Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.51 no.7 Texcoco oct./nov. 2017
Crop Science
Effect of salicylic acid on growth, nutritional status, and performance of maize (Zea mays)
1Edafología, Colegio de Postgraduados. Carretera Federal México-Texcoco km 36.5. 56230. Montecillo, Texcoco, Estado de México, México. (tucuch.cesar@colpos.mx) (alcantar@colpos.mx) (vvolke@colpos.mx) (tlibia@colpos.mx).
2Campo Experimental Valles altos de Jalisco. INIFAP. Km.8 de la Carretera Tepatitlán-Lagos de Moreno. 47600. Tepatitlán de Morelos, Jalisco, México. (salinas.yolanda@inifap.gob.mx).
3Recursos Naturales-Centro de Investigación Científica de Yucatán Chuburna de Hidalgo Mérida. 97200. Yucatán, México. (larque@cicy.mx).
Salicylic acid (AS) may modulate several physiological and biochemical processes in plants, and may contribute to improve crop yield. These processes are also induced in maize (Zea mays) cultivation, but their effects on bioproductivity are not very well known. Therefore, we evaluated the effect of applying AS to maize leaves on growth, nutrient status, and yield of the local variety Xmejen-nal, State of Yucatan, Mexico. We conducted two independent experiments with randomized complete block designs, under field conditions, using two AS concentrations, 0.1 and 1 µM, and distilled water as control. Seven day-old plants were sprayed daily with this solution, for a week. The following variables were measured during harvest: plant height and diameter; above-ground dry biomass; cob diameter, length, and weight; grain yield (g plant-1); nitrogen, phosphorus (P2O5) and potassium (K2O) content, in above-ground tissue and grain. Applying 1 µM of AS increased (p≤0.05) grain yield per plant, total dry biomass, and N, P2O5 and K2O content. Additionally, spraying maize plants with 1 µM and 0.1 µM AS resulted in 29 and 21 % increases (p≤0.05) in cob length, respectively, as compared to the control. Grain yield showed a highly significant and positive correlation with all cob variables, suggesting that the increase of grain yield may be linked with the effect of AS on the cob.
Key words: Zea mays; biomass; cob; nitrogen; phosphorus; potassium
El ácido salicílico (AS) participa en diversos procesos fisiológicos y bioquímicos en algunas especies de plantas, favoreciendo el rendimiento. En el cultivo de maíz (Zea mays) estos procesos también son favorecidos, pero se conoce poco sobre los efectos causados en la bioproductividad. Por tal razón, el efecto de AS aplicado a las hojas de maíz se evaluó en el crecimiento, el estatus nutrimental y la producción de grano, de la variedad local Xmejen-nal, estado de Yucatán, México. Dos experimentos independientes se realizaron con arreglo de bloques completos al azar, en condiciones de campo, usando dos concentraciones de AS (0.1 y 1 µM) y agua destilada como testigo. Las soluciones se asperjaron a plántulas de 7 d de edad, durante 7 d. Los resultados de ambos experimentos se analizaron mediante ANDEVA y las medias se compararon con la prueba de Tukey (p≤0.05). Al momento de la cosecha se midió: altura y diámetro de la planta; biomasa seca aérea; diámetro, longitud y peso de la mazorca; rendimiento del grano (g planta-1); contenido de nitrógeno (N), fósforo (P2O5) y potasio (K2O) en tejido aéreo y grano. La aplicación de 1 µM de AS aumentó (p≤0.05) la producción del grano por planta, la biomasa seca total y el contenido de N, P2O5 y K2O. Además, la longitud de la mazorca de las plantas asperjadas con 1 µM y 0.1 µM de AS fue 29 % y 21 % fue mayor (p≤0.05) que el testigo. El rendimiento del grano presentó una correlación altamente significativa y positiva con todas las variables de la mazorca, lo cual indica que el aumento del rendimiento del grano está ligado al efecto del AS sobre la mazorca.
Palabras clave: Zea mays; biomasa; mazorca; nitrógeno; fósforo; potasio
Introduction
Salicylic acid (AS) is a phenolic compound resulting from the secondary metabolism (Hayat et al., 2010), present in vegetable tissues (Rasking, 1992). Salicylic acid regulates plant growth and may increase crop yield, when low concentrations are supplied exogenously (Rivas-San Vicente and Plasencia, 2011). This compound may increase length, weight, perimeter, and root area (Gutiérrez-Coronado et al., 1998; Larqué-Saavedra et al., 2010), may modify its morphology (Echevarría-Machado et al., 2007), and may also increase stalk’s fresh and dry biomass (Villanueva-Couoh et al., 2009), while improving the nutrient status of the plant (Khan et al., 2010).
Physiological processes triggered by AS include stomatal closure (Larqué-Saavedra, 1978), improvement of carboxylation and nitrate reductase activity (Fariduddin et al., 2003), increase of photosynthetic activity (Arfan et al., 2007; Sánchez-Chávez et al., 2011), and reduction of photochemical capacity (Janda et al., 2012). Additionally, it stimulates the production of secondary metabolites (Bennet and Wallsgrove, 1994), such as capsicine (Sandoval-Rangel et al., 2011), bacoside A (Largia et al., 2015), withaferin A (Piñeros-Castro et al., 2009), flavonoids (Pachecho et al., 2013), and total phenols (Burbano and Garcés, 2007; Rodrigues-Brandao et al., 2014).
Applying low AS concentrations increases the yield and quality of pepper (Capsicum annuum), (Capsicum chinense), tomato (Solanum lycopersicum), cucumber (Cucumis sativus), and papaya (Carica papaya) (Martín-Mex et al., 2013). AS protects rice (Oryza vulgare), wheat (Triticum aestivum), and barley (Hordeum vulgare) against the damages caused by cadmium (Cd) (Fatima et al., 2014), arsenic (As) (Singh et al., 2015), lead (Pb) (Chen et al., 2007), and boron (B) (El-Feky et al., 2014). Additionally, it induces the appearance of proteins linked to plant pathogenesis (PR) -which are responsible for systemic acquired resistance-; it stimulates the activity of the peroxydase (EC 1.11.1.7), β-1,3-glucanase (EC 3.2.1.58), and catalase (EC 1.11.1.6) enzymes (Shi et al., 1999; Mutlu et al., 2013); and it contributes to the accumulation of magnesium (Mg), calcium (Ca), and potassium (K) (El Tayeb and Ahmed, 2010). It also improves vigour and height of seedlings (Anwar et al., 2013), fresh and dry weight of the roots and shoots (Deef, 2007) fresh and dry biomass (Haya et al., 2005; Tucuch et al., 2015), chlorophyll (a and b) and carbohydrate content (El-Feky et al., 2014), as well as grain yield in rice and wheat (López et al., 1998; Tavares et al., 2014).
Applying AS to the leaves of maize seedlings exposed to saline stress accelerates the enzymatic activity of Rubisco, increases photosynthetic activity, activity of chlorophylls a and b, carotenoids and carbohydrates concentrations, length and fresh and dry weight of roots, as well as height, fresh and dry biomass of plants aerial part and foliage area, under salinity conditions (Khodary, 2004). Additionally, it contributes to the accumulation of N, K+, Fe2+, Mg2+, and Cu2+ in plant tissue, under the same saline stress conditions (Gunes et al., 2007; Fahad and Bano, 2012).
AS participation in physiological and biochemical processes in plants -particularly in graminaceous- suggests that this compound may induce greater grain yield in maize crops. In order to verify this hypothesis, the objective of this study was to evaluate the effect of applying low foliar AS concentrations to maize leaves during the seedling stage on grain yield and its components.
Materials and Methods
In order to analyze the effect of AS on plant growth, grain yield, N, P2O5 and K2O contents in (above-ground) shoot tissue and grain in maize plants, two open-air experiments were carried out in the Centro de Investigación Científica de Yucatán (CICY). The first experiment began on August 30, 2013, and the second, in January 19, 2014. Both experiments used local Xmejen-nal (Nal-telXTuxpeño) native maize seeds, which are very important in Yucatan. The seeds were sown in the ground, in 1-m wideX10-m long beds; the plants were sown 0.30 m apart from each other, in 0.50 m rows.
For both experiments (1 and 2), 0.1 and 1 µM AS concentration were prepared following the methodology described by Gutiérrez-Coronado et al. (1998). Additionally, distilled water was used as control spray. The treatments were applied daily for a week on the leaves of the seedlings, up to dew point, with 24 h application intervals, at 08:00 h, starting 7 d after sowing (dds). After the plants were sprinkled with AS for the last time, the only activities that were carried out were weeding, watering -to keep the ground humid at field capacity-, and one fertilizer application (17 N, 17 P2O5, 17 K2O, 20 g plant-1, 20 dds).
The following data was collected 140 dds (end of the experiment) for both experiments: plant height; distance from the stalk base to the apex terminal; stalk diameter, measured 10 cm from the soil; dry weight of total biomass; length, diameter, and weight of the cob; and grain yield per plant; using an analytical balance (Sartorius, BL3100), and a digital flexometer and vernier caliper (Truper, CALDI-6MP). In order to determine N, P2O5, and K2O concentrations in tissue and grain, the above-ground part of the plant was taken, and 100 g of grain from each treatment were placed in an oven (Binder, FED720), at 70 °C, until a constant weight was reached; they were ground and analyzed in the laboratory. Nitrogen concentration was determined using the micro-Kjeldahl method, while P2O5 and K2O concentration was measured using readings from extracts taken from diacid wet digestion, as described by Alcántar and Sandoval (1999), in an atomic spectroscopy equipment with plasma induction (ICP-OES, Agilent 725-OES, Australia). The concentration of each element in tissue and grain and the weight of dry above-ground biomass and maize grains were used to estimate total contents.
The statistical design was randomized complete blocks with 20 repetitions and one plant as experimental unit. The data collected belong to 4 and 10 plants, for experiments 1 and 2, respectively. ANOVA was used to analyze the results of both experiments and means were compared using the Tukey test (p≤0.05). Additionally, yield data were correlated with cob variables and nutritional content. Statistical analysis were carried out using the SAS software (2004).
Results and Discussion
The average temperature for experiment 1 fluctuated between 21 and 28 °C, and was higher in the early stages of cultivation, and lower at the end. On the contrary, in experiment 2, lower temperatures were recorded in the early stages, while the higher temperatures were recorded at the end, with an interval between 20 and 25 °C. The average rainfall during the whole maize development cycle was 5 mm d-1, because experiment 1 was carried out during the 2013 spring-summer cycle, when the overall rainfall was 599 mm. However, during experiment 2, rainfall was scarce (overall: 159 mm), and support irrigation was used most of the time (two irrigations every second day, up to saturation point), since the experiment was carried out during the 2014 autumn-winter cycle.
Table 1 includes the results for plant height, stalk diameter, and dry weight for the total dry biomass for both experiments. Plant height and stalk diameters means were higher when AS concentrations were added, but only in experiment 1 the application of 1 µM SA resulted in greater height (14.3 %) and diameter (41.2 %) with regard to control (p≤0.05). In both experiments, total dry biomass increased 48 % (p≤0.05) with 1 µM of AS, with regard to control.
Table 1 Effect of applying salicyclic acid to the leaves of native Xmejen-nal maize plants, on plant height, stalk diameter, and above-ground dry biomass, in two experiments carried out in the CICY, during the 2013 and 2014 cycles.

AP: plant height; DT: stalk diameter; BT: total dry biomass. Means with different letters in a column for each experiment are statistically different (Tukey, p≤0.05).
The results show that AS stimulates growth, development, and biomass accumulation in mature maize plants, which is in full agreement with those published by Farahbakhsh and Saiid (2011) and Singh et al. (2015a) in maize under salt stress, and by Zamaninejad et al. (2013), in this species under drought stress. Similarly, our results match those reported in wheat (Sayede and Mujahed, 2016; Tucuch et al., 2015), papaya (Martín-Mex et al., 2012), cucumber (Singh and Chatuverdi, 2012), pepper (Qados, 2015), and coriander (Coriandrum sativum) (Hesami et al., 2012).
The length of plant cobs were sprinkled with AS is different (p≤0.05) than the control, regardless of the AS concentration sprayed in both experiments: the 1 and the 0.1 µM AS concentrations surpassed control by 29 and 21 %, respectively (Table 2). With regard to other cob variables -particularly grain production (Table 2)-, only the 1 µM AS concentration was higher (p≤0.05). The grain length, width, and thickness values did not show significant differences between treatments, in neither experiment.
Table 2 Effect of applying salicyclic acid to the leaves of native Xmejen-nal maize plants, on cob and the grain parameters, in two experiments carried out in the CICY, during the 2013 and 2014 cycles.
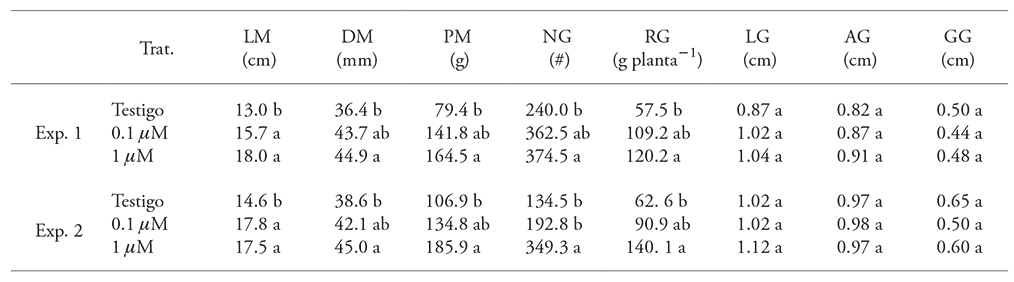
LM: cob length; DM: cob diameter; PM: cob weight; NG: number of grains; LG: grain length; AG: grain width; GG; grain thickness; RG: grain yield per cob. Means with different letters in a column for each experiment are significantly different (Tukey, p≤0.05).
Our results also agree with those reported by El-Wahed et al. (2006), who found out that applying 1, 2, or 3 mM AS in maize leaves 45 dds, increased cob length and diameter. This has a positive effect in grain yield (up to 25.7 %). Additionally, this information confirms the results reported by Zamaninejad et al. (2013), who observed the same grain yield increase pattern (59 % more than control), when 1 mM of AS is sprinkled in plants with 10 to 12 leaves exposed to drought conditions. Chávez-Servia et al. (2004) carried out a morphological characterization of the same maize variety and found out that length and diameter values of the cob and grain yield match the results of control plants and are lower than those of plants treated with AS in our study.
The data in Table 3 shows that grain yield has a highly significant and positive correlation with all cob variables, except for grain thickness, which had a negative behavior in both experiments and it was not significant for experiment 1; additionally, grain width response was not significant for experiment 2. These results are similar to those reported by El-Wahed et al. (2006) and Zamaninejad et al. (2013) who correlated grain yield with cob length and diameter in maize plants treated with AS, indicating that grain yield increase is linked with the effect of AS on the cob.
Table 3 Correlation between grain yield and cob variables in two experiments with Xmejen-nal native maize, carried out in the CICY, during the 2013 and 2014 cycles.
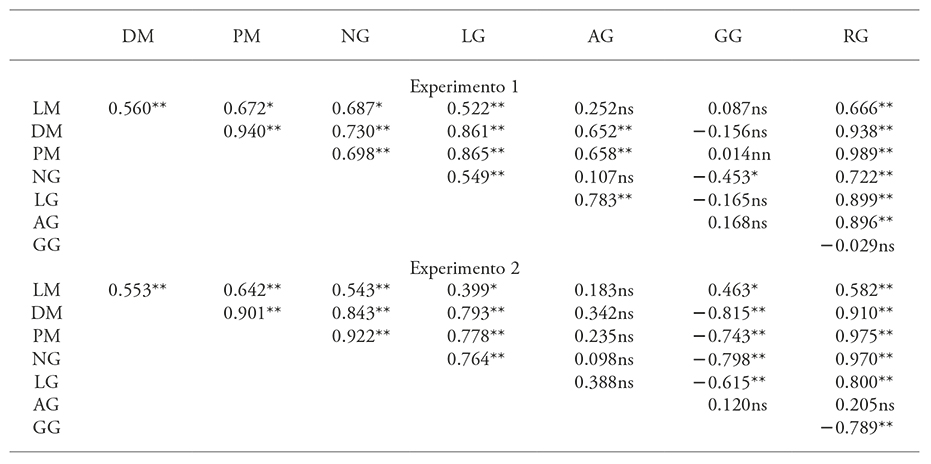
LM: cob length; DM: cob diameter; PM: cob weight; NG: number of grains; LG: grain length; AG: grain width; GG: grain thickness; RG: grain yield per cob. *p≤0.05; **p≤0.01; ns: not significant.
Table 4 includes the results of the N, P2O5, and K2O contents in above-ground dry biomass and in grain for both experiments. The 1 µM AS treatment contributed to the accumulation of these three elements (p≤0.05); additionally, in both experiments, the average increase of N, P2O5, and K2O was 75, 85, and 67% in above-ground dry biomass, and 59, 129, and 116 % in grain, respectively.
When 0.1 µM AS was applied, the effect between experiments did not show a consistent behavior. In experiment 1, AS affected (p≤0.05) above-ground dry biomass: the content of N and K2O increased by 37.5 and 59.0 %, respectively; however, there were no significant effects in experiment 2. In grains, N and P2O5 concentrations in experiment 1 were significantly greater (79 and 29 %, respectively) than control; K2O values in both experiments were 29 % higher.
Table 4 Average N, P2O5, and K2O content in above-ground dry biomass and grain, in two experiments, in maize (var. Xmejen-nal) crops, treated with salicyclic acid.
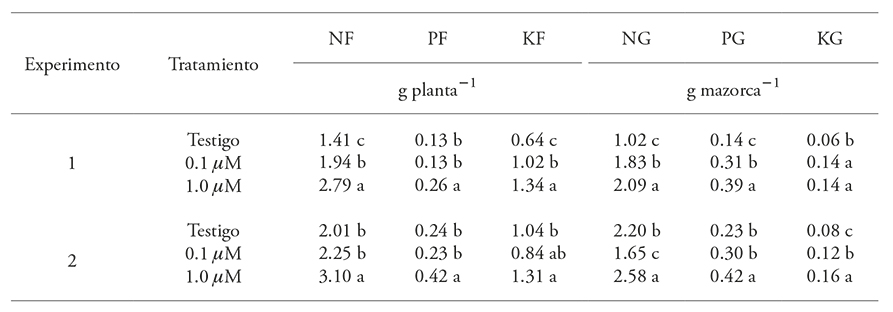
NF: leaf nitrogen; PF: leaf phosphorus; KF: leaf potassium; NG: grain nitrogen; PG: grain phosphorus; and KG: grain potassium. Means with different letters in a column for each experiment are significantly different (Tukey, p≤0.05).
Vazirimehr and Rigi (2014) reviewed the responses of several agricultural important crops treated with AS, under salinity conditions, and reported that the total ion content in treated plants was higher than that found in control plants. Gunes et al. (2005) reported that the N, P2O5, K2O, Mg, and Mn content in maize increased, under different stress conditions, which is consistent with our results. Data in our experiment might be explained because AS promoted greater root development (for the same species and variety), increasing the exploration area in the soil and, therefore, the nutrient absorption rate (Tucuch-Haas et al., 2016).
When N, P2O5, and K2O content was correlated with grain yield and above-ground dry biomass (Table 5), values were significantly different, except for P in above-ground dry biomass. Therefore, we can assume that there is a greater absorption of these elements and that they contribute to greater above-ground dry biomass and grain yield. We must emphasize that there is a greater photosynthesis rate for this same species, when AS is applied on the crop’s canopy during the seedling stage (Khodary, 2004), which would also contribute to improving grain yield and above-ground dry biomass.
Table 5 Correlation of N, P2O5 y K2O contents in tissue and grain, between above-ground dry biomass and grain weight, in two experiments with maize var. Xmejen-nal native maize, carried out in the CICY, during the 2013 and 2014 cycles.

BA: above-ground dry biomass; RG: grain yield; NF: leaf nitrogen; NG: grain nitrogen; PF: leaf phosphorus; PG: grain phosphorus; KF: leaf potassium; and, KG: grain potassium. *p≤0.05; **p≤0.01; ns: not significant.
The results of the effect of AS in maize confirm the impact of this growth regulator on graminaceous production, as pointed out by López et al. (1998) and Tavares et al. (2014). Additionally, this confirms their usefulness in the bioproductivity of other species, such as C. annunm (Elwan and El-Hamahny, 2009; Martín-Mex et al., 2004; Martín et al., 2005), S. lycopersicum (Javaheri et al., 2012), and C. papaya (Martín-Mex et al., 2012).
Conclusions
Salicylic acid increases the production of total dry biomass, grain yield, as well as N, P2O5, and K2O contents in tissue and grain, when the canopy of maize seedlings is sprinkled with AS. Both salicyclic acid doses tested produced longer cobs. Maize grain yield is linked with the effect of AS on the cob
Literatura Citada
Alcántar G., G., y M. Sandoval V. 1999. Manual de Análisis Químico de Tejido Vegetal. Publicación especial 10. Sociedad Mexicana de la Ciencia del Suelo. Chapingo. México. Chapingo, México. 156 p. [ Links ]
Anwar, S., M. Iqbal, S. H. Raza, and N. Iqbal. 2013. Efficacy of seed preconditioning with salicylic and ascorbic acid in increasing vigor of rice (Oryza sativa L.) Seedling. Pak. J. Bot. 45: 157-162. [ Links ]
Arfan, M., H. R. Athar, and M. Ashraf. 2007. Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress? J. Plant Physiol. 164: 687-694. [ Links ]
Bennet, R. N., and R. M. Wallsgrove. 1994. Secondary metabolites in plant defense mechanisms. New Phytol. 127: 617-633. [ Links ]
Burbano, C., y F. Garcés. 2007. Control del virus de la hoja amarilla de la caña de azúcar (SCYLV) mediante técnicas de cultivo de tejidos en la variedad CR74-250. Rev. Tecnol. ESPOL 20: 0257-1749. [ Links ]
Chávez-Servia, J. L., T. C. Camacho-Villa, y L. A. Burgos-May. 2004. Patrones de variabilidad genotípica de maíz y su potencial para mejorar la producción en Yucatán. Memoria del foro-taller Problemática campesina, retos y perspectivas de la investigación y el servicio para el mejoramiento de la milpa en Yucatán. 2004. Mérida Yucatán. pp: 22-30. [ Links ]
Chen, J., C. Zhu, L. Li, Z. Sun, and X. Pan. 2007. Effects of exogenous salicylic acid on growth and H2O2 metabolizing enzymes in rice seedlings under lead stress. J. Environ. Sci. 19: 44-49. [ Links ]
Deef, H. E. 2007. Influence of salicylic acid on stress tolerance during seed germination of Triticum aestivum and Hordeum vulgare. Advan. Biol. Res. 1: 40-48. [ Links ]
Echevarría-Machado, I., R. M. Escobedo-G.M, and A. Larqué-Saavedra. 2007. Responses of transformed Catharanthus roseus roots to femtomolar concentrations of salicylic acid. Plant Physiol. Biochem. 45: 501-507. [ Links ]
El Tayeb, M. A., and A. N. Ahmed. 2010. Response of wheat cultivars to drought and salicylic acid. Am-Euras. J. Agron. 3: 01-07. [ Links ]
El-Feky, S. S., F. A. El-Shintinawy, and E. M. Shaker. 2014. Role of CaCl2 and salicylic acid on metabolic catabolic and productivity of boron stressed barley (Hordeum vulgare L.). Int. J. Curr. Microbiol. App Sci. . 3: 368-380. [ Links ]
El-Wahed, M. S. A., A. A. Amin, and El-Sh. M. Rashad. 2006. Physiological effect of some bioregulators on vegetative growth, yield and chemical constituents of yellow maize plants. World J. Agric. Sci. 2: 149-155. [ Links ]
Elwan, M. W. M, and M. A. M. El-Hamahmy. 2009. Improved productivity and quality associated with salicylic acid application in greenhouse pepper. Sci. Hortic. 122: 521-526. [ Links ]
Fahad, S., and A. Bano. 2012. Effect of salicylic acid on physiological and biochemical characterization of maize grown in saline area. Pak. J. Bot . 44: 1433-1438. [ Links ]
Farahbakhsh, H., and M. S. Saiid. 2011. Effects of foliar application of salicylic acid on vegetative growth of maize under saline conditions. Afr. J. Plant Sci. 5: 575-578. [ Links ]
Fariduddin, Q., S. Hayat, and A. Ahmad. 2003. Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica 41: 281-284. [ Links ]
Farzane, M. H., R. Monem, S. M. Mirtaheri, and S. F. Kashani. 2014. Effect of Salicylic acid on germination and growth seedling of 10 variety barley (Hordeum vulgare L.) under drought stress. Int. J. Biosci. 5: 445-448. [ Links ]
Fatima, R. N., F. Javed, and A. Wahid. 2014. Salicylic acid modifies growth performance and nutrient status of rice (Oryza sativa) under cadmium stress. Int. J. Agric. Biol. 16: 1083-1090. [ Links ]
Fayez, K. A., and S. A. Bazaid. 2014. Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J. Saudi Soc. Agri. Sci. 13: 45-55. [ Links ]
Gunes, A., A. Inal, M. Alpaslan, N. Cicek, E. Guneri, F. Eraslan, and T. Guzelordu. 2005. Effects of exogenously applied salicylic acid on the induction of multiple stress tolerance and mineral nutrition in maize (Zea mays L.). Arch. Agron. Soil Sci. 51: 687-695. [ Links ]
Gunes, A ., A. Inal , M. Alpaslan , F. Eraslan , E. G. Bagci, andN. Cicek . 2007. Salicylic acid changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J. Plant Physiol . 164: 728-736. [ Links ]
Gutiérrez-Coronado, M. A., C. Trejo-López, and A. Larqué-Saavedra . 1998. Effects of salicylic acid on the growth of roots and shoots in soybean. Plant Physiol. Biochem . 36: 563-565. [ Links ]
Habibi, G. 2012. Exogenous salicylic acid alleviates oxidative damage of barley plants under drought stress. Acta Biol. Szeged. 56: 57-63. [ Links ]
Hayat, Q., S. Hayat , M. Irfan, and A. Ahmad . 2010. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 68: 14-25. [ Links ]
Hayat, S., Q. Fariduddin, B. Ali, and A. Ahmad . 2005. Effect of salicylic acid on growth and enzyme activities of wheat seedlings. Acta Agron. Hung. 53: 433-437. [ Links ]
Hesami, S., E. Nabizadeh, A. Rahimi, and A. Rokhzadi. 2012. Effects of salicylic acid levels and irrigation intervals on growth and yield of coriander (Coriandrum sativum) in field conditions. Environ. Exp. Biol. 10: 113-116. [ Links ]
Janda, K., E. Hideg, G. Szalai, L. Kovács, and T. Janda. 2012. Salicylic acid may indirectly influence the photosynthetic electron transport. J. Plant Physiol . 169: 971-978. [ Links ]
Javaheri, M., K. Mashayekhi, A. Dadkhah, and F. T. Zaker. 2012. Effects of salicylic acid on yield quality characters of tomato fruit (Lycopersicum esculentum Mill). Int. J. Agric. Crop Sci. 4: 1184-1187. [ Links ]
Khan, N.A., S. Syeed, A. Masood, R. Nazar, andN. Iqbal . 2010. Application of salicylic acid increases contents of nutrients and antioxidative metabolism in mungbean and alleviates adverse effects of salinity stress. Int. J. Plant Biol. 1: 1-8. [ Links ]
Khodary, S. E. A. 2004. Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int. J. Agri. Biol. 6: 5-8. [ Links ]
Largia, M. J. V., G. Pothiraj, J. Shilpha, and M. Ramesh. 2015. Methyl jasmonate and salicylic acid synergism enhances bacoside a content in shoot cultures of Bacopa monnieri (L.). Plant Cell Tiss. Organ Cult. 122: 9-20. [ Links ]
Larqué-Saavedra, A., R. Martín-Mex, A. Nexticapan-Garcéz, S. Vergara-Yoisura, y M. Gutiérrez-Rendón. 2010. Efecto del ácido salicílico en el crecimiento de plántulas de tomate (Lycopersicon esculentum Mill.). Rev. Chapingo Ser. Hortic. 16: 183-187. [ Links ]
Larqué-Saavedra, A. 1978. The antitranspirant effect of acetylsalicylic acid on Phaseolus vulgaris L. Physiol. Plant 43: 126-128. [ Links ]
López T., R., V. Camacho R., y M.A.Gutiérrez C . 1998. Aplicación de ácido salicílico para incrementar el rendimiento agronómico en tres variedades de trigo. Terra Latinoam. 16: 43-48. [ Links ]
Martín-Mex, R., R. López-Gutiérrez, J. Medina-Arceo, J. Cruz-Campos, A. Nexticapan-Garces, F. González-Rodríguez, y A. Larqué-Saavedra . 2004. Incremento en la productividad de chile habanero (Capsicum chinense Jacq.) por aspersiones de ácido salicílico. Primera Convención Mundial del Chile. León, Guanajuato, México. 2004. pp: 326. [ Links ]
Martín M., R., A. Nexticapan G., L. Vega M., A. Baak P., y A. Larqué S. 2005. Efecto del ácido salicílico en la floración y productividad de chile habanero (Capsicum chinense Jacq.). Segunda Convención Mundial del chile. Zacatecas, Zacatecas, México. 2005. pp: 325-326. [ Links ]
Martín-Mex, R ., A. Nexticapan-Garcéz , R. Herrera-Tuz, S. Vergara-Yoisura , y A. Larqué-Saavedra . 2012. Efecto positivo de aplicaciones de ácido salicílico en la productividad de papaya (Carica papaya). Rev. Mex. Cienc. Agric. 18: 1637-1643. [ Links ]
Martín-Mex, R ., A. Nexticapan-Garcéz , and A. Larqué Saavedra. 2013. Potential benefits of salicylic acid in food production. In: Hayat S., A. Ahmad , and M. N. Alyemeni (eds). Salicylic Acid. Springer publishers, Dortdrech, The Netherlands. pp: 299-213. [ Links ]
Mutlu, S., Ö. Karadagoglu, Ö. Atici, and B. Nalbantoglu. 2013. Protective role of salicylic acid applied before cold stress on antioxidative system and protein patterns in barley apoplast. Biol. Plant 57: 507-513. [ Links ]
Pacheco, A. C., et al. 2013. Salicylic acid-induced changes to growth, flowering and flavonoids production in marigold plants. Glob. J. Med. Plant Res. 7: 3158-3163. [ Links ]
Piñeros-Castro, Y., A. Otálvaro-Álvarez, y M. Velásquez-Lozano. 2009. Efecto de la aplicación de elicitores sobre la producción de 4B-hidroxiwithanólido E, en raíces transformadas de Physalis peruviana L. Universitas Scientiarum 14: 23-28. [ Links ]
Pirasteh-Anosheh, H., G. Ranjbar, Y. Emam, andM. Ashraf . 2014. Salicylic-acid-induced recovery ability in salt-stressed Hordeum vulgare plants. Turk. J. Bot. 38: 112-121. [ Links ]
Qados, A. M. S. A. 2015. Effects of salicylic acid on growth, yield and chemical contents of pepper (Capsicum annuum L.) plants grown under salt stress conditions. Intl J. Agri. Crop Sci. 8: 107-113. [ Links ]
Raskin, I. 1992. Role of salicylic acid in plants. Annu. Rev. Plant physiol. Plant Mol. Biol. 43: 439-463. [ Links ]
Rivas-San Vicente, M., and J. Plasencia. 2011. Salicylic acid beyond: its role in plant growth and development. J. Exp. Bot. 1: 1-18. [ Links ]
Rodrigues-Brandao, I., et al. 2014. Salicylic acid on antioxidant activity and betacyan in production from leaves of Alternanthera tenella. Ciencia Rural 44: 1893-1898. [ Links ]
Sánchez-Chávez, E. et al. 2011. Efecto del ácido salicílico sobre biomasa, actividad fotosintética, contenido nutricional del chile jalapeño. Rev. Chapingo Ser. Hortic. 17: 63-66. [ Links ]
Sandoval-Rangel, A., et al. 2011. Influencia de ácidos orgánicos sobre el crecimiento, perfil bromatológico y metabolitos secundarios en chile piquín. Terra Latinoam . 29: 395-401. [ Links ]
SAS, 2004. Statistical Analysis System Institute. SAS Proceeding Guide, Version 8.1. SAS Institute. Cary, NC. USA [ Links ]
Sayede, H. E. A., and H. M. Mujahed. 2016. Exogenous application of salicylic acid for stimulates germination, growth and yield production of wheat (Triticum aestivum L.) plant under Water Stress. Int. J. Life Sci. 5: 88-104. [ Links ]
Shi, S., M. Pan, T. Lu, H. Chen, M. Zhu, and J. Liu. 1999. Analysis of salicylic acid induced proteins in rice. Tsinghua Sci. Technol. 4: 519-1523. [ Links ]
Singh, P. K., and V.K. Chaturvedi. 2012. Effects of salicylic acid on seedling growth and nitrogen use efficiency in cucumber (Cucumis sativus L.). Plant Biosyst. 146: 302-308. [ Links ]
Singh, A. P., et al. 2015. Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front. Plant Sci. 6: 1-28. [ Links ]
Singh, P. K ., S. K. Shahi, and A. P. Singh. 2015a. Effects of salt stress on physico-chemical changes in maize (Zea mays L.) plants in response to salicylic acid. Indian J. Plant Sci. 4: 2319-3824. [ Links ]
Tavares L. C., C. Araújo, S. de Olivera, A. Pich, and F. Amaral V. 2014. Treatment of rice seeds with salicylic acid: seed physiological quality and yield. J. Seed Sci. 36: 356-356. [ Links ]
Tucuch H., C. J., G. Alcántar G., y A. Larqué S. 2015. Efecto del ácido salicílico en el crecimiento de la raíz y biomasa total de plántulas de trigo. Terra Latinoam . 33: 63-68. [ Links ]
Tucuch-Haas, C. J. et al. 2016. Efecto del ácido salicílico sobre el crecimiento de raíz de plántulas de maíz. Rev. Mex. Ciencias Agric. 7: 709-716. [ Links ]
Vazirimehr, M. R., and K. Rigi. 2014. Effect of salicylic acid in agriculture. Int. J. Plant Anim. Environ. Sci. 4: 291-296. [ Links ]
Villanueva-Couoh, E., G. Alcántar-González, P. Sánchez-García, M. Soria-Fregoso, y A. Larqué-Saavedra . 2009. Efecto del ácido salicílico y dimetilsulfóxido en la floración de Chrysanthemun morifolium (Ramat) Kitamura en Yucatán. Rev. Chapingo Ser. Hortic. 15: 25-31. [ Links ]
Zamaninejad, M., S. K. Khorasani, M. J. Moeini, and A. R. Heidarian. 2013. Effect of salicylic on morphological characteristics, yield and yield components of corn (Zea mays L.) under drought condition. Eur. J. Exp. Bio. 3: 153-161 [ Links ]
Received: May 2016; Accepted: May 2017











 texto en
texto en 


