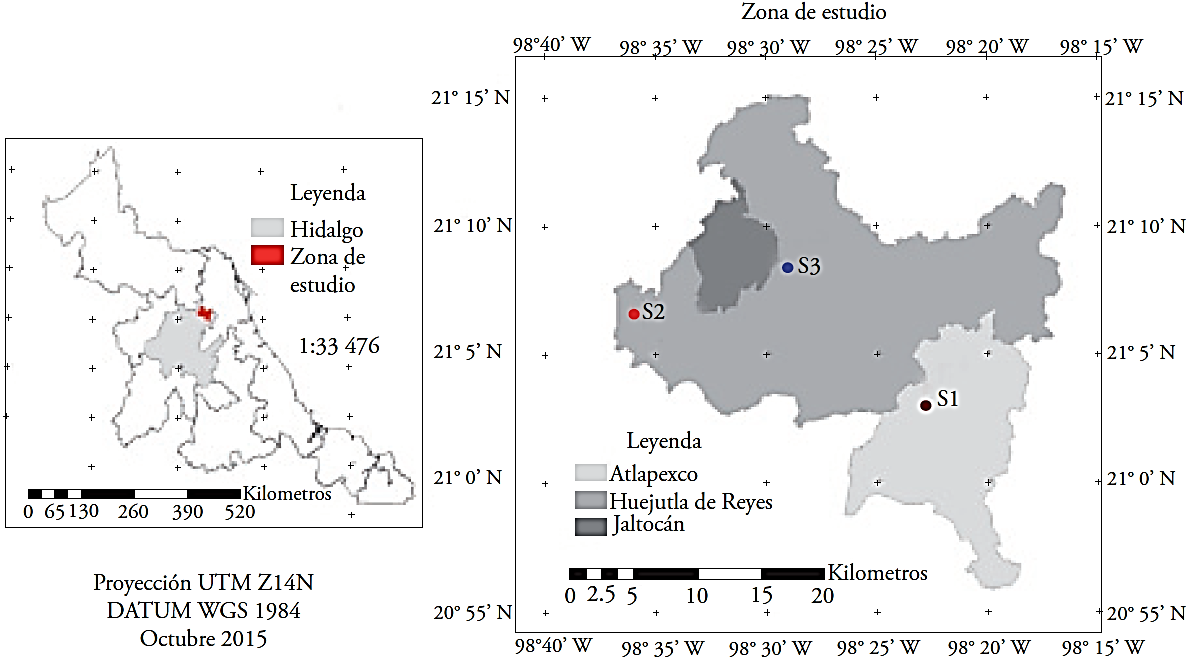
Figure 1 Geographic location of the collection sites of vanilla structures in the Huasteca Hidalguense, México. S1: Huizotlaco, S2: Coacuilco, S3: Contepec.
Table 1 Location of plant populations of Vanilla planifolia Jacks. ex Andrews in the Huasteca Hidalguense, México.
| Municipio | Localidad | Longitud (grados) | Latitud (grados) | Altitud (m) | Clima |
| Atlapexco | Huizotlaco | -98.38 | 21.05 | 285 | Am (f) Cálido húmedo, temperatura media anual mayor de 22 ºC y temperatura del mes más frío mayor de 18 ºC. |
| Huejutla | Coacuilco | -98.60 | 21.11 | 400 | A (f) Cálido húmedo, temperatura media anual mayor de 22 ºC y temperatura del mes más frío mayor de 18 ºC. |
| Huejutla | Contepec | -98.49 | 21.14 | 352 | (A)C(m)(f) Semicálido húmedo del grupo C, temperatura media anual mayor de 18 ºC, temperatura del mes más frío menor de 18 ºC. |
Quantitative analysis by thin-layer chromatography (TLC)
Preparation of extracts. From crushed fresh leaves, stems and flowers, and hexane and methanol in 1:5 proportion (tissue:solvent), extracts with different polarity were obtained (Recio-Iglesias, 1999); the extract from the cured pod was obtained only with methanol because of the low availability of the sample (Jadhav et al., 2009). The samples were placed in a sonicator (Auto Science, model A5515OB) for 30 min, at a frequency of 5.5, they were kept in maceration at room temperature for 24 h, filtered and stored in glass vials at -20 °C until the time of their analysis.
Identification of groups of compounds. 15 to 20 μL of the extracts were applied to silica gel plates 60, F254 (Sigma-Aldrich). The eluents and chromogenic agents were specific for the detection of each group of metabolite (Wagner and Bladl, 1996); in the case of flavonoids the plates were visualized with ultraviolet light (UVLMS-38 El series 3UV™ Lamp) at 365 nm.
Quantitative analysis
Leaves and stems were obtained from the superior and inferior ends and the middle region of a meter of cutting from the plant, and small pieces were sectioned, extracted with methanol and kept in the same manner as described before. The humidity content was determined for each tissue to express the concentration of the secondary metabolites based on dry matter (DM).
Total phenolic compounds. These compounds were quantified with the method proposed by Singleton et al. (1999), at 725 nm in a UV-Vis spectrophotometer (Evolution 300, Thermo Scientific). The standard curve (y=1.6571x-0.016, R²=0.9932) was prepared with gallic acid (Sigma). The results were expressed in mg gallic acid equivalent per 100 g of dry matter.
Total tannins. These compounds were quantified with the method described by Makkar et al. (1993), at 725 nm in a UV/ VIS spectrophotometer (Evolution 300 Thermo Scientific). The results were expressed in mg of tannic acid (Sigma) per 100 g of dry matter, based on the standard curve equation (y=1.6571x-0.016, R²=0.9932).
Condensed tannins. These compounds were quantified with the method proposed by Makkar et al. (1993), at 550 nm in a UV/VIS spectrophotometer (Evolution 300 Thermo Scientific). The results were expressed in mg of tannic acid (Sigma) per 100 g of dry matter, according to the following equation:
where A is absorbance, 78.26 is the correction factor, % DM is the percentage of dry matter.
Flavonoids. These compounds were quantified according to the method suggested by Chang et al. (2002) at 415 nm in a UV/ VIS spectrophotometer (Evolution 300, Thermo Scientific). The results were expressed in equivalent mg of quercetin (Sigma) per 100 g of dry matter, according to the standard curve equation (y=6.0143x-0.0084, R²=0.9984).
The curing process of fruit was carried out following the description by Xochipa-Morante et al. (2016).
Statistical analysis
Contingency tables were obtained to analyze the results of the qualitative tests by thin-layer chromatography. ANOVA was performed for the concentration of total phenolic compounds, total tannins, condensed tannins and flavonoids, from nine repetitions of leaf and stem, and four of flower and cured pod, from each collection site. The difference between means per collection and between tissues was evaluated with the Tukey test (α=0.05) with the SAS statistical package version 9.0 (SAS Institute Inc., 2002).
Results and Discussion
Qualitative analysis by thin-layer chromatography
The most abundant phytochemical groups, identified by TLC, varied between the tissues (Table 2): 1) terpenoids (17 to 20 bands) are produced commonly in vegetative tissues, flowers and occasionally roots (Dudareva et al., 2004); their presence in vanilla could be because it is the most abundant and diverse group of plant secondary metabolites in chemical structure, and their role is important in plant-insect, plant-pathogen, and plant-plant interactions (Paschold et al. 2006); 2) saponins (16 to 18 bands) are a type of terpenoid; they usually participate actively in the regulation processes of plant growth and the variations in their distribution, composition and concentration in plants is attributed to the species’ reactions to the environment (Moses et al., 2014), and 3) flavonoids (14-18 bands); their synthesis in plants is recognized as a reaction to infection from microorganisms (Dixon et al., 1983), and they help combat oxidative stress and act as growth regulators (Kumar and Pandey, 2013). In general, the leaves presented a higher diversity of metabolites (19 to 22 bands), perhaps because of their higher metabolic activity, since in addition to photosynthesis, exposure to light modulates the synthesis and presence of phytochemicals that protect plants against pathogens or herbivores; these compounds may even be deposited outside the leaves as waxes and cutin (Vivanco et al., 2005).
Table 2 Number of bands per chemical group, observed in thin-layer chromatography, in Vanilla planifolia leaf (H), stem (T), flower (F) and cured pod (Vb) from three collection sites in the Huasteca Hidalguense, México.
| Sitio de recolecta | Tejido | Flav* | Sap | Tan | Alc | CFT | Terp** | Total |
| Número de bandas | ||||||||
| Huizotlaco | H | 5 | 5 | 1 | 2 | 2 | 5 | 20 |
| T | 4 | 4 | 1 | 2 | 1 | 2 | 14 | |
| F | 5 | 4 | 1 | 2 | 1 | 4 | 17 | |
| Vb | 1 | 3 | 1 | 2 | 2 | 6 | 15 | |
| Total | 15 | 16 | 4 | 8 | 6 | 17 | 66 | |
| Coacuilco | H | 6 | 4 | 0 | 2 | 2 | 5 | 19 |
| T | 6 | 5 | 1 | 2 | 1 | 5 | 20 | |
| F | 5 | 4 | 1 | 2 | 2 | 3 | 17 | |
| Vb | 1 | 3 | 1 | 2 | 2 | 5 | 14 | |
| Total | 18 | 16 | 3 | 8 | 7 | 18 | 70 | |
| Contepec | H | 4 | 7 | 0 | 2 | 2 | 7 | 22 |
| T | 4 | 7 | 0 | 2 | 2 | 5 | 20 | |
| F | 5 | 2 | 0 | 2 | 2 | 3 | 14 | |
| Vb | 1 | 2 | 1 | 2 | 3 | 5 | 14 | |
| Total | 14 | 18 | 1 | 8 | 9 | 20 | 70 | |
*Methanol extracts of flavonoids, saponins, tannins, alkaloids and total phenolic compounds; **hexanic extract of terpenoids.
The presence of metabolites by tissue depended on the collection site. The flavonoids showed more bands (6) in leaves and stems from Coacuilco; the leaves and stems from Contepec stood out for the higher number of saponins (7 bands), terpenoids in leaf (7), and total phenolic compounds in cured pod (3). In the tissues from the three collection sites there were less bands (1-2) of tannins and alkaloids (Table 2). Shanmugavalli et al. (2009) documented similar results, since they detected traces of these two groups in leaves and stems of V. planifolia under natural conditions, in India.
Quantitative analysis
The principal groups of phytochemicals in tissues showed coefficients of variation (CV) of between 7 and 15 %, the highest corresponded to condensed tannins. In all the variables, highly significant differences were observed (p<0.0001) (Table 3).
Table 3 Mean, coefficient of variation (CV) and mean squares of phytochemical components per collection site and tissue, and their interaction in Vanilla planifolia from the Huasteca Hidalguense, México.
| Variable (mg·100 g-1 MS) | Media | CV (%) | Cuadrados medios | |||
| Sitio | Tejido | Sitio*Tejido | Error | |||
| Compuestos fenólicos totales | 302.844 | 7.580 | 44 666.961*** | 1 008 918.109*** | 7936.734*** | 526.935 |
| Flavonoides | 94.236 | 10.940 | 12 654.398*** | 16 701.632*** | 2241.404*** | 106.287 |
| Taninos totales | 56.197 | 11.021 | 12 99.700*** | 103 69.071*** | 1011.244 *** | 38.367 |
| Taninos condensados | 23.179 | 14.250 | 332.960*** | 5223.171*** | 822.119*** | 10.909 |
*** p < 0.0001 and CV: coefficient of variation.
The concentration of phytochemical components varied widely between the collection sites. The plant material from Contepec had the highest concentration of total phenolic compounds, and total tannins. The material from Coacuilco had the highest concentration of flavonoids and condensed tannins, and from Huizotlaco it presented the lowest concentration of all the components evaluated (Table 4).
Table 4 Chemical components per collection site and in Vanilla planifolia plant tissues of the Huasteca Hidalguense, México.
| Factor | Compuestos fenólicos totales | Flavonoides | Taninos totales | Taninos condensados |
| (mg·100 g-1 MS) | ||||
| Sitio de recolecta | ||||
| Huizotlaco | 252.352c | 88.168b | 50.236b | 22.897b |
| Coacuilco | 318.977b | 120.417a | 64.009a | 27.529c |
| Contepec | 335.099a | 74.915c | 54.345b | 19.654a |
| DMS | 15.952 | 7.164 | 4.119 | 2.444 |
| Tejido | ||||
| Hoja | 166.245d | 127.023a | 52.414b | 40.992a |
| Tallo | 212.372c | 64.243c | 46.250c | 28.572b |
| Flor | 298.692b | 71.026c | 41.145c | 8.336c |
| Vb | 749.608a | 111.860b | 102.141a | 0.382d |
| DMS | 21.503 | 9.632 | 5.664 | 3.202 |
Average values with the same letter in a column and per factor are not statistically different (Tukey p(0.05). Vb: cured pod and DMS: minimum significant difference.
The cured pod presented the highest concentration of total phenolic compounds (Table 4), apparently because they contribute widely (1 000 to 3 000 mg 100 g-1 DM) to the aroma of the cured pods (Shina et al., 2008) and favor the antioxidant activity of cured pod extracts (Rojas-López and Cañizares-Macías, 2013).
The leaf was the tissue with highest concentration of flavonoids, but its content was considerably less than in tea (Camelia sinensis) (611 mg 100 g-1 DM), which has high antioxidant capacity (Pereira et al., 2014) (Table 4). The cured pod showed the highest concentration of total tannins, but corresponded to less than half in some of fresh fruits, such as cranberry (233 mg 100 g-1) (Vázquez-Flores et al., 2012), or even fruits that are cured like roasted coffee (270 mg 100 g-1) (Savolainen, 1992). The leaves showed the highest concentration of condensed tannins and the stems followed; these compounds can contribute to the protection of the tissues, which like others are exposed to pathogens, herbivores and UV radiation (Brillouet et al., 2013).
The highest concentration of total phenolic compounds was observed in flowers and cured pod from Contepec, compared to the other collection sites and tissues. This agreed with the results of the aroma components (mainly phenolic compounds) in cured pods from the same collection site, which showed high concentration of vanillic acid and medium concentration of vanillin (Delgado-Alvarado et al., 2016). The leaves, stems and flowers from Coacuilco and the cured pod from Contepec showed a higher concentration of flavonoids and total tannins (Table 5). The highest concentration of condensed tannins was shown by stems from Coacuilco, leaves from Huizotlaco, and flowers and cured pod from Contepec (Table 5). These differences between collection sites can be attributed to the conditions of each site; Coacuilco is at a higher altitude compared to the other sites (Table 1), so the temperature differs and can generate stress in the plants and favor the production of these compounds.
Table 5 Concentration of total phenols (CFT), flavonoids (Flav), total tannins (TT) and condensed tannins (TC) in Vanilla planifolia tissues collected in localities of the Huasteca Hidalguense, México.
| Sitio de recolecta/ tejido | CFT | Flav | TT | TC |
| (mg·100 g-1MS) | ||||
| Huizotlaco | ||||
| Hoja | 125.591d | 122.708a | 52.326b | 46.106a |
| Tallo | 160.024c | 48.295c | 37.424c | 23.152b |
| Flor | 183.469b | 74.947b | 37.731c | 4.586c |
| Vb | 719.115a | 113.388a | 86.869a | 0.213c |
| DMS | 22.545 | 17.382 | 8.323 | 7.357 |
| Coacuilco | ||||
| Hoja | 194.510d | 143.505a | 55.525bc | 33.969b |
| Tallo | 252.850c | 120.361ab | 66.858b | 52.775a |
| Flor | 335.290b | 81.059b | 43.430c | 7.123c |
| Vb | 731.490a | 107.914c | 97.265a | 0.407d |
| DMS | 53.63 | 24.411 | 13.177 | 6.418 |
| Contepec | ||||
| Hoja | 164.500d | 108.773a | 49.390b | 42.048a |
| Tallo | 224.240c | 42.779b | 34.468c | 16.051b |
| Flor | 377.320b | 57.071c | 42.275bc | 13.30b |
| Vb | 798.220a | 114.280a | 122.290a | 0.526c |
| DMS | 30.507 | 7.059 | 8.808 | 3.872 |
Means with the same letter in each variable and for each collection site are not statistically different (Tukey p(0.05). Vb: cured pod.
Cola parchycarpa and Cola lepidota grow in different geographic and environmental conditions and the collection site determines the presence and concentration of phytochemicals in the vegetative structures (Ene-Obong et al., 2016). Also, in V. planifolia the differences between tissues can also be associated to the development stage and growth conditions of the plant, since the younger tissues tend to concentrate a higher amount of phytochemicals (Palama et al., 2010). These metabolites are usually transported from the root to the stems and the leaves, via xylem or phloem, and stored in reproductive structures such as flowers and pods (Wink and Schimmer, 2010).
Based on this, the collection region of greatest interest could be Coacuilco, because the leaves and stems collected there presented the highest concentration of total flavonoids and total tannins, and Contepec because the pods and flowers presented the highest concentration of total phenolic compounds and condensed tannins. In contrast, all the plant tissues in Huizotlaco showed the lowest concentration of secondary metabolites. These results allow suggesting the convenience of researching the effect of other factors that favor the synthesis of metabolites in the vanilla plant.
Conclusions
The concentration of phytochemical components varied in function of the tissue and the collection site of vanilla. Among the tissues, the leaf had the highest concentration of flavonoids and condensed tannins, the cured pod had the highest concentration of total phenolic compounds and total tannins. The plants collected in Coacuilco and Contepec had higher content of phenolic compounds, flavonoids and tannins (total and condensed) and those from Huizotlaco had a different profile with the lowest concentrations of the phytochemicals.
Literatura citada
Anilkumar, A.S. 2004. Vanilla cultivation: a profitable agri-based enterprise. Kerala Calling: 26-30. [ Links ]
Brillouet, J.-M., C. Romieu, B. Schoefs, K. Solymosi, V. Cheynier, H. Fulcrand, J. Verdeil, and Conéjéro, G. 2013. The tannosome is an organelle forming condensed tannins in the chlorophyllous organs of Tracheophyta. Ann. Bot. 112: 1003-1014. [ Links ]
Bruman, H. 1948. The culture history of Mexican vanilla. Hisp. Am. Hist. Rev. 28: 360-376. [ Links ]
Castro-Bobadilla, G. and J. G. García-Franco. 2007. Vanilla (Vanilla planifolia Andrews) crop systems in the Totonacapan area of Veracruz, Mexico: Biological and productivity evaluation. J. Food Agric. Environ. 5: 136-139. [ Links ]
Chang, C., M. Yang, H. Wen, and J. Chern. 2002. Estimation of total flavonoids content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 10: 176-182. [ Links ]
CONABIO (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad). 2012. Portal de Geoinformación. Disponible en: Disponible en: http://www.conabio.gob.mx/informacion/gis/ Fecha de consulta: Agosto 2015. [ Links ]
Delgado-Alvarado, A., G. Andrade-Andrade, B. H. Herrera-Cabrera, M. L. Arévalo-Galarza. 2016. Perfil del aroma de vainilla beneficiada (Vanilla planifolia Jacks. ex Andrews) de la Huasteca hidalguense, México. Agroproductividad 9 (suplemento):15-16 [ Links ]
Dixon R. A., P. M. Dey, and C. J. Lamb. 1983. Phytoalexins: enzymology and molecular biology. Adv. Enzymol. Relat. Areas Mol. Biol. 55:1-136. [ Links ]
Dudareva, N., E. Pichersky, J. Gershenzon. 2004. Biochemistry of plant volatiles, Plant Physiol. 135: 1893-1902. [ Links ]
Ene-Obong, H. N., H. O. Okudu, and U. V. Asumugha. 2016. Nutrient and phytochemical composition of two varieties of Monkey kola (Cola parchycarpa and Cola lepidota): An underutilized fruit. Food Chem. 6: 194-203. [ Links ]
Hossain, M. M. 2011. Therapeutic orchids: traditional uses and recent advances-an overview. Fitoterapia 82: 102-140. [ Links ]
Jadhav, D., B. N. Rekha, P. R. Gogate, and V. K.Rathod. 2009. Extraction of vanillin from vanilla pods: A comparison study of conventional soxhlet and ultrasound assisted extraction. J Food Eng. 93: 421-426 [ Links ]
Klimes, I., and D. Lamparsky. 1976. Vanilla volatiles -a comprehensive analysis. Int. Flavours Food Addit. 7:272-291. [ Links ]
Kumar, S., and A. K. Pandey. 2013. Chemistry and biological activities of flavonoids: An overview. Scientific World J. 2013:1-16. [ Links ]
Makkar, H. P. S., M. Blummel, N. K. Borowy, and K. Becker. 1993. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food. Agric. 61: 161-165. [ Links ]
Mazid, M., T. A. Khan, and F. Mohammad. 2011. Role of secondary metabolites in defense mechanisms of plants. Biol. Med. 3: 232-249. [ Links ]
Moses, T., K. K. Papadopoulou, and A. Osbourn. 2014. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit. Rev. Biochem. Mol. Biol. 49: 439-462. [ Links ]
Pagare, S., M. Bhatia, N. Tripathi, S. Pagare, and Y. K. Bansal. 2015. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 9: 293-304. [ Links ]
Palama, T. L., I. Fock, Y. H. Choi, R. Verpoorte, and H. Kodja. 2010. Biological variation of Vanilla planifolia leaf metabolome. Phytochemistry. 71: 567-573. [ Links ]
Paschold, A., R. Halitschke, and I. T. Baldwin. 2006. Using ‘mute’ plants to translate volatile signals. Plant J. l45: 275- 291. [ Links ]
Pereira, V. P., F. J. Knor, J. C. R. Vellosa, and F. L. Beltrame. 2014. Determination of phenolic compounds and antioxidant activity of green, black and white teas of Camelia sinensis (L) Kuntze, Theaceae. Rev. Bras. Med. Campinas. 16: 490-498. [ Links ]
Pérez G., R. M. 2010. Orchids: A review of uses in traditional medicine, its phytochemistry and pharmacology. J. Med. Plants Res. 4: 592-638. [ Links ]
Pérez-Silva, A., E. Odoux, P. Brat, F. Ribeyre, G. Rodríguez-Jiménez, V. Robles-Olvera, M. A. García-Alvarado, and Z. Günata. 2006. GC-MS and GC-olfactometry analysis of aroma compounds in a representative organic aroma extract from cured vanilla (Vanilla planifolia G. Jackson) beans. Food Chem. 99: 728-735. [ Links ]
Recio-Iglesias, M. C. 1999. Métodos generales de extracción y purificación de principios activos de drogas. In: Farmacognosia general. Ed. A.M. Villar del Fresno, Madrid, pp: 83-98. [ Links ]
Rojas-López, A. and M. Cañizares-Macías 2013. Antioxidant Capacity in Vanilla Extracts Obtained by Applying Focused Microwaves. Food Nutr. Sci. 4: 244-253. [ Links ]
SAS Institute Inc. 2002. SAS/STAT® 9.0. User’s guide. Cary, NC. SAS Institute Inc. 421 p. [ Links ]
Savolainen, H. 1992. Tannin content of tea and coffee. J. Appl. Toxicol., 12: 191-192. [ Links ]
Shanmugavalli, N., V. Umashankar, and Raheem. 2009. Antimicrobial activity of Vanilla planifolia. Indian J. Sci. Technol. 2: 37-40. [ Links ]
Sharma, A., S. C. Verma, N. Saxena, N. Chadda, N. P. Singh, and A. K. Sinha. 2006. Microwave and ultrasound assisted extraction of vanillin and its quantification by high performance liquid chromatography in Vanilla planifolia. J. Sep. Sci. 29: 613-619. [ Links ]
Singleton, V. L., R. Orthofer, R. M. Lamuela-Raventos. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method. Enzymol. 299: 152-178. [ Links ]
Sinha, A. K., U. K. Sharma, and N. Sharma. 2008. A comprehensive review on vanilla flavor: extraction, isolation and quantification of vanillin and others constituents. Int. J. Food Sci. Nutr. 59: 299-326. [ Links ]
Sun, R., J. N. Sacalis, C. K. Chin, and C. C. Still. 2001. Bioactive aromatic compounds from leaves and stem of Vanilla. J. Agric. Food Chem. 49: 5161-5164. [ Links ]
Vázquez-Flores, A. A., E. Álvarez-Parrilla, J. A. López-Díaz, A. Walll-Medrano, y L. A. de la Rosa. 2012. Taninos hidrolizables y condensados: naturaleza química, ventaja y desventajas. Tecnociencia Chihuahua 6: 84-93. [ Links ]
Vivanco, J. M., E. Cosio, V. M. Loyola-Vargas, y H. E. Flores. 2005. Mecanismos químicos de defensa en las plantas. Investigación Ciencia 341: 68-75. [ Links ]
Wagner, H., and S. Bladt. 1996. Plant Drug Analysis: A thin Layer Chromatography Atlas. 2d. Ed. Springer Verlag. New York. [ Links ]
Wink, M., and O. Schimmer. 2010. Introduction. In: M. Wink. Annual plant review. Function and biotechnology of plant secondary metabolites. Wiley-Blackwell, United Kingdom, pp: 1-16. [ Links ]
Xochipa-Morante, R. C., A. Delgado-Alvarado, B. E. Herrera-Cabrera, J. S. Escobedo-Garrido, y L. Arévalo-Galarza. 2016. Influencia del proceso de beneficiado tradicional mexicano en los compuestos del aroma de Vanilla planifolia Jacks. ex Andrews. Agroproductividad 9: 55-62. [ Links ]
Received: October 2016; Accepted: April 2017











 texto en
texto en 


