Introduction
Apuleia leiocarpa (Vogel) J. F. Macbride is a tree species of the Fabaceae family native to Atlantic Rainforests and extensively distributed in Brazil. It also occurs naturally in Peru (Backes and Irgang, 2004), Uruguay (Sobral et al., 2006), northeastern Argentina (Martinez-Crovetto, 1963), southern Bolivia and eastern Paraguay (Lopez et al., 1987). It produces wood of excellent quality and high economic value, which is used for external structures, construction and carpentry (Carvalho, 2003). Thus, despite its broad natural distribution, the interest of the wood industry on harvesting A. leiocarpa, places the species at high risk of extinction (Rio Grande do Sul, 2014).
Production of apuleia seedlings is limited by some of its inherent characteristics, such as irregular fruiting overtime and seeds with tegument impermeability to water, which can delay germination (Carvalho, 2003). In addition to seminiferous reproduction, most forest species are heterozygous at many loci and highly heterogeneous populations, because of the process of plant reproduction by allogamy. An alternative option used in forestry to obtain superior and homogeneous reproductive materials for forest plantations is vegetative propagation (Xavier et al., 2013), which justifies the higher cost of clonal seedlings. Studies aimed toward finding alternative production technologies are important and micropropagation may be a good option, since it is successfully applied to a number of tree species (Erig and Schuch, 2005).
Adventitious shoots produced in the multiplication stage in micropropagation can be rooted in vitro or ex vitro; however, the tissue capacity for root formation depends on several endogenous and / or exogenous factors and their interaction (Rocha et al., 2008). The type of explant influences adventitious rooting responses of micropropagated plants, and its selection is based on seeking vegetative propagules that can provide better response to in vitro culture (Xavier et al., 2013).
In vitro rooting is characterized by induction of adventitious roots in elongated shoots, kept in culture medium supplemented with auxins, in order to obtain complete plantlets that will be subsequently acclimatized (Oliveira et al., 2013). For ex vitro rooting, explants might be treated with auxins and planted in substrate for root formation concomitant to acclimatization. Indolebutyric acid (IBA) has shown high effectiveness in promoting adventitious roots on plantlets of forest species (Xavier et al., 2013).
Acclimatization is essential to obtain new plantlets (Wendling et al., 2006) and consists of transferring complete plants produced in vitro to an external environment. This process should be modified gradually and with care. To minimize stress at this stage and maximize survival, it is important to manage adequately air relative humidity, substrate conditions, temperature and light (Oliveira et al., 2013). The substrate used during acclimatization should present adequate porosity and high water retention capacity (Girardi and Pescador, 2010), so the choice of substrate is determinant for effective acclimatization in micropropagated plantlets (Skrebsky et al., 2006).
The hypothesis for this study was that the type of explant, concentration of IBA applied and substrate composition influences the rhizogenic process and acclimatization. The objetive of this study was to evaluate the effect of type of explants and concentration of IBA on in vitro rooting and influence of type of explants and substrate composition on ex vitro rooting and acclimatization of apuleia micropropagated plantlets.
Materials and Methods
The experiments were conducted from September 2012 to February 2013 in the Department of Plant Sciences of the Federal University of Santa Maria in Brazil. In experiments using substrate, one sample of each composition was submitted to physical analysis, according to the method described in Norm nº 14 of the Brazilian Ministry of Agriculture, Livestock and Food Supply to obtain data of dry density, aeration porosity and water retention capacity under suction of a 10 cm water column.
Explants for in vitro and ex vitro experiments
Aseptic seedlings, obtained from the in vitro germination of A. leiocarpa seeds, from three mother plants, were established in Wood Plant Medium (WPM) (Lloyd and McCown, 1980) for 15 d (Figure 1A). The aerial part the seedlings were sectioned into nodal segments (Figure 1B), which were kept for 30 d in WPM medium supplemented with 8.8 μM of benzylaminopurine (BAP), and then transferred to BAP-free medium for another 30 d. The shoots formed by the end of this period (Figure 1C) were sectioned, again into nodal segments, with only one-bud and used for in vitro and ex vitro rooting (Figure 1D). The root system and the aerial part remaining in the aseptic seedlings, called microstumps, were maintained in WPM medium supplemented with 8.8 μM BAP for 30 d and then transferred to BAP-free medium where they remained for another 30 d (Figure 1E). The shoots formed from the microstumps (Figure 1F) were sectioned into one-bud micro-cuttings, which were also used for in vitro and ex vitro rooting experiments (Figure 1G). Thus, the explants differed in origin. Nodal segments were produced from the aerial segments, whereas micro-cuttings were produced from microstumps formed from the root system and the remaining aerial part.
In vitro rooting and acclimatization
For in vitro rooting, nodal segments and one-bud micro-cuttings, with 1.0 to 1.5 cm in length, were grown in glass culture tubes (50 mL) containing approximately 10 mL WPM medium supplemented with 30 g L-1 sucrose, 6 g L-1 agar, 0.1 g L-1 inositol and 1.5 g L-1 activated charcoal. Culture media was sterilized by autoclaving during 20 min at 121 °C and 1 atm pressure. The pH was adjusted to 5.8 before autoclaving and all cultures were maintained in a growth room at temperature of 25±2 °C on a 16 h photoperiod under light intensity of 14.3 μE m-2 S-1 supplied by fluorescent lamps. Indolebutyric acid (IBA) concentrations of 0, 4.9, 9.8, 14.7 and 19.6 μM were tested. The experiment was a factorial 2x5 (type of explant and IBA concentration), with five replications of four explants. Survival and rooting percentages and number and length (cm) of roots were evaluated after 60 d in culture.
For acclimatization, the explants rooted in vitro in five concentration of IBA were removed from the glass culture tubes. The roots were washed in running water and randomly transferred to individual plastic pots (330 mL), with approximately 110 g substrate; this was previously sterilized by autoclaving for 1 h. Equal proportions of commercial substrate + vermiculite + coarse sand and commercial substrate + vermiculite were evaluated. In all treatments, pine bark commercial substrate (H.DECKER®), medium vermiculite (particles between 0.50 and 1.19 mm diameter) and coarse sand (particles between 1.0 and 3.0 mm diameter) were used. The containers were kept in a growth room in plastic trays (55 x 34 x 15 cm) covered with transparent PVC film. Irrigation was applied daily using distilled water to maintain substrate humidity. Once a week, irrigation applied contained nutrient solution with (mmol): 0.61 potassium nitrate, 1.0 calcium nitrate, 1.67 magnesium sulfate, 0.14 ammonium nitrate, 0.14 potassium monophosphate and 0.16 5% chelated iron. The micronutrients were added to the nutrient solution in a prepared solution (mmol): 0.015 sodium molybdate, 0.089 boric acid, 0.125 copper sulfate, 0.123 manganese sulfate and 0.028 zinc sulfate. The experiment was a complete random design, with two treatments and eight replications of four containers with one plantlet. After 30 d of growth, survival percentage, height of the aerial part (cm) and number of leaves were evaluated.
Ex vitro rooting and acclimatization
Nodal segments and one-bud micro-cuttings (Figure 1), with 1.0 to 1.5 cm length, excised from shoots produced during in vitro multiplication, were put with the base immersed in a solution with 0 or 4920 μM IBA, for 10 s. Then they were grown in plastic containers (50 mL) containing approximately 20 g of substrate, previously sterilized by autoclaving for 1 h. Equal proportions of the compositions of substrate were tested: commercial substrate + vermiculite + coarse sand, commercial substrate + vermiculite, and commercial substrate + coarse sand. Pine bark commercial substrate and medium grain vermiculite were used. The containers were kept in trays covered by transparent PVC film for 7 d in a room at 25±2 °C and 16 h photoperiod under 14.3 μE m-2s-1 light intensity supplied by fluorescent lamps. Two irrigations with distilled water were applied daily to maintain substrate humidity. Then, the cultures were transferred to a moist chamber in an acclimatized greenhouse where they were grown for 45 d. The experimental design was complete random with a factorial arrangement 2 x 2 x 3 (explant type, IBA concentration and substrate composition), with five replication of five containers with one explants. After 7, 15, 30, and 45 d in a moist chamber, survival and rooting percentage and number and length (cm) of roots were evaluated in the explants.
The percentage data were transformed by the arc-sine function of
Results and Discussion
In vitro rooting and acclimatization
For in vitro rooting, there was no interaction between explant type and IBA concentration for all variables analyzed at 60 d. For survival, rooting and root length, there were differences for explant type, with the best results found in nodal segments (97.5 % survival, 28.9 % rooting and 6.2 cm mean root length) as compared to micro-cuttings (Table 1). Both nodal segments and micro-cuttings are originated from pre-formed meristematic organs (axillary bud), which generally present satisfactory growth and uniformity (Watt et al., 2003). We believe that the different responses among the explant types do not derive from differing morphogenic competences, since they are formed from the same meristematic tissue, but rather because they present differing endogenous concentrations of phytohormones. Auxin synthesis takes place in the plant apex and presents unidirectional movement in the parenchymatous tissues toward the root apex (Taiz and Zeiger, 2013). Because of this the greater rooting capacity in the A. leiocarpa nodal segments may be related to a higher quantity of endogenous auxin in comparison to that of the micro-cuttings, since this phytohormone acts as a cell signaler in rooting. Contrasting from our results, Pimentel et al. (2016) found that micro-cuttings explants of Handroanthus heptaphyllus showed better in vitro rooting response compared to nodal segments.
Table 1 Survival and rooting percentages and number and length of roots formed from nodal segments and micro-cuttings of Apuleia leiocarpa grown in vitro for 60 days in WPM medium with five concentrations of IBA.

† Values within the same factor with different letter are statistically different (p≤0.05. SE: Standard error; CV: Coefficient of variation
IBA concentrations did not influence the evaluated variables, except for root length, where the best results were observed in explants grown in IBA-free medium or with 9.8 μM IBA (Table 1). Many forest species present difficulties of adventitious rooting even in culture medium supplemented with auxins, indicating that other factors influence the differentiation of the tissues during this process (Souza and Junghans, 2006). In addition, it is possible that the concentrations of IBA used were not adequate to favor in vitro rooting of apuleia. Inefficient in vitro rooting was also observed inLavandula angustifolia(Miller) treated with 0.5, 1.0 and 2.0 μM of IBA (Machado et al., 2013).
In the acclimatization of A. leiocarpa plantlets produced in vitro, the substrate compositions evaluated did not affect survival, height of the aerial part and number of leaves, with a mean of 73.4 % surviving plantlets after 30 d of growth (Table 2). In the acclimatization of Cydonia oblonga Mill. micropropagated plantlets, there was 81.8 % survival at 30 d when commercial substrate and vermiculite (v/v 1:1) were used (Erig et al., 2004). Stryphnodendron adstringens Mart. Coville plantlets obtained from in vitro rooting presented 41 % survival when acclimatized in commercial substrate and vermiculite (v/v 1:1), at 30 d of growth (Nicioli et al., 2008). The combination of soil and vermicomposting (v/v 3:1) resulted in 80 % survival of Azadirachta indica A. Juss plantlets after 30 d of acclimatization (Reddyet al., 2006). An average survival of 73 % was observed in Pterocarpus santalinus L. plantlets during acclimatization in a combination of organic manure and sand (v/v 1:1) (Balaraju et al., 2011). In our study, the acclimatization conditions provided during the transition from heterotrophic to autotrophic state may have been adequate to favor survival of plantlets.
Table 2 Survival percentage, height of aerial part and number of leaves of Apuleia leiocarpa plantlets rooted in vitro and acclimatized for 30 days in growth room.

†Values with different letter are statistically different (p≤0.05). SE: Standard error; CV: coefficient of variation
¶Equal proportions (v/v) of commercial substrate (CS), vermiculite (V) and coarse sand (S)
Ex vitro rooting and acclimatization
There was no significant interaction between explant types, IBA concentration or substrate for all variables analyzed at 7, 15, 30, and 45 d. Root formation occurred only at 30 d of growth; however, without differences among explant types, IBA concentration or substrate for any of the variables analyzed. At 45 d of evaluation, survival was affected only by explant type, with the best response found in nodal segments when compared to micro-cuttings (Table 3). For ex vitro rooting, the best responses were in the nodal segments (14.6 % rooting), 4,920 μM IBA (12 % of rooted explants) and in commercial substrate + vermiculite+ coarse sand (14 % rooted explants) (Table 3).
Table 3 Survival and rooting percentage of Apuleia leiocarpa nodal segments and micro-cuttings treated with IBA and grown ex vitro for 45 days in different substrates.
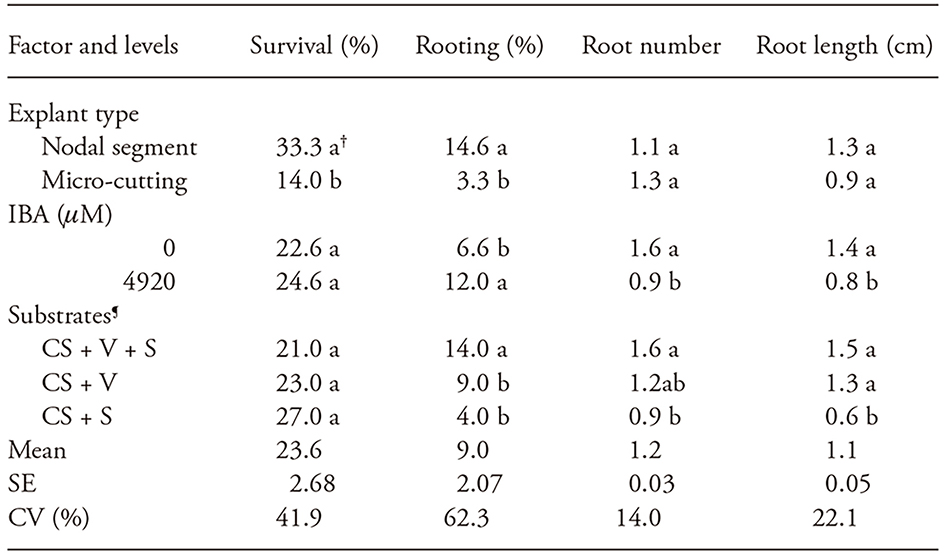
†Values with different letter are statistically different (p≤0.05). SE: Standard error; CV: coefficient of variation
¶Equal proportions (v/v) of commercial substrate (CS), vermiculite (V) and coarse sand (S)
Despite the small number of experimental units evaluated in our study, these results suggest that nodal segments respond better to ex vitro rooting when compared to micro-cuttings. In addition, for ex vitro rooting in Apuleia leiocarpa, IBA use is necessary, contrary to findings for in vitro rooting. In shoots obtained from in vitro multiplication of Tectona grandis L. treated with 4,920 μM IBA and grown in commercial substrate or vermiculite 100 % ex vitro rooting was observed at 30 d of growth, whereas in those not treated with IBA, 85 % rooting was observed in vermiculite and 95 % rooting in commercial substrate (Fermino et al., 2011). Micro-cuttings of T. grandis treated with 2460 μM of IBA resulted in 85 % rooting in vermiculite at 30 d (Ramesh et al., 2009). Micro-cuttings of Dalbergia sissoo Roxb. treated with 984 μM IBA presented a 90 % of ex vitro rooting in a commercial peat, perlite and vermiculite (Vibha et al., 2014).
For root number and length, there were IBA concentration and substrate composition effects at 45 d of growth (Table 3). The greatest number and length of roots were obtained in cultures not treated with IBA, with averages of 1.6 and 1.4 cm, or grown in commercial substrate + vermiculite + sand or in commercial substrate+ vermiculite (Table 3). Based on physical analysis, the composition of equal proportions of commercial substrate + vermiculite + sand presented dry density of 770.7 kg m-3, aeration porosity of 25.3 % and water retention capacity of 38.7 %. Whereas commercial substrate + vermiculite presented 277.8 kg m-3 dry density, 19.9 % aeration porosity and 57.2 % water retention capacity and commercial substrate + sand presented dry density of 997.1 kg m-3, 29.6 % aeration porosity and 30.9 % water retention capacity. In commercial substrate + vermiculite + sand and commercial substrate + sand, higher dry density was reported in comparison to reference values between 400 and 500 kg m-3 (Bunt, 1973). This result may be explained by the presence of sand in these mixtures, which is a highly dense component. The three combinations of substrates tested in our study presented adequate aeration porosity, within the range recommended by De Boodt and Verdonck (1972) between 20 and 40 %. For production of Sesbania virgata Cav. Pers seedlings, aeration porosity of up to 25 % increased root production after 150 d (Delarmelina et al., 2014).
Higher water retention capacity values were observed in the commercial substrate + vermiculite + sand and commercial substrate + vermiculite compositions, which may be related to the lower proportion of sand in comparison to commercial substrate + sand. Thus, the greater Apuleia leiocarpa explants rooting in commercial substrate + vermiculite + sand (Table 3) may be associated to higher water retention capacity provided by this substrate composition.
The results of our study demonstrate that Apuleia leiocarpa is a species with difficult rooting in vitro and ex vitro considering that the highest rooting percentages were 28.9 % and 14.0 %, respectively. In both in vitro and ex vitro rooting, nodal segments presented higher rooting capacity when compared to micro-cuttings. However, even though there was significantly higher rooting in nodal segments, both explants presented rooting in vitro. In addition, considering that maintenance of microstumps may be an important strategy for increasing multiplication rate in A. leiocarpa, additional studies should be performed to find solutions to minimize these differences. In relation to the use of auxin, the concentrations of IBA evaluated did not alter rooting percentage in vitro, but did favor ex vitro rooting. In ex vitro rooting, 14% of the explants rooted in commercial substrate + vermiculite + sand, showing adjustments are needed to better define ideal conditions for ex vitro rooting and acclimatization of A. leiocarpa plantlets. In addition, these plantlets can be utilized in micro-clonal hedges (for plantlet production by micro-cuttings), which would make their mass production technically viable.
Conclusión
Use of IBA is not necessary for in vitro rooting of Apuleia leiocarpa nodal segments and micro-cuttings and acclimatization was not affected by substrate compositions. For ex vitro rooting and acclimatization of A. leiocarpa plantlets, nodal segments can be treated with 4920 μM IBA and grown in commercial substrate + vermiculite +coarse sand, but further adjustments of these and other factors are required to increase rooting percentage and survival of plantlets after the acclimatization period.











 text in
text in 



