Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.51 no.4 Texcoco Mai./Jun. 2017
Crop science
MACF-IJ, automated method for measuring color and leaf area through digital images
1Facultad de Agricultura del Valle del Fuerte, Universidad Autónoma de Sinaloa, Calle 16 Avenida Japaraqui S/N, 81110. Juan José Ríos, Ahome, Sinaloa quintana.josegpe@gmail.com.
2Genética. Campus Montecillo. Colegio de Postgraduados, 56230. Estado de México.
3Campo Experimental Valle del Fuerte, Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP). Km 1609, Carretera Internacional México-Nogales, 81110. Juan José Ríos, Guasave, Sinaloa.
4Facultad de Agronomía y Veterinaria, Universidad Autónoma de San Luís Potosí. Carretera San Luis Potosí-Matehuala km 14.5, 78321. Soledad de Graciano Sánchez, San Luis Potosí.
Leaf color and area are important characteristics in plant physiology and genetic studies, but data collection is laborious and requires optical integrators and colorimeters. Nowadays, data collection could be automated through digital image analysis (DIA). Free software for DIA to measure leaf area or color is available, but user intervention is required to record dimensions or leaf color. This study shows the Macf-IJ routine for DIA to measure leaf area and color. Results from DIA of seven geometric figures were compared to physical measurements with a LI-COR 3000® Leaf Area Integrator. Additionally, leaf area was measured in 13 herbaceous plants, and color and relative chlorophyll content (RCC) was recorded for Rhynchosia minima (L.) DC with a SPAD 502®. DIA was performed with Digitizer 4.3.5® and Black Spot Leaf. Duration of each measurement was recorded for each case. The area measured with the integrator for any figure varied less than 8.96 % with Macf-IJ and less than 1.82 % with photographs. R2 between methods was 0.999 (p≤0.001). With exception of five grasses, leaf area measured with the integrator correlated well with the Macf-IJ (r>0.989, p≤0.001); Macf-IJ, Black Spot Leaf and Digimizer® had an R2 greater than 0.99 (p≤0.001). Duration was not significantly different between methods, but Macf-IJ registered additional data such as length (cm), width (cm), circularity (0-1), and red (R), green (G), and Blue (B) colors. There was correlation between RCC and green (r=-0.946, p≤0.01), red (r=-0.941) and blue (r=-0.881) colors and between RGB obtained the Digimizer® and CRC (r=-0.939, -0.942 and -0.811for red, green and blue colors, respectively). Macf-IJ took 4.43 s to measure leaves, while RCC took 38.37 s (p≤0.001). Macf-IJ is a quick and precise method that measures area, dimensions and leaf color through scanned images and digital photography.
Key words: leaf area; leaf color; digital images; LI-COR 3000; SPAD 502
El color y área foliar (AF) son importantes en estudios fisiológicos y fitogenéticos, pero su obtención es laboriosa, incluso con integradores y colorímetros. Ambas características pueden medirse con análisis de imágenes digitales (AID), pues, aunque existen programas gratuitos específicos para obtener el AF éstos requieren intervención del usuario, no registran las dimensiones ni coloración foliar. El objetivo de este estudio fue mostrar el funcionamiento de Macf-IJ para medir área y color foliar mediante AID. Los resultados se compararon con mediciones manuales y del integrador Li-Cor 3000® del área de siete figuras geométricas. También AF se midió en 13 herbáceas; el color y el contenido relativo de clorofila (CRC), con SPAD 502®, en Rhynchosia minima (L.) DC. El AID se realizó con Digimizer 4.3.5® y Black Spot Leaf. El tiempo se registró para cada caso. El área obtenida con integrador para una misma figura mostró variación inferior a 8.96 %, con Macf-IJ y con fotografías fue menor a 1.82 %; el R2 entre métodos fue 0.999 (p≤0.001). El AF se correlacionó entre el integrador y Macf-IJ (r>0.989, p≤0.001); excepto en cinco gramíneas, Macf-IJ, Black Spot Leaf y Digimizer® presentaron R2 ≥0.99 (p≤0.001). El tiempo no mostró significancia entre métodos, pero Macf-IJ registra longitud (cm), anchura (cm), perímetro (cm), circularidad (0-1), así como el color rojo (R), verde (G) y azul (B). Existió correlación del CRC con el verde (r=-0.946, p≤0.01), con el rojo (r=-0.941) y el azul (r=-0.881), entre el RGB obtenido con Digimizer® e CRC (r=-0.939, -0.942 y -0.811 para rojo, verde y azul, respectivamente). Macf-IJ utilizó 4.43 s por hoja y 38.37 s para CRC (p≤0.001). Macf-IJ es un método rápido y preciso para medir el área, dimensiones y color foliar mediante imágenes escaneadas y fotografía digital.
Palabras clave: área foliar; color; imágenes digitales; Li-Cor 3000; SPAD 502
Introduction
In most plants, the leaf is the main photosynthetic organ that performs important energy transfer and gas exchange functions (Dornbusch and Andrieu, 2010). Leaf area size is highly relevant to ecophysiological studies as an indicator of plant growth and development: it relates to interception of solar radiation, CO2 exchange, evapo-transpiration rate and photosynthetic efficiency (Backhaus et al., 2010; Misle et al., 2013).
Leaf area may be estimated using ruled paper, gravimetric techniques, manual and photoelectric measurements, calculation by leaf length and width (Pandey and Singh, 2011), or a combination of one or more of these procedures. Empirical models constructed with collected data estimate leaf area in a precise and non-destructive manner. However, if leaf size and shape vary greatly, estimated values might be incorrect (Bylesjö et al., 2008). Besides, conventional leaf area estimation methods are laborious and prolonged (Chaudhary et al., 2012; Varma and Osuri, 2013) as each leaf must be measured separately.
Leaf area measured with an electronic device is precise (in mm2) and immediate, and the device may be portable. However, usage of these devices on small leaves, like those found on wheat or early-developing leaves, is rather complicated (Cogliatti et al., 2010). For research in Mexico, the price of these devices is a limiting factor for widespread usage. Additionally, devices like the LI-COR 3000 (LICOR Biosciences, Lincoln, Nebraska, USA) do not measure leaf perimeter, width or length.
Digital image analysis (DIA) and processing is an alternative method to measure leaf area. Low cost devices, like desktop image scanners, could be employed for image acquisition (Varma and Osuri, 2013). Additionally, leaf color, an indicator of the nutritional state, health and senescence of the leaves (Riccardi et al., 2014; Wang et al., 2014), could be estimated instead of using portable reflectometers such as SPAD 502® (Soil Plant Analysis Development. Konica-Minolta Inc. Osaka, Japan). Easlon and Bloom (2014) pointed out that methods based on DIA are fast and precise, and they they are replacing equipment that use light obstruction to estimate the leaf area.
Leaf morphological traits and color can be obtained with widely used commercial software like Matlab® (MathWorks, Inc. Natick, Massachusetts, USA) (Dornbusch and Andrieu, 2010; Price et al., 2011; Ali et al., 2012; Corney et al., 2012; Chaudhary et al., 2012; Wang et al., 2014). A free-software option to record leaf characteristics is ImageJ (Warman et al., 2011; Green et al., 2012; Schneider et al., 2012; Maloof et al., 2013; Easlon and Bloom, 2014; Sauceda et al., 2015).
Leaf area can also be measured with Black Spot Leaf created in Python (Python Software Foundation), a free and specific software that runs under MS-DOS® (Microsoft Corporation, Redmond, Washington, USA). Black Spot Leaf can only calculate total area per image (Varma and Osuri, 2013). Instead, LAMINA, a software developed under Java®, can measure leaf area, length, width, and leaf area loss (Bylesjö et al., 2008). Easy Leaf Area, also Python software, can estimate total area per image, as well as area and length per leaf (Easlon and Bloom, 2014). LeafJ, a complement for ImageJ, calculates area and dimensions for leaves and petioles (Maloof et al., 2013).
However, the software noted above do not measure leaf color and require user intervention along several parts of the process that consumes more time. Greater automation could facilitate leaf area and color measurement, thus this research developed an ImageJ routine (Macf-IJ) that measures leaf area and leaf color through digital images. The objetive of our study was to perform a detailed description of the operation of the Macf-IJ routine and compare its precision and efficiency against other methods reported in the scientific literature. Our hypothesis was that the Macf-IJ routine estimates morphological variables and leaf color with precision, in one single pass.
Materials and Methods
Macf-IJ is a routine or macro developed under ImageJ 1.50b and is accessible in the toolbar or in a selectable command through the Plugins menu. In either case, the user can process and analyze individual images or all the contents in a folder, with just one click. The routine requires digitized images of the leaves. The images should preferably be scanned flat-faced on the middle of the scanner bed and saved in image format (jpeg, tiff, bmp, pgm, png). Alternatively, digital photographic images could be used. These photographs should frame the leaves on a white background, capture the image with the highest possible camera balance, and include a red object of known area in each image as a calibration reference.
This routine employs the Color_Transformer.java plug-in to record the color in the popular CIELab space that measures reflectance and transmittance of objects (Graeff et al., 2008). According to Rodríguez et al. (2012), it is used in colorimeters, spectrophotometers and radiometers. The use of such space allows for color comparison between devices that analyze coloration (Whan et al., 2014).
The routine automatically sets the scale based on image resolution. Required measurements are defined with the Set Measurements command... The process initiates by duplication of the original image and conversion of the duplicate to an 8-bit grayscale image (run(“8-bit”)). The image is then segmented through adjustment of color thresholds with the default method (setAutoThreshold (“Default”)). This process creates a binary image that differentiates between background (black) and leaves or regions of interest (white). Image particle analysis starts with the Analyze Particles... function, with size restriction of 0.5 to 500 cm2. Holes inside the canopy are excluded and regions of interest are added to the manager (Roi Manager).
The color in the RGB (Red, Green, Blue) space is obtained from the original image. The color image is separated into the three channels with the command Make Composite, alternatively the image could be transformed to the CIELab space (a more common method in commercial colorimeters), through RGB transformation with the Color Transformer complement. In both cases, for each color component, the analysis of the regions of interest added to the manager is executed (roiManager (“Measure”)). For each leaf, the values of area (cm2), length (cm), width (cm), perimeter (cm), and circularity (0 a 1) are recorded, as well as the intensity of red (R), green (G), blue (B) and the average color (RGB). The set of observations for each variable is stored in arrays, so they can be used afterwards in a of personalized results table.
Results are automatically saved in a comma delimited file (.csv), with the prefix Leaf Area Results, plus the name of the processed image (without the jpg extension). This file can be opened by any electronic spreadsheet (MS Excel®, Gnumeric, OpenCalc OpenOffice, and LibreOffice Calc). In the first column, called Sample, the image name is shown, followed by the duration (s), date and hour of the sample. A summary table, named Leaf Area Summary, is also created and shows total number of analyzed leaves, average leaf area (cm2), leaf length (cm), leaf width (cm), leaf perimeter (cm), leaf circularity (0 to 1), leaf color and duration (s) of image analysis.
The precision and speed of the Macf-IJ routine was assessed by comparison of data recorded with the LI-COR 3000® integrator, and the software Black Spot Leaf and Digimizer 4.3.5® (MedCalc Software bvba; Ostend, Belgium). Digimizer 4.3.5® measures color, it is free, and it facilitates image analysis. The duration for each measurement was registered for each case. Fresh leaves were randomly collected from 13 species (Table 1) from gardens at Colegio de Posgraduados, Montecillo Campus, Estado de Mexico. The species were identified based on the morphological description from the National Commission for the Knowledge and Use of Biodiversity (CONABIO, for its Spanish acronym, 2009). Area for each leaf was measured using the LI-COR integrator, and then it was digitalized with an HP® scanner, model M1132 MFP.
Color leaf images (RGB) were saved in jpeg format, at 300 dpi resolution, and dimensions of 2550 x 3507 pixels. Image acquisition lasted an average of 40 s, and file size was around 655 kb. Leaf color in the CIELab and RGB spaces was evaluated in the 13 species (data not shown), but only Rhynchosia minima (L.) DC, commonly named least snout-bean, was used for practical deductions for color comparison (RGB). Sampled leaves were under virus attack and were intense green, yellow and spotted. Relative chlorophyll content (RCC) was also quantified on these leaves with a SPAD 502®, and the duration of sampling was registered in both cases.
The area of seven geometric shapes was measured manually and with the aid of the LI-COR Li-3000®; in both cases, each shape was measured at least 30 times. These results were compared to DIA results of the scanned shapes at 100, 150, 200, 300 and 400 dpi resolution and to photographs from a Fujifilm® digital camera, model Finepix Z20fd (Fujifilm Corporation, China) in jpeg format. Digital images were captured under shaded conditions using ambient lighting. A white background was used, and a circular, red object, with a 3.09 cm diameter was placed in the frame as reference to calibrate the scale. This object was removed from the analysis by circularity restriction. The length of time required to take the photograph was 3.5 s, plus 1.5 s used to display it on the camera screen. Average file size was 1.08 MB. Image analysis was carried out in a computer with a 2.7GHz Intel Pentium® G630 processor, 3 GB of RAM, and Windows 8® 64-bit operating system.
Data analysis was performed with InfoStat 2014 (Di Rienzo et al., 2014). Data normality was determined via the test described by Shapiro and Wilk (1965), while homogeneity of variances was verified with the Barlett test (1937). The variable “Duration” did not fulfill the normality and homogeneity of variances tests, and it was transformed by reciprocal transformation. ANOVA and Tukey’s means comparison (p≤0.05) were performed for the length of time required to obtain leaf color and RCC. Other variables were compared through descriptive statistics, simple Pearson correlations (r), determination coefficients (R2), root mean squared errors (RMSE) and mean relative errors (MRE).
Results and Discussion
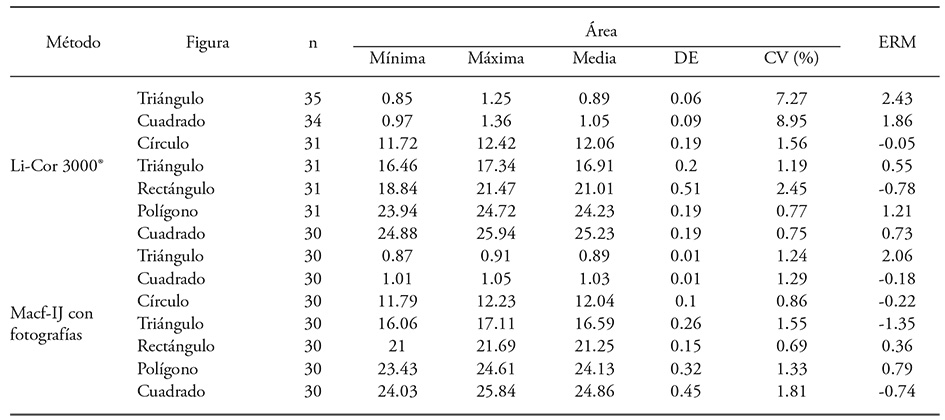
Regarding precision, the area of the small geometrical shapes varied less than 8.96 % when measured with the LI-COR LI-3000®, compared to less than 1.82 % variation when measured with photographs and the Macf-IJ routine (Table 2). The small triangle had the greatest mean relative error (MRE= 2.43 %) between the integrator and the manual procedure for the area, with standard deviation (SD) of 7.45 %. Easton y Bloom (2014) reported an MRE of -11.56 % ± 2.16 % for the LI-3000®, and they claim this method is less convenient than DIA to measure the area of small leaves.
Table 2 Descriptive statistics of the area of seven geometric shapes measured with an LI-3000® and DIA (Macf-IJ) of digital photographs.
N: Number of observations; SD: standard deviation; CV: coefficient of variation; MRE: mean relative error with respect to manual measurement.
According to our results, MRE was smaller and similar between the LI-3000® readings and the DIA of photographs (Table 2). This behavior could be the result of repeated readings when the operator considered the value erroneous: for the small triangle, 23.3 % of the readings were repeated. Small geometrical shapes project a shadow from the transparent band on the integrator that is recorded as additional area. Such error affects larger shapes too, but to a lesser degree. Occasionally, area is lost due to shape folding in the integrator bands. Another source of error for registering individual leaves occurs when the operator forgets to reset the integrator counter.
Average area of the geometric shapes showed a high correlation between manually measured values and those measured with the LI-3000® (R2=0.999, p≤0.001), with a mean relative error (MRE) of 0.83 % and a standard deviation (SD) of 1.08 %. Comparison between the values obtained with the Digimizer® software and the manual method showed that MRE was -0.50 % ± 0.85 (DE) and R2=0.999 (p≤0.001), while with the Macf-IJ MRE it was 0.32 % ± 1.0 and R2=0.999 (p≤0.001).
The MRE values in our study are smaller than those reported by Easlon and Bloom (2014). The authors compared the calculated area by a gravimetric technique and the area obtained with ImageJ and found that the corresponding MRE’s were 1.67 % and 7.21 %, respectively. These results indicate that the area was underestimated with ImageJ due to shadows produced by the scanner. The elimination of shadows prior to image analysis by software (GIMP, GNU Image Manipulation Program, for example) is implied. This procedure is not practical or necessary because ImageJ can differentiate between image background and sample leaves or objects of interest.
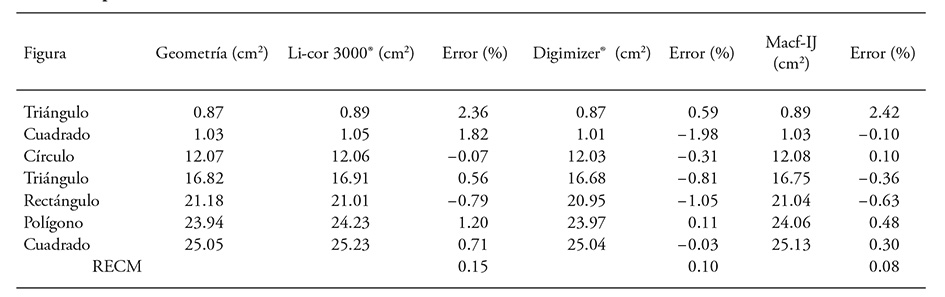
Compared to manual measurement, the highest accuracy was obtained analyzing scanned images with Macf-IJ (RMSE = 0.08), followed by Digimizer® (Table 3). These results agree with Ali et al. (2012), who obtained higher relationship between the manual area measurements of five geometric shapes and image analysis by Optileaf (developed with Matlab®), compared to areas measured with LI-3100®.
Table 3 Comparison of manually measured area with a LI-3000®, and through image analysis (300 dpi) of seven geometric shapes.
RMSE: root mean squared error.
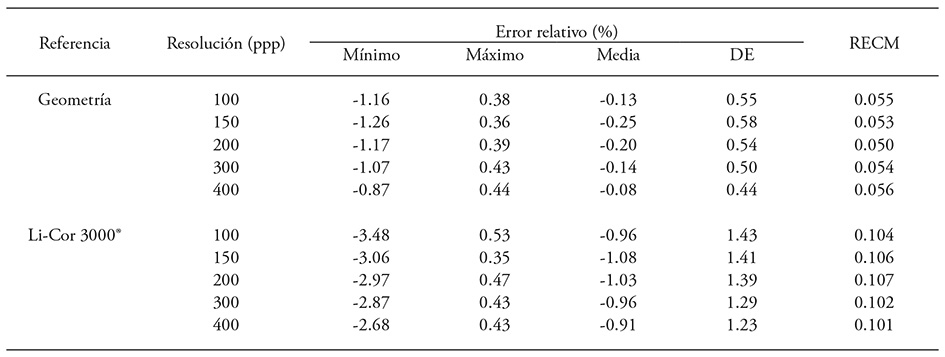
Relative area differences between the five image resolutions were less than 0.25 % compared to manual calculations. Areas measured with the LI-3000® had a relative mean error which varied from 0.91 % to 1.08 % (Table 4). The area of the geometric shapes in all resolutions were accurate compared to the manual measurements (RMSE≤0.056) and to the integrator values, even though RMSE was almost double (Table 4). The coefficient of determination between the measured area in each resolution and the value measured by geometry was 1.0, while the coefficient of determination for the LI-3000® values and geometry values was 0.999. These results are similar to those described by Bradshaw et al. (2007) who reported an R2 of 0.972 between area of soybean leaves (Glycine max L.) measured with an integrator (LI-3100®) and the image analysis with Adobe® Photoshop® (Adobe Systems Incorporated, San José, CA.) with resolution of 300, 600 and 1200 dpi.
Table 4 Percentage differences and RMSE between the area obtained with Macf-IJ in at different resolutions compared to the manual method and to LI-3000®.
n=7; SD: standard deviation; RMSE: root mean squared error.
Acquisition of 100 dpi images took 17.5 s; 150, 200 and 300 dpi images required between 36.4 s and 40.3 s; and a 400 dpi image took 106.8 s. There is a close relationship between image resolution and time required for DIA with Macf-IJ (r=0.99, p≤0.01), as pointed out by Dornbusch and Andrieu (2010). They reported that processing time depends on image size and resolution. Our results also agree with Chen et al. (2011) who reported that when performing DIA with Adobe® Photoshop® to estimate leaf area of Euonymus japonicas, greater resolution digital photographs increase DIA duration. Thus, DIA on lower than 300 dpi images would decrease digitalization time and file size.
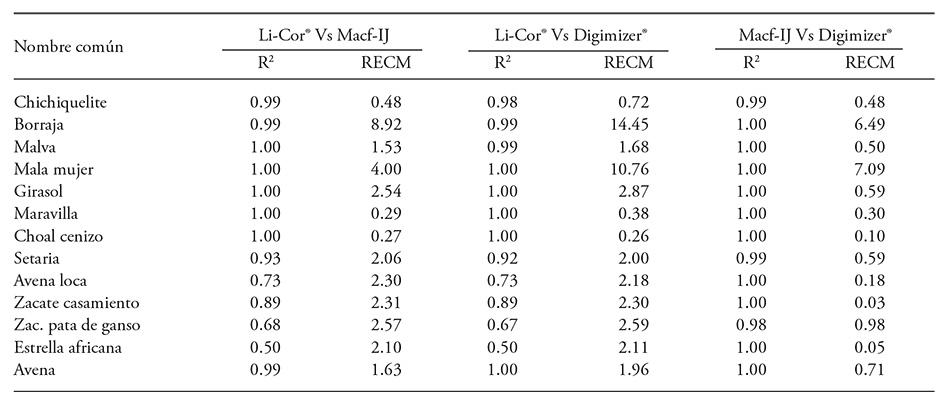
For most species, leaf area recorded with the LI-3000® showed high correlation with the area obtained by the Macf-IJ routine. Except for estimated areas on five grass species, R2 was greater than 0.98 (Table 5). Green et al. (2012) showed similar results using leaves of Brassica rapa; they achieved R2 of 0.979 between the area obtained with the PhenoPhyte software and the area measured with an integrator. Results also coincide with the high correlation (r=0.999) between the area measured with Optileaf and an LI-3100® in 40 leaves of different form and size, as shown by Ali et al. (2012). Chaudary et al. (2012) also obtained an accuracy above 99 % between the area of 70 leaves measured with Matlab 2010a® and through estimation with ruled paper. Green et al. (2012) reported a close relationship between area values obtained with PhenoPhyte and ImageJ (r=0.998); in this research, the coefficients observed between Digimizer® and Macf-IJ (Table 5) were similarly close.
Table 5 Coefficient of determination and RMSE between th leaf area obtained with LI-3000® and with DIA (300 dpi) of leaves of 13 plant species.
RMSE: root mean squared error.
Buffalo bur nightshade and sow thistle leaves are difficult to measure using a LI-3000®. Buffalo bur leaves have long and sharp thorns. Damage to the LI-3000® belt was prevented by sampling only 15 leaves, and additional time was spent preventing damage. Sow thistle leaves tended to fold as they passed on the transparent belt and required repetition of the measurement which took additional time (23.4 s). Despite careful handling and repetition, area miscalculation is reflected in higher RMSE (Table 5). Either situation did not interfere with DIA, but digitalization time increased due to greater leaf size (Table 6) that allowed only only one or two leaves to be captured per image.
Table 6 Average leaf area and measurement duration with a LI-3000® and with DIA (300 dpi) of leaves from 13 different plants species.
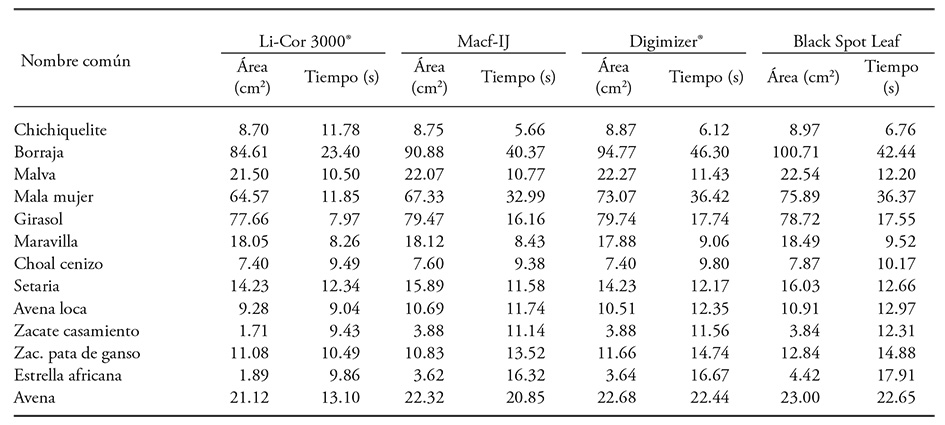
Leaf area for Mexican lovegrass and African stargrass measured with LI-3000® tended to be less (Table 6). Compared to DIA, the difference was 55.8 % and 47.8 %, respectively, and it was caused by leaves folding along the length of the central rib. A comparable situation was reported in wheat by Sauceda et al. (2015) who indicated that correct measurement of leaf area with DIA is difficult when leaves of this cereal whither and lose turgor. Unwanted folding is solved by fixing the leaf with transparent adhesive tape; however; this extra process requires additional time but prevents overlap (Dornbusch and Andrieu, 2010). In grasses, leaf folding is a serious setback that hinders measurement with a LI-3000®. Mean leaf area values per species obtained with Black Spot Leaf and Macf-IJ had an R2=0.994 (p≤0.001), but the Black Spot Leaf software over-estimated leaf area in most cases (Table 6).
Average measurement duration with the LI-3000® per leaf was 11.35±3.95 s, while Macf-IJ required 16.07±10.03 s, Digimizer® took 17.45±11.57 s, and Black Spot Leaf needed 17.57±10.58 s; there was no significant difference (p>0.134) among methods though. These results do not coincide with O’neal et al. (2002). They reported that measurement of total leaf area and damaged leaf area on five soybean leaves (Glycine max L.) required less time with DIA (3:25 ± 0:03, min:s) than with a LI-3000® (6:10 ± 0:10). The Macf-IJ routine records area, width, length, perimeter and color (RGB) of the leaf in 41.4 s per image, which includes image digitalization and analysis. Analysis duration is similarly reported by Easlon and Bloom (2014) when the image is captured with a digital camera (about 35 s) and analyzed with Easy Leaf Area (5 s). However, this duration contrasts with Davidson’s report (2011), that image digitalization with a scanner took around five minutes plus three minutes for ImageJ analysis. The additional analysis time might be caused by manual leaf selection prior to measurement as per the author’s recommendation. Macf-IJ identifies and measures leaves automatically. Digital photographs analysis took 6.0 s for capture and 3.87 s for Macf-IJ analysis; Easlon y Bloom (2014) reported longer duration of the analysis.
The duration of DIA indicates the complexity of the analysis, number of variables that need to be processed (image size, image resolution, software, hardware), and processing type (manual or automatic). About 7.5 s were need to acquire leaf color in CieLab; 80 % longer than the wmeasurement in the RGB space (1.4 s). Dornbusch and Andrieu (2010) employed a computer with an INTEL® Xeon® (2 GHz) processor and MATLAB 7.04® to record leaf based on width and length in almost 15 s.
DIA duration also depends on leaf area size: more leaves can be digitalized in one image if they are smaller. Divine nightshade plant required less digitalization time (5.59 s) per leaf (two images included 40 leaves) and 5.66 s for processing. The common lambsquarters plant took 9.38 s per leaf, while the marvel of Peru plant needed 8.43 s (Table 6). In these cases, a maximum of three images were obtained. This method is limited by the number of leaves that can be scanned at once, but a more complete leaf profile can be acquired (length, width, perimeter, circularity and color.
Relative chlorophyll content (RCC) measured with the SPAD 502® showed a relationship to the green channel (G, r=-0.946, p≤0.01), red channel (R, r=-0.941) and blue channel (B, r=-0.881). Correlation coefficients between RGB channels quantified with Digimizer® and RCC were -0.939, -0.942 and -0.811 for red, green and blue, respectively. These results coincide with conclusions by Wang et al. (2014) in rice (Oryza sativa L.) who found lower correlation coefficients when RCC was plotted against red (r=-0.68), green (r=-0.67) and blue (r=-0.48) color content. They also indicate that color analysis is a simple method for assessing nitrogen status in the rice plant. Teoh et al. (2012) also reported a strong relationship between RCC with red (r=-0.97) and green (r=-0.82) colors in rice leaves. Riccardi et al. (2014) found the same tendency when the RGB color of photographic images and the corresponding RCC was plotted against leaf chlorophyll concentration from amaranth (Amaranthus sp.) and quinoa (Chenopodium quinoa Wild.) leaves. A higher correlation occurred among RGB values and chlorophyll concentration in the leaf (r=-0.96) than between RCC measured with the SPAD 502® and chlorophyll concentration (r=0.92).
Dimensional and color analysis of the leaves with the Macf-IJ routine required less time (4.43 s) than RCC (p≤0.001) measurement, even if only one lecture per leaf was taken. For example, snoutbean leaves with an average size of 10.35 cm2 took 24.2 s per leaf, but 30.15 s for leaves of 4.72 cm2 average area. Recording three readings per leaflet on the same leaves required additional time, from 49.52 to 59.22 s respectively (DMS=7.53, p≤0.05). However, accuracy between RCC and the green color improved with three measurements per leaf, as the coefficient of determination increased from 0.893 to 0.927, while the coefficient of determination for the red color increased from 0.881 a 0.917, and for the blue color from 0.774 to 0.867. These results highlight the effect of leaf size on RCC measurement and the number of SPAD 502® observations needed for greater confidence to represent actual leaf conditions. Chang and Robison (2003) mention reliable RCC values in maize leaves need multiple SPAD 502®, at the expense of more time.
The SPAD 502® sensor did not measure the yellow color, which happened when the green color component (G), acquired with DIA, showed a value higher than 150. This limitation is evident when leaves contain areas with chlorosis, like Rhynchosia minima (L.) DC., that trigger failed readings (the SPAD 502® sensor marks an error) and increases the time required to obtain the RCC. Additionally, intense color variation on the leaf affects the relationship between color components (RGB) and SPAD 502® readings, because the sensor samples 6.0 mm2 on the leaf and is limited to 12 mm reach. According to Ali et al. (2012), the thickness of the leaves, biotic stresses, and health are affect these readings. Riccardi et al. (2014) mention that RGB values captured through digital photographs directly on the field is a faster, more effective, and less expensive method than SPAD 502® readings to estimate chlorophyll content in amaranth and quinoa leaves.
Conclusions
Macf-IJ is an easy, fast and accurate method to measure color and area of leaves through scanned images and digital photography. Image processing and analysis is thus automated, and the software provides leaf dimensions and color of the complete leaf blade. The hypotesis that Macf-IJ measures, exactly and in one step, leaf color and morphological variables, was supported.
Macf-IJ measures color in smaller leaves, like Rhynchosia minima (L.) DC., quicker than obtaining chlophyll relative content with the SPAD 502®. Additionally, color acquisition with Digimizer® and Macf-IJ is advantageous, especially when there are chlorotic areas or color variations in the leaf blade
Literatura Citada
Ali, M. M., A. Al-Ani, D. Eamus, and D. K. Y. Tan. 2012. A New image-processing-based technique for measuring leaf dimensions. American-Eurasian J. Agric. Environ. Sci. 12: 1588-1594. [ Links ]
Backhaus, A., A. Kuwabara, M. Bauch, N. Monk, G. Sanguinetti, and A. Fleming. 2010. LEAFPROCESSOR: A new leaf phenotyping tool using contour bending energy and shape cluster analysis. New Phytol. 187: 251-261. [ Links ]
Bartlett, M. 1937. Properties of sufficiency and statistical tests. Proceedings of the Royal Society of London. Series A, Math. Phys. Eng. Sci. 160: 268-282. [ Links ]
Bradshaw, J. D., M. E. Rice, and J. H. Hill. 2007. Digital analysis of leaf surface area: Effects of shape, resolution, and size. J. Kansas Entomol. Soc. 80: 339-347. [ Links ]
Bylesjö, M. et al. 2008. LAMINA: A tool for rapid quantification of leaf size and shape parameters. BMC Plant. Biol. 8: 82. [ Links ]
Chang, S. X., and D. S. Robison. 2003. Nondestructive and rapid estimation of hardwood foliar nitrogen status using the SPAD-502 chlorophyll meter. For. Ecol. Manage. 181: 331-338. [ Links ]
Chaudhary, P., S. Godara, A. N. Cheeran, and A. K. Chaudhari. 2012. Fast and accurate method for leaf area measurement. Int. J. Comput. Appl. 49: 22-25. [ Links ]
Chen, B., Z. Fu, Y. Pan, J. Wang, and Z. Zeng. 2011. Single leaf area measurement using digital camera image. In: Li, D., Y. Liu, Y. Chen (eds.). Computer and Computing Technologies in Agriculture IV. 4th IFIP TC 12 Conference, CCTA 2010, Nanchang, China, October 22-25, 2010, Selected Papers, Part II. Springer Berlin Heidelberg. 345: 525-530. [ Links ]
Cogliatti, D. H., M. F. Cataldi, y F. Iglesias. 2010. Estimación del área de las hojas en plantas de trigo bajo diferentes tipos de estrés abiótico. Agriscientia 27: 43-53. [ Links ]
CONABIO (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad). 2009. ¿Cómo identifico una planta? ¿Cómo identifico una planta? http://www.conabio.gob.mx/malezasdemexico/0claves/0claves-inicio.htm (Consulta: febrero, 2015). [ Links ]
Corney, D.P.A., H.L.Tang, J.Y.Clark, Y. Hu, and J. Jin 2012. Automating digital leaf measurement: The tooth, the whole tooth, and nothing but the tooth. PLoS ONE 7: doi: 10.1371/journal.pone.0042112. [ Links ]
Davidson, A. 2011. Measuring leaf perimeter and leaf area. Prometheus wiki. http://prometheuswiki.org/tiki-index.php?page=Measuring+leaf+perimeter+and+leaf+area (Consulta: octubre, 2015). [ Links ]
Di Rienzo, J. A., F. Casanoves, G. Balzarini, L. González, M. Tablada, y W. Robledo. 2014. InfoStat, versión 2014, Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. [ Links ]
Dornbusch, T., and B. Andrieu. 2010. Lamina2Shape-An image processing tool for an explicit description of lamina shape tested on winter wheat (Triticum aestivum L.). Comput. Electron. Agr. 70: 217-224. [ Links ]
Easlon, H. M., and A. J. Bloom. 2014. Easy Leaf Area: Automated digital image analysis for rapid and accurate measurement of leaf area. Appl. Plant. Sci. 2: 1400033. doi: 10.3732/apps.1400033. [ Links ]
Graeff, S., J. Pfenning, W. Claupein, and H. P. Liebig. 2008. Evaluation of image analysis to determine the N-fertilizer demand of broccoli plants (Brassica oleracea convar. botrytis var. italica). Adv. Opt. Technol. 2008: doi:10.1155/2008/359760. [ Links ]
Green, J. H., H. Appel, E. Rehrig, J. Harnsomburana, J. Chang, P. BalintKurti, and C. Shyu. 2012. PhenoPhyte: A flexible affordable method to quantify 2D phenotypes from imagery. Plant Method 8: 45. [ Links ]
Maloof, J. N., K. Nozue, M. R. Mumbach, and C. M. Palmer. 2013. LeafJ: An ImageJ plugin for semi-automated leaf shape measurement. J. Vis. Exp. 71: e50028. doi:10.3791/50028. [ Links ]
Misle, E., B. Kahlaoui, M. Hachicha, and P. Alvarado. 2013. Leaf area estimation in muskmelon by allometry. Photosynthetica 51: 613-620. [ Links ]
O’neal, M. E., D. A. Landis, and R. Isaacs. 2002. An inexpensive, accurate method for measuring leaf area and defoliation through digital image analysis. J. Econ. Entomol. 95: 1190-1194. [ Links ]
Pandey, S. K., and H. Singh. 2011. A Simple, cost-effective method for leaf area estimation. J. Botany. Article ID 658240. doi:10.1155/2011/658240. [ Links ]
Price, C. A., O. Symonova, Y. Mileyko, T. Hilley, and J. S. Weitz. 2011. Leaf extraction and analysis framework graphical user interface: Segmenting and analyzing the structure of leaf veins and areoles. Plant Physiol. 155: 236-245. [ Links ]
Riccardi, M., G. Mele, C. Pulvento, A. Lavini, R. d’Andria, and S.-E. Jacobsen. 2014. Non-destructive evaluation of chlorophyll content in quinoa and amaranth leaves by simple and multiple regression analysis of RGB image components. Photosynth. Res. 120: 263-272. [ Links ]
Rodríguez P., F. J., L. Gómez, M. Melgosa, B. Gordillo, M. L. González , and F. J. Heredia. 2012. Ripeness estimation of grape berries and seeds by image analysis. Comput. Electron. Agr . 82: 128-133. [ Links ]
Sauceda A., C. P. et al. 2015. Un método preciso para medir severidad de roya de la hoja (Puccinia triticina Eriksson) en trigo. Rev. Fitotec. Mex. 38: 427-434. [ Links ]
Schneider, C. A., W. S. Rasband, and K. W. Eliceiri. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Method. 9: 671-675. [ Links ]
Shapiro, S. S., and M. B. Wilk. 1965. Analysis of variance test for normality (complete samples). Biometrika 52: 591-611. [ Links ]
Teoh, C. C., D. A. Hassan, M. M. Radzali, and J. J. Jafni. 2012. Prediction of SPAD chlorophyll meter readings using remote sensing technique. J. Trop. Agric. Food Sci. 40: 127-136. [ Links ]
Varma, V., and A. M. Osuri. 2013. Black Spot: A platform for automated and rapid estimation of leaf area from scanned images. Plant Ecol. 214: 1529-1534. [ Links ]
Wang, Y., D. Wang, P. Shi, and K. Omasa. 2014. Estimating rice chlorophyll content and leaf nitrogen concentration with a digital still color camera under natural light. Plant Method. 10: 36. [ Links ]
Warman, L., A. T. Moles, and W. Edwards. 2011. Not so simple after all: Searching for ecological advantages of compound leaves. Oikos 120: 813-821. [ Links ]
Whan, A.P., A.B.Smith, C.R.Cavanagh, J.-P.F.Ral, L.M. Shaw, C.A. Howitt, and L. Bischof. 2014. GrainScan: A low cost, fast method for grain size and colour measurements. Plant Method. 10: 23. [ Links ]
Received: June 2016; Accepted: November 2016











 texto em
texto em 



