Introduction
he commercial cultivation of strawberry (Fragaria x ananassa) in Mexico began in the late 1940s in the state of Guanajuato and in the 1950s in the crop extended to the state of Michoacan in the Zamora Valley, and later to other regions such as Panindicuaro and Maravatio. Production of this crop has expanded significantly in the north, especially in the state of Baja California (Medina y Aguirre, 2004; Sánchez, 2008).
Mexico grows more than 9000 ha of strawberry and more than 50 % of this crop is grown in the state of Michoacan, mainly in the valleys of Zamora-Jacona and Maravatío (SIAP, 2014). Among the most important diseases is gray mold caused by the fungus Botrytis cinerea, which can cause severe losses when conditions favor disease development, especially if they coincide with the flowering period (Sutton, 1998). Given the national and international increase in demand for this berry, the cultivated area has increased in recent years. The advancement in technology for strawberry production has also favored an increase in the intensity of fungicide applications to manage the gray mold, because the geographic area for strawberry production has favorable conditions for the disease development.
Cultural management of the disease is based on the establishment of healthy plants and crop debris removal. However, chemical fungicides, especially those with a specific mode of action, provide a better disease management. Among these fungicides, iprodione (dicarboximide), thiophanate-methyl (benzimidazole), pyraclostrobin and azoxystrobin (QoI), fenhexamide (hydroxianilide), cyprodinil + fludioxonil (phenylpyrrole + anilinopyrimidine) and pyrimethanil (anilinopyrimidine) are classified as medium-high risk fungicides for the selection of resistance in B. cinerea (FRAC, 2008).
Resistance of B. cinerea to single-site fungicides with different modes of action is documented, even shortly after the development and use of these fungicides in the late 1960s (Leroux, 2007). There are reports about development of resistance of B. cinerea to benzimidazole and dicarboximide fungicides (Yourman and Jeffers, 1999; Lennox and Spotts, 2003; Li et al., 2007; Leroux, 2007; Banno et al., 2008), as well as to QoI (Quinone Outside Inhibitors), anilinopyrimidines, hydroxianilides and phenylpyrroles (Myresiotis et al., 2007; Grabke et al., 2014). Applying different discriminatory doses against strawberry isolates from California (USA), Mercier et al. (2010) found that 92 % of the strawberry isolates were resistant to thiophanate-methyl, 25 % to fenhexamide, 28 % to cyprodinil + fludioxonil and 66 % to pyraclostrobin + boscalid. Out of the active ingredients tested, 85 % of the isolates were resistant to at least two of them. Similarly, in a study with 353 blueberry, raspberry and currant isolates of B. cinerea in Germany, 40.5 % of the isolates were highly resistant to thiophanate-methyl, 64 % to iprodione, 45 % to fenhexamide, 76.8 % to trifloxystrobin, 21 % to boscalid and 14 % to cyprodinil. In addition, 18 % of the isolates were resistant to the five tested active ingredients, and between 6 % and 23 % of the isolates were highly or moderately resistant to one of the five tested active ingredients (Weber, 2011). In Florida (USA), resistance to multiple fungicides was reported in B. cinerea, frequently associated with subpopulations that are resistant to three fungicides (boscalid-QoI-anilinopyrimidines or boscalid-QoI-hydroxianilides), revealing the wide distribution of B. cinerea resistance to multiple fungicides that contain the most common point mutations associated with each of these chemical groups (Amiri et al., 2014). Similar results were obtained in isolates from the Carolinas (USA), where 66.7 % of the collected B. cinerea isolates were resistant to pyraclostrobin and boscalid-pyraclostrobin, in most cases related to the G143A mutation conferring high levels of resistance to strobilurin and H272R and H272Y to boscalid (Fernández-Ortuño and Schnabel, 2012).
Benzimidazole fungicides are systemic, broad-spectrum fungicides used commercially for controlling plant diseases since the early 1960s and include benomyl, carbendazim, thiabendazole and thiophanate-methyl (Leroux, 2007). These fungicides inhibit mycelial growth and cause distortions of germ tubes, interfering with the polymerization of the β-tubulin protein to inhibit cell division. Resistance is the result of at least three point mutations, including those arising at positions 198 and 200 of the β -tubulin gene (Yarden and Katan, 1993; Leroux, 2007; Banno et al., 2008).
Dicarboximide fungicides inhibit conidial germination but mainly act by inhibiting mycelial growth (Leroux, 2007). Point mutations were identified in the MAP/histidine kinase gene, which is associated with osmotic signal transduction, conferring different degrees of fungicide resistance (Cui et al., 2002; Oshima et al., 2002; Oshima et al., 2006; Leroux et al., 2002; Cui et al., 2004). In addition, the gene encoding a class III histidine kinase (BOS1) was sequenced from dicarboximide-susceptible and resistant isolates in which two types of mutations are detected: a mutation at position 365 where serine replaced isoleucine (I365N) and one at position 369 where proline replaced glutamine (Q369P) (Ma et al., 2007). However, studies using isolates from North Carolina, South Carolina and Florida indicate that moderately resistant isolates are associated with the N373S and Q369P B. cinerea mutations, while those with low resistance are associated with I365S or I365N, but no specific mutation was associated with highly resistant isolates, suggesting the existence of other mutations that confer this phenotype (Grabke et al., 2014).
Strawberry producers from the state of Michoacan noticed a lack of efficacy of commonly used fungicides in the management of gray mold. Some of the active ingredients of these fungicides have been on the market for more than 30 years, as is the case for benzimidazole and dicarboximide. The sensitivity status of B. cinerea to these fungicides is unknown, but it is likely that the resistance of this pathogen to these group of fungicides is widely distributed in this strawberry production area.
The objective of this research was to determine the distribution of B. cinerea sensitivity in isolates from two major strawberry producing areas in the state of Michoacan (Maravatio and Zamora-Jacona valleys) to the fungicides thiophanate-methyl (benzimidazole) and iprodione (dicarboximide). Knowing the sensitivity status of this fungus in these producing areas will directly impact the production costs for producers, reducing the selection pressure on fungi populations and providing a basis for the design of fungicide resistance management programs to maximize the effectiveness of these tools in an integrated disease management approach.
Material and Methods
Sampling sites
The isolates of B. cinerea were collected from strawberry fruits with symptoms of gray mold and leaves in the Maravatío (19° 52’ 44.2092’’ N, 100° 24’ 29.595’’ W) and Zamora-Jacona (19° 59’ 9.0168’’ N, 102° 18’ 58.9608’’ W) valleys during the 2009 and 2010 growing seasons.
Monosporic isolates
Small pieces of diseased tissue from strawberry fruits were disinfested with a solution of 1 % sodium hypochlorite for 2 min, rinsed with sterile distilled water, blotted dry in the hood for 10 min and placed in Petri dishes containing PDA medium (Difco Lab. Sparks, MD, USA). The plates were incubated under fluorescent light for 5 d at room temperature (22±2 °C) to promote sporulation. From sporulated cultures, a suspension of 1x103 conidia mL-1 was prepared, and 0.1 mL suspension was placed on Petri dishes with water agar (2 %) medium and incubated at room temperature for 6 h. After incubation, the germinated conidia were isolated and placed individually in Petri dishes with PDA medium. Once the cultures covered the plates, they were stored at 4 °C for use in the sensitivity tests. Sixty two monosporic isolates (Table 1) were obtained.
Morphological and phylogenetic identification
The preliminary identification of the isolates was performed based on colony color, conidia morphology and conidiophores (Elad et al., 2007). To confirm the identification, eight randomly selected isolates were obtained from the field collections listed in Table 1 (Z7A-1-3, Z7A, Z16C-2-3, Z14A, Z14A-2, Y-1, Z7C and Z7C-2) and the isolates were grown on PDA at room temperature (22±2 °C) for 7 d. The phylogenetic identification was performed via the PCR amplification of the ITS region of ribosomal DNA using the primers ITS4 and ITS5 (White et al., 1990). A micropipette was used to place a portion of mycelium of each isolate in Eppendorf tubes with 30 µL of a lysis solution developed at the Colegio de Posgraduados (Silva-Rojas, personal communication, 2009). The tubes were placed on a Peltier Thermal Cycler PTC 200 (Bio-Rad, USA) at 95 °C for 5 min and centrifuged at 12 000 x g for 5 min. In new tubes, 45 µL of HPLC water for PCR and 5 µL of supernatant were centrifuged for 2 min. From this solution, 5 µL of each DNA solution from each isolate was used as template DNA. The mixture reaction was prepared in a final volume of 25 µL containing 2 U of Taq DNA polymerase, 10X Taq DNA polymerase buffer, 10 pM of each primer and 200 mM dNTPs (Promega, USA). The amplification program was as follows: one cycle of initial denaturation at 95 °C for 4 min, followed by 35 cycles with denaturation at 95 °C for 2 min, one cycle at 56 °C for 1 min and 72 °C for 2 min; and a final extension cycle at 72 °C for 7 min.
The PCR products were verified by agarose gel electrophoresis using 1.5 % 1X TAE buffer (Tris-Acetate-EDTA) at 87 V cm-1 for 1 h. The gel was stained with ethidium bromide and the bands were visualized on the Infinity 1000/26MX Xpress (Vilber Lourmat, Germany) photo documentation system. Before sequencing, the PCR products were purified using ExoSAP-IT (Affymetrix, USA) following the manufacturer’s instructions. The sequencing reaction was prepared using the Big Dye Terminator v3.0 (Applied Biosystems, USA), and the fragments were resolved by capillary electrophoresis on the Genetic DNA Analyzer Model 3730 (Applied Biosystems, USA). The regions corresponding to both sequences were assembled and edited with the BIOEDIT v7.2.0 software (Hall, 1999). The consensus sequences of each isolate were compared with those in the GenBank database, NCBI (https://www.ncbi.nlm.nih.gov), with the option BLASTN 2.2.19 (Zhang et al., 2000).
Fungicides
Cercobin M® (79 % active ingredient, BASF Mexicana, Zapopan, Jalisco, Mexico) and Rovral 50 PH® (50 % active ingredient, Bayer Crop Science in Mexico, DF) were used as sources of the active ingredients thiophanate-methyl and iprodione, respectively.
Inhibition assay of mycelial growth
The PDA culture medium was prepared and kept in a water bath at 60 °C until use. Separately, a stock solution of 10 000 µg mL-1 AI of each fungicide was prepared, and serial dilutions of 1:10, 1:100 and 1:1000 were made in 250-mL sterile flasks. The appropriate proportion of each of these dilutions was mixed with the PDA medium to obtain final fungicide concentrations of 0, 0.1, 0.5, 1, 10, 50 and 100 µg mL-1 for iprodione, and 0, 0.1, 1, 0, 100, 1000 and 2000 µg mL-1 AI for thiophanate-methyl. Approximately 10 mL of fungicide-amended media were poured into 60 x 0.5 mm Petri dishes.
From the margins of 5-day-old colonies, 0.5-cm diameter mycelial plugs were placed in the center of four 6-cm Petri dishes containing fungicide-amended media. These plates were sealed with Parafilm®, placed in Ziploc® plastic bags and incubated in the dark in the laboratory (22±2 °C) for 72 h. After incubation, the diameter of the colonies was measured in two directions for each of the tested doses and averaged. The mycelium growth (mm) of each isolate tested in the four replicates was averaged and used to calculate the relative growth (RG) in relation to the average mycelial growth in the plates not amended with fungicide for each of the concentrations. The means of micelial growth were used to estimate the effective dose 50 (EC50). Each assay was performed twice.
Inhibition assay on conidial germination
The same fungicide concentrations that were used for the mycelial growth assay were evaluated in water agar medium. Colonies from 8- to 10-day-old cultures were used to prepare a conidial suspension (1x103 conidia mL-1) with sterile distilled water. From this suspension, 0.1 mL was placed in a Petri dish with culture medium + fungicide for each concentration and incubated in the dark for 14 h. After incubation, the numbers of germinated and non-germinated conidia were counted in a sample of 100 conidia in the visual field of a microscope (Carl Zeiss, Germany) at 40 x magnification for each concentration. A conidium was considered germinated when the germ tube length was equal to or greater than three times its width (Yourman and Jeffers, 1999). As in the mycelial growth assay, four replicates were used for each dose of fungicide-isolate combination. Using these data, the conidial germination relative to on the plates not amended with fungicide was calculated. This assay was performed twice.
Data analysis
From these data, the effective dose that inhibited 50 % (EC50) of the mycelial growth or conidial germination was estimated by adjusting the log-logistic model with an upper asymptote to the data using the procedure NLMIXED SAS (ver. 9.2) for each of the tested isolates. This model is expressed as follows:
where y is the relative growth of mycelium or conidial germination, α is the upper asymptote (maximum possible value of y), β is the slope of the curve at the inflection point, and EC50 is the dose at which the relative growth/conidial germination is reduced by 50 % (Schanbenberger and Pierce, 2002; Rebollar-Alviter et al., 2007). This model is a re-parameterization of the log-logistic model in terms of EC50 (Schanbenberger and Pierce, 2002). The starting values of the parameters for the iterations were assigned arbitrarily. Because not all of the starting values resulted in convergence for every isolate, different sets of starting values were used to achieve convergence. The pattern of residuals served as an indicator of the model’s goodness of fit (Rebollar-Alviter et al., 2007).
Sensitivity distribution
Box plots and histograms of the EC50 values were used to illustrate the sensitivity distribution of mycelium growth and conidial germination. The estimated moments and normality tests were obtained using the SAS Proc Univariate (ver. 9.2). The data were diagnosed to check for the presence of outliers in the distribution. A log10 transformation was applied to the data to achieve normality when possible. If, after performing the transformation, the data did not fit into a normal distribution, the extreme values were removed to estimate the parameters of interest but were kept to illustrate the distribution.
To determine any significant differences between the sensitivity of the isolates from both of the sampling areas, the sensitivity distributions in the conidia and mycelium of each fungicide in each studied region were evaluated by comparing the EC50 distribution using the Kolmogorov-Smirnov test for two populations with the SAS Procedure NPAR1WAY (ver. 9.2).
Results and Discussion
Morphological and phylogenetic identification
The samples were used for the identification of isolates from two strawberry-producing regions in Michoacan corresponded to B. cinerea. The sequences of the amplification products of the ITS region of ribosomal DNA from eight isolates as obtained by PCR showed 100 % identity to the sequences in GenBank (accessions EU128648 and EU128649).
Sensitivity of Botrytis cinerea to iprodione
The sensitivity distribution of the mycelium to iprodione was bimodal (Figure 1A) and did not adjust to a normal distribution after the Log10 transformation, ranging from 3 µg mL-1 to 0.21 µg mL-1 with a median of 0.74 µg mL-1, while that of the conidia ranged from 0.47 µg mL-1 to 22.59 µg mL-1 with a median of 3.73 µg mL-1, similar to those results reported by Chen et al. (2011). However, to determine whether the sensitivity distributions in mycelia in the two study areas were different given the bimodal behavior, the datasets were analyzed separately. The statistical comparisons using the Kolmogorov-Smirnov 2-sample test of the sensitivity distributions of B. cinerea mycelium in both of the study regions indicated significant differences (p≤0.001, Ks=3.44) between the Zamora-Jacona and Maravatío valleys. In contrast, the distributions of conidia from both of the sampling areas were normal (Log10 EC50) and did not show significant differences (Figure 1B); therefore, the data from the two areas were pooled together. The sensitivity of mycelium from the Maravatío Valley isolates ranged from 0.23 to 0.89 µg mL-1 with a median of 0.35 µg mL-1, whereas that from the Zamora-Jacona Valley isolates ranged from 0.22 µg mL-1 to 3.0 µg mL-1 with a median of 1.5 µg mL-1, indicating that the latter was almost 5 times less sensitive than were those from Maravatío Valley. Myresiotis (2007) obtained an EC50 in a range between 0.1 to 1.42 µg mL-1 and considered resistant those isolates with an EC50≥1 µg mL-1. Banno et al. (2008) reported as sensitive those isolates with an EC50< 0.4 µg mL-1 and reported different degrees of resistance associated with different mutations in those isolates with an EC50 > 0.4 µg mL-1. Grabke et al. (2013) used discriminatory doses of 5 and 50 µg mL-1 to detect isolates that were highly susceptible, susceptible, moderately resistant and highly resistant, as evaluated using the germ tube length of the conidia. The isolates with low levels of resistance occurred at the highest frequency and were mainly associated with the mutations I365S and I365N of the gene bos1. Based on the studies of Banno et al. (2008) carried out on mycelium, 46 % of Maravatío Valley isolates and 94 % of those from Zamora-Jacona Valley would be considered resistant to iprodione. However, no genotyping studies were performed to confirm the presence of specific point mutations.
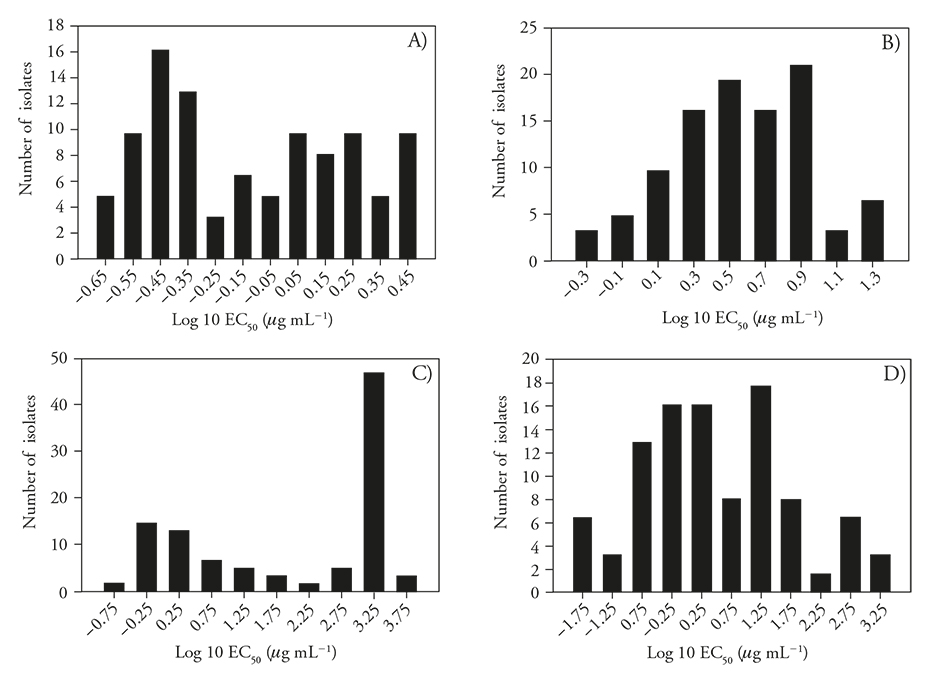
Figure 1 Sensitivity distribution (log10 EC50) of B. cinerea isolates (N=62) from two strawberry-producing areas in Michoacan, Mexico. A) Iprodione sensitivity distribution based on mycelium, B) iprodione sensitivity distribution based on conidial germination, C) thiophanate-methyl sensitivity distribution based on mycelial growth, and D) thiophanate-methyl sensitivity distribution based on conidial germination.
The iprodione sensitivity distribution of B. cinerea in the conidial germination assays showed a wide distribution of EC50 values. The sensitivity ranged from 0.47 to 22.60 µg mL-1. Grabke et al. (2013) and Amiri et al. (2013) considered isolates to be moderately resistant to resistant if they grew under iprodione concentrations greater than 5 µg mL-1 (EC50>5 µg mL-1). Based on these results, 45 % of the isolates from both of the study areas have some degree of resistance. Analyzing data from the sampling areas separately, 36 % of the isolates from Maravatío Valley were resistant, while 53 % of the isolates from Zamora-Jacona Valley exceeded the indicated discriminatory dose, confirming again that isolates from this area tend to be more tolerant to the tested fungicides based on bioassays in both the conidia and mycelia.
The isolates from Zamora-Jacona Valley were less sensitive to iprodione than were those from Maravatío Valley. These results can be partly explained by the advancement of technology in both areas, which may be related not only to the number of fungicide applications per season (15 to 20) but also to the intrinsic biological aspects of the populations of the pathogen in that area. In contrast, Maravatío Valley is an area of low technology that is undeveloped and without ongoing technical assistance for strawberry producers, who grow strawberry mainly for national and local markets. It is likely that under these conditions, pathogens are subject to lower selective pressures than under the conditions of the Zamora-Jacona Valley.
Sensitivity of Botrytis cinerea to thiophanate-methyl
The sensitivity distribution of B. cinerea based on mycelial growth was clearly bimodal (Figure 1C), separating the two areas of study with the highest frequency of isolates at both ends of the distribution (Figure 2C). In contrast, the sensitivity distribution based on conidial germination was adjusted to a normal distribution when considering both of the study areas. The data from comparing the thiophanate-methyl sensitivity distributions of mycelium between the two study areas indicated that these areas were significantly different (p≤0.002, Ks=1.86) and that the median EC50 from Zamora-Jacona Valley was almost 667 times that from Maravatío Valley. However, there were no significant differences (p=0.13, Ks=1.17) between the sensitivity distributions of the conidia, although the median was 9 times higher in the valley of Zamora-Jacona, partly indicating the lack of correlation between the sensitivities of the conidia and mycelium with this group of fungicides. Fungal mycelia growth shows the greatest sensitivity to benzimidazole fungicides than does the germination of conidia (Leroux, 2007).
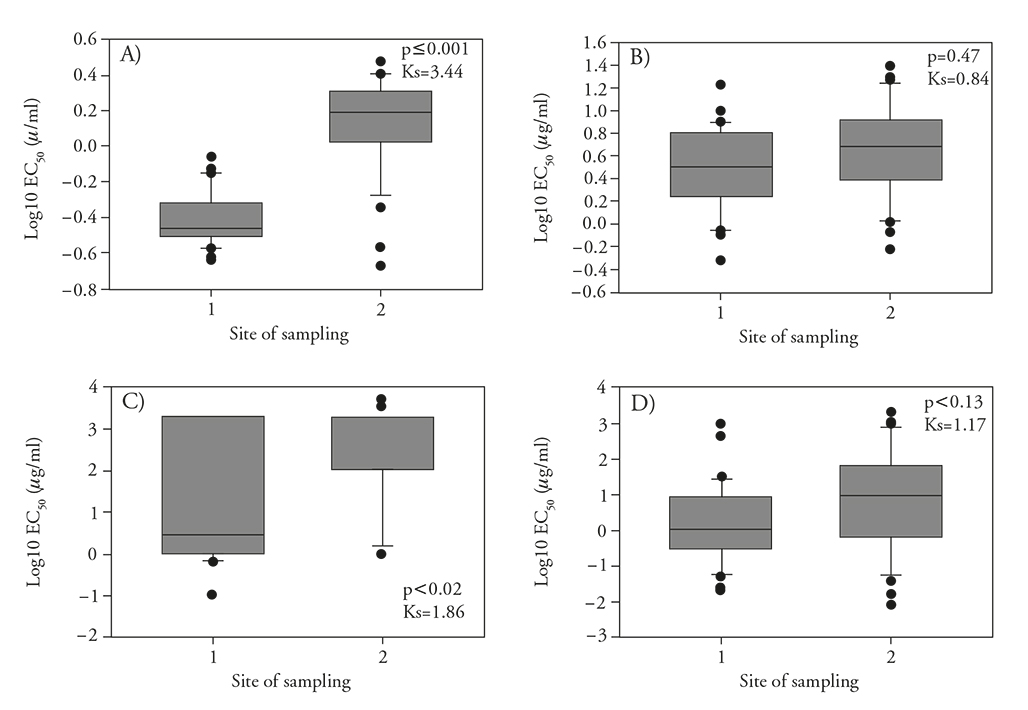
Figure 2 Sensitivity distribution of Botrytis cinerea isolates from two strawberry-producing areas (1=Maravatío Valley, n=30; 2=Zamora-Jacona Valley, n=32) in Michoacan, Mexico. A) Iprodione sensitivity distribution of mycelium, B) iprodione sensitivity distribution of conidia, C) thiophanate-methyl sensitivity distribution of mycelium, and D) thiophanate-methyl sensitivity distribution of conidia. Within each figure, the Kolmogorov-Smirnov p-value and test statistic from two independent samples is shown.
Due to the bimodal shape of the histogram, a mycelium-based analysis was performed for both sampling areas separately. The mycelium sensitivity distribution of the Maravatío Valley isolates was dispersed (Figure 2C), with EC50 values ranging from 0.11 to > 2000 µg mL-1 with a median of 3.11 µg mL-1. This distribution was not normal, even after the removal of outliers and after the log10 transformation. The distribution of the Zamora-Jacona Valley isolates was also dispersed, with EC50 values ranging from 0.82 to > 2000 µg mL-1 with a median of 2000 µg mL-1 without fitting to a normal distribution after the log10 transformation.
The distribution of the conidial EC50 values, considering isolates from the sampling areas, ranged from 0.01 to greater than 2000 µg mL-1 with a median of 2.01 µg mL-1. In Maravatío Valley, the distribution ranged from 0.02 to 892 µg mL-1 with a median of 1.13 µg mL-1, whereas in the Zamora-Jacona Valley, the values were more scattered, ranging from 0.01 to 2000 µg mL-1 with a median of 9.7 µg mL-1, which is 9 times greater than the median EC50 of the Maravatío Valley isolates. In both the conidial and mycelial assays, the EC50 values of the Zamora-Jacona Valley isolates exceeded those of the Maravatío Valley isolates.
The data indicate a high level of B. cinerea resistance to thiophanate-methyl in both of the strawberry-producing areas, but this resistance was significantly higher in the Zamora-Jacona Valley isolates than in the Maravatío Valley isolates. In both areas, the use of benzimidazole fungicides, especially thiabendazole, benomyl and thiophanate-methyl, on a calendar basis is common.
Studies defining the base line sensitivity of mycelium to B. cinerea showed that isolates with EC50 values greater than 50 µg mL-1 are resistant (Luck and Guilling, 1995; LaMondia and Douglas, 1997; Yourman and Jeffers, 1999). Based on this criterion, 81 % of the Zamora-Jacona Valley isolates were resistant, whereas 34 % of the Maravatío Valley isolates fell into this category. Using a discriminatory dose of 50 µg mL-1, Mercier et al. (2010) found that 92 % of the isolates were resistant to thiophanate-methyl in strawberry fields in Oxnard, California. Based on studies of conidial germ tube elongation, Weber and Hahn (2011) considered isolates to be moderately resistant with an EC50 of 4.67 to 16.1 µg mL-1 and resistant with an EC50 greater than 28 µg mL-1. Fernández-Ortuño and Shnabel (2012), applying a discriminatory dose of 100 µg mL-1, found that 85 % of strawberry isolates from South Carolina were resistant to thiophanate-methyl, similar to the results of our study. All of the isolates characterized by Fernández-Ortuño and Shnabel (2012) as resistant, expressed the E198A (alanine to glutamate at position 198) point mutation.
Benzimidazole fungicides have a greater effect on mycelial growth than on conidial germination, associated with the distortion of germ tubes, which is an indicator of the disruption of cellular microtubules (Davidse and Ishii, 1995; Leroux, 2007). It is likely that these mutations are widely distributed in both of the examined areas in Mexico, but genotyping studies have not yet been performed.
Our data revealed the existence of B. cinerea populations that are resistant to dicarboximide and benzimidazole fungicides in two of the most important strawberry-producing areas of Mexico with contrasting socio-economic and technological conditions. Data from sensitivity studies in both of the sample areas show that isolates from Zamora-Jacona Valley had the highest frequency of resistance to both fungicides, especially to thiophanate-methyl.
Conclusions
Resistance of B. cinerea to thiophanate-methyl and iprodione is widely distributed in the strawberry-producing areas in Michoacan, with differences in sensitivity between the isolates from both areas; the sensitivity was more marked with the thiophanate-methyl fungicide. As shown by separate analyses of the two study areas, the Zamora-Jacona Valley isolates were more resistant than were those from Maravatio Valley. These results will contribute to the establishment of integrated disease management programs of gray mold of strawberry and serve as the basis for implementing anti-resistance strategies for dicarboximide and benzimidazole fungicides.











 texto em
texto em 




