Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Agrociencia
On-line version ISSN 2521-9766Print version ISSN 1405-3195
Agrociencia vol.51 n.3 Texcoco Apr./May. 2017
Natural Renewable Resources
Above-ground net primary productivity in commercial Eucalyptus urophylla S. T. Blake plantations in Huimanguillo, Tabasco, Mexico
1Postgrado en Ciencias Forestales, Colegio de Postgraduados. 56230. Carretera México-Texcoco Km. 36.5. Montecillo, Estado de México. (adrian.hernandezr90@gmail.com).
2Campo Experimental Chetumal, INIFAP. 77900. Km. 25, Carretera Chetumal-Bacalar, Chetumal, Quintana Roo.
3Research Forester, US Forest Service. 11 Campus Blvd. Ste. 200 Newtown Square, United States.
4GRANFLOR. Avenida Carlos Gomes, 1200 CJ. 502-Bairro Mont Serrat-Porto Alegre, Rio Grande del Sur, Brasil.
The above-ground net primary productivity (ANPP) is an indicator of the plantations’ performance. The objective of this study was to estimate litterfall production and ANPP in commercial Eucalyptus urophylla S. T. Blake forest plantations in Huimanguillo, Tabasco, Mexico. The null hypothesis was that ANPP decreases as age increases. In order to estimate ANPP, we considered: 1) The annual total litterfall production (TLP), according to the information from 112 litter-traps, distributed in 1-7 years old plantations, and monthly litterfall measurements; 2) the increment in biomass per site, estimated with the initial and final biomass of 28 sampling sites (500 m2), distributed in plantations with a completely randomized experimental design. Biomass equations reported for this species were used to estimate the TLP and the biomass increment, both by component and total. ANOVA and Tukey’s multiple comparison test were applied in order to detect TLP and ANPP differences by plantation age. The plantations’ TLP average was 4.289 Mg ha-1 year-1; the highest production was achieved at 7 years-old plantation (5628 Mg ha-1 year-1). Peak litterfall was obtained from April to June (37.9 % of the annual total). The lower production matched the beginning of the rainy season (July to September). The average ANPP was 26.26 Mg ha-1 year-1, equivalent to an 84.0 % and a 16.0 % increment in biomass and litterfall, respectively. The highest production was achieved in one- and two-years plantations. Plantations show higher biomass growth in younger age.
Key words: Above-ground biomass; litterfall; biomass increment
La productividad primaria neta aérea (PPNA) es un indicador del rendimiento de las plantaciones. El objetivo del estudio fue estimar la producción de hojarasca y PPNA en plantaciones forestales comerciales de Eucalyptus urophylla S. T. Blake en Huimanguillo, Tabasco, México. La hipótesis nula fue que la PPNA disminuye con el aumento de la edad. Para estimar la PPNA se consideró: 1) la producción total anual de hojarasca (PTH), estimada con la información de 112 trampas de captura, distribuidas en plantaciones de 1 a 7 años de edad, y mediciones mensuales de caída de hojarasca; 2) el incremento en biomasa por sitio, estimado con la biomasa inicial y final de 28 sitios de muestreo de 500 m2, distribuidos en las plantaciones con un diseño experimental completamente al azar. La estimación de la PTH e incrementos en biomasa se realizó por componentes y total con las ecuaciones de biomasa reportadas para la especie. Para detectar las diferencias en PTH y PPNA por edad se aplicó ANDEVA y la prueba Tukey para comparación de las medias. El promedio de la PTH en las plantaciones fue 4.289 Mg ha-1 año-1, la producción mayor fue a la edad de 7 años (5.628 Mg ha-1 año-1). El pico de caída de hojarasca se obtuvo de abril a junio (37.9 % del total anual). La producción menor correspondió al inicio de los meses de lluvia (julio a septiembre). La PPNA promedio fue 26.26 Mg ha-1 año-1, equivalente a 84.0 % de incremento en biomasa y 16.0 % de caída de hojarasca, y la mayor se presentó en plantaciones con edades de uno y dos años. Las plantaciones presentan crecimiento mayor de biomasa en edades tempranas.
Palabras clave: Biomasa aérea; incremento en biomasa; hojarasca
Introduction
The increase in the area of commercial or restoration forest plantations has contributed to decrease the pressure over natural forests (FAO, 2006). Commercial plantations partially offer the forest raw materials that would otherwise be required from forest resources and represent an economic and social production alternative (Alice et al., 2004; FAO, 2015). In addition -compared to natural forests-, the offer of plantation timber has competitive advantages, as a result of lower production costs and timber prices, and it is an alternative that reduces the pressure over natural resources -mainly over tropical forests, which suffer one of the highest deforestation and degradation rates in the world compared to other ecosystems (Velázquez et al., 2002; FAO, 2006). Therefore, the natural forest resources consumption has been largely replaced by commercial forest plantations (CFP) products (FAO, 2015).
Living biomass is an indicator of the productivity, energy potential, and carbon absorption capacity of forest plantations. This indicator shows the plantation’s development status and the performance potential of a single species in terms of volume (m3 ha-1), in a specific place, at a certain age, and with a particular management type (Castañeda-Mendoza et al., 2012). CFPs’ productivity must be estimated in order to find out their biomass production dynamics. Gross primary productivity (GPB) is an indicator that includes the total amount of new organic matter (biomass) fixed by plants in a time interval and given area, without taking into consideration the losses generated by respiration. In order to find out the amount of biomass that was fixed or destined to each structure of the plant (in a time interval), it is recommended to estimate the above-ground net primary productivity (ANPP), which is the GPB minus the total respiration required for plant growth within the ecosystem (Grier et al., 1989; Clark et al., 2001a; Li et al., 2015).
In order to obtain an accurate estimation of ANPP in CFP, we need to substract the estimation of the trees’ respiration and to consider as ANPP the integration of two factors: the total increment of living biomass and the biomass losses as a result of the fall of fine detritus (litterfall) in a time interval (Clark et al., 2001a; Salas and Infante, 2006; Smith and Smith, 2007). Litterfall is fine plant material accumulated over the soil (leaves, flowers, fruits, seeds, and branches). Its degradation is a process that allows the nutrients to circulate in the ecosystem and maintains the soil fertility (Salas and Infante, 2006; González et al., 2013; Marmolejo et al., 2013). The positive integration of these two components to estimate NPP represents the increment in the biomass accumulation in CFPs in a fixed time (Miquelajauregui, 2013). The biomass increment enables the quantification of timber-yielding stocks in natural forests and plantations, a value necessary to offer products to the forest sawmill or pulp industry.
The objective of our study was to estimate the litterfall production and the above-ground net primary productivity in commercial Eucalyptus urophylla S. T. Blake forest plantations in Huimanguillo, Tabasco, Mexico. This was done in order to find out the productivity biomass dynamics in the plantations in a year of growth. The null hypothesis was: as age increases, ANPP decreases.
Materials and Methods
Study area
The study was carried out in commercial E. urophylla plantations established in the municipality of Huimanguillo, Tabasco, Mexico (17° 55’ N, 94° 06’ W, at 30 m average altitude) (Figure 1). The climate is hot and humid (annual average), with copious summer rains, 2500 mm mean annual precipitation, and 26 °C mean annual temperature. The plantations are grown in Phaeozem soil type ( INEGI, 2005).
Plantations characterization
The study was carried out in 1-7 years plantations. The space between plants and rows is 2×3 or 2.5×3.5 m, with an average density of 1667 and 1143 trees per hectare. The plants are improved E. urophylla clones (Table 1) and their purpose is to supply the domestic pulp market.
Filed data collection
Litterfall production
A random sampling system of 28 sites, 500 m2 each, was established in the E. urophylla plantations, placing four sites by plantation age. During one year (August 2014-July 2015), the litterfall biomass was collected monthly in 112 1-m2 (collection area) litter-traps (Arriaza, 2006; Martínez et al., 2006). Four litter-traps were set in each site, with a total of 16 litter-traps per planting age. The monthly measurements covered the four seasons of the year, which allowed us to detail the dynamics of annual litterfall production in the plantations.
In order to avoid decomposition, the litterfall of each trap was dried in a drying oven, at 70 °C during 72 h. The material of each litter-trap was separated into leaves, branches (diameter <2.0 cm), fruits, and bark, and its dry weight was recorded. With these data, the monthly litterfall rate (Mg ha-1 month-1) of each structure was calculated, using the estimated average per age. The annual litterfall rate (Mg ha-1 year-1) was estimated adding up the monthly measurements per site. This method has been used in microphyll desert scrublands, tropical rainforests, temperate forests, and plantations of E. saligna, Albizia facaltaria, Pinus greggi, P. cembroides, P. patula, P. taeda, and Bambusa oldhamii (Binkley and Ryan, 1998; Pérez et al., 2006; Quinto et al., 2007; Navar and Jurado, 2009; Castañeda-Mendoza et al., 2012; Gutiérrez et al., 2012; González et al., 2013; Kotowska et al., 2015).
Above-ground net primary productivity (ANPP)
The ANPP calculation in Mg ha-1 year-1 included the integration of the increment in organic matter and the production of litterfall, over a given time interval (Clark et al., 2001b; Hanson et al., 2003; Kotowska et al., 2015).
ANPP was estimated using the method described by Clark et al. (2001a), which considers the total biomass increment, the individuals’ mortality, the joint growth of evaluated trees, and the litterfall production in the ecosystem, assuming a continuous replacement rate, as represented by the following expression:
The individual biomass was estimated using the allometric equations developed by Hernández-Ramos et al. (unpublished results) for E. urophylla in the study area (Table 2). The values of Diameter at breast height (Dn) and total height (At) of the trees measured in July 2014 and August 2015, at 28 sampling sites, were used for these equations.
Table 2 Allometric equations used to estimate the total and per components above-ground biomass of Eucalyptus urophylla, in Huimanguillo, Tabasco, Mexico.

Dn: Diameter at breast height (1.30 m) (cm), At: total height (m).
Biomass increment (Mg ha-1 year-1): total and by structural component biomass (trunk, branches, and leaves) was estimated at the beginning (t1) and at the end (t2) of the analysis period. Biomass of the trees lost by mortality was added to the second sampling. The increase is defined as the difference in biomass between t2 and t1, according to the following equation (Klepac, 1983; Clark et al., 2001a):
where: Btt2't1: total above-ground biomass calculated in time 1 and 2 (Mg ha-1 year-1).BΘ: biomass of trees lost by mortality (Mg ha-1 year-1).
Annual fine litterfall: it was estimated adding the total litterfall (g m-2 month-1) that fall monthly per component in 1 m2, during 12 months, in order to complete one E. urophylla growth cycle in plantation conditions.
Comparative analysis of ANPP and litterfall production
Each sampling site was considered as a completely randomized distribution experimental unit. Each site’s age was considered as the primary factor (treatment), the month was the secondary factor, and the litterfall production was the dependent variable. Means of total and per component ANPP litterfall production were analyzed with ANOVA. The comparative test included the monthly and annual results, in order to determine the higher and lower production season. The production differences between plantations were analyzed using Tukey’s multiple comparison test. Statistical analyzes were performed in SAS 9.4 statistical software.
Results and Discussion
Litterfall production rate by structural component and total
There was a great variation in the annual total litterfall production (TLP) between different age plantations. The annual total litterfall production estimated ranged from 4.068 to 5.628 Mg m-2 year-1 for 1-7 years plantations. The largest production was observed in the 7-years plantation, followed by the 2-years plantation, and the smaller values belonged to the 4- and 5-years plantations. The remaining plantations had similar TLP values (Figure 2).

Figure 2 Annual total litterfall production, leaves, branches (A) and fruits (B), in Eucalyptus urophylla plantations, in Huimanguillo, Tabasco, Mexico.
The annual total and per component litterfall productions were statistically different (p=0.05) between ages (Table 3). Litterfall low values in 4- and 5-years plantations can be attributed to a greater competition with the understory, generated by the stoppage of weed control practices, given the species’ dominance at that age, and by the clonal differences between plantations.
Table 3 Total and by structural component average annual litterfall production (g m-2 year-1) in Eucalyptus urophylla plantations, in Huimanguillo, Tabasco, Mexico.
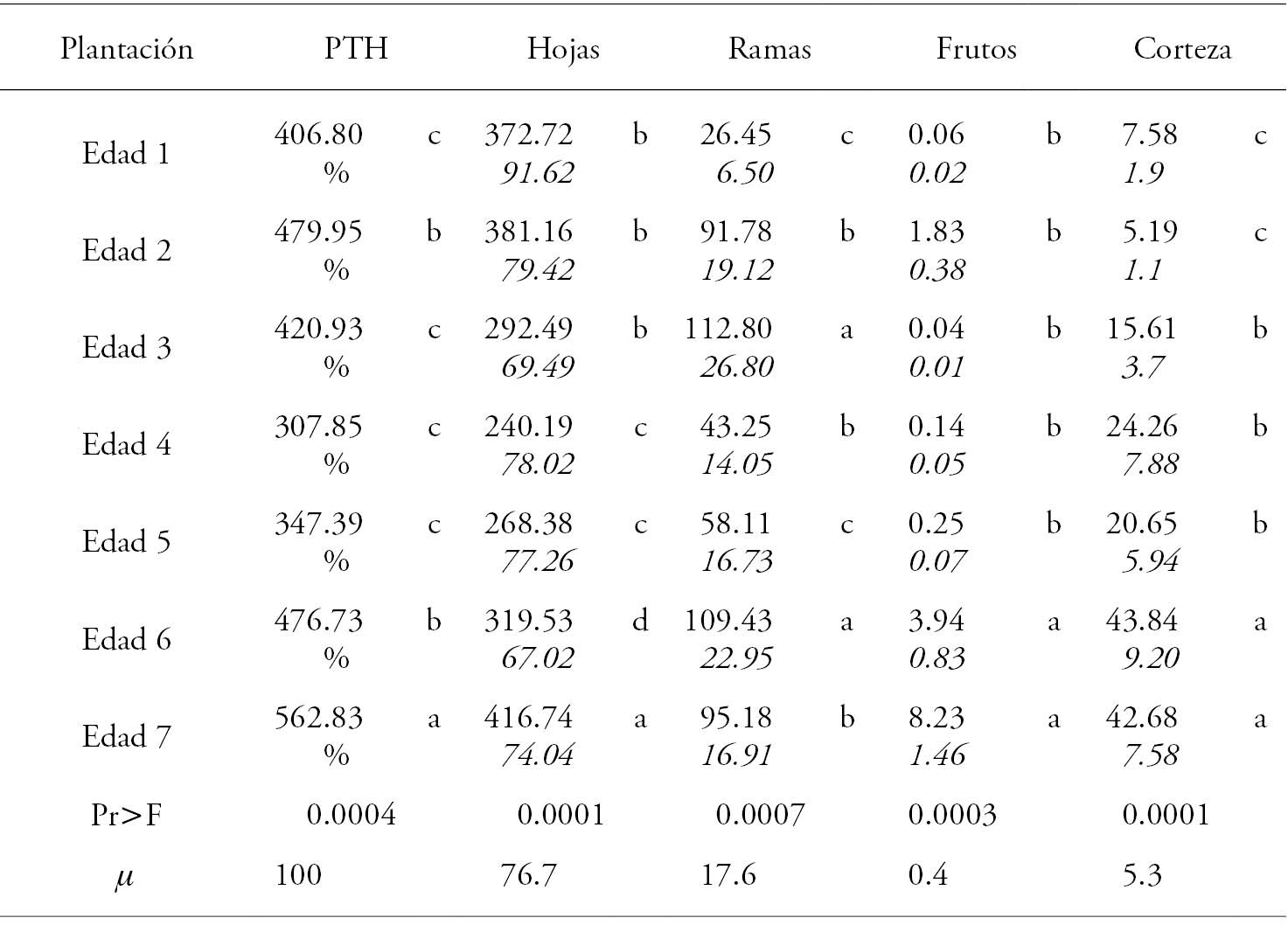
PTH: annual total litterfall production. Means with a different letter in each column are statistically different; µ: PTH proportion mean for each component.
The production trends per component (leaves, branches and fruits) were similar to those of the overall production. The largest number of fallen leaves was found in the 7-years plantation and the lowest in the 4- and 5-years plantations. The branches maximum value was presented in the 3-years plantations and the minimum in the 1-years plantations. The highest fruit production occurred in the 6- and 7-years plantations.
Leaves made the greatest contribution to litterfall, representing 91.62 to 67.02 % in 1 to 7 years plantations. The second largest contribution was made by branches, with a minimum and maximum of 6.5 and 26.80 %, respectively. Fruits represented less than 10 % of TLP (Table 4).
Table 4 Average monthly litterfall production (g m-2 month-1) in Eucalyptus urophylla plantations, in Huimanguillo, Tabasco, Mexico.

PTH: total litterfall production. Means with a different letter in each column are statistically different.
The average annual litterfall production (4.289 Mg ha-1 year-1) was similar to the production in commercial plantations of temperate climate species, such as 13 and 15 years P. patula plantations without fertilization (5.768 and 4.365 Mg ha-1 year-1) in the north of the State of Veracruz (Vásquez et al., 2015); 26-years-old P. radiata, with the vegetation associated with 3.206 Mg ha-1 year-1, in the Valdivia region, Chile (Huber and Oyarzún, 1983). Gutiérrez et al. (2012) reported lower litterfall production values in P. greggii and P. cembroides plantations established in Coahuila, Mexico (1.072 and 0.976 Mg ha-1 year-1), which is understandable because P. greggii and P. cembroides are slow growing species, compared to E. urophylla.
In species established in tropical regions (specifically in Junin, Peru), Gamarra (2001) reported that E. globulus Labill, produced 4.99 Mg ha-1 year-1 of litterfall. Compared to plantations in temperate regions, plantations in tropical climates show higher production at a younger age. This is attributed to the fact that tropical regions are more productive than temperate ones, due to the high water availability and higher temperatures, which are variables that significantly influence the tropical species’ growth; the opposite occurs in temperate ecosystems (Gómez and Gallopin, 1991).
Monthly litterfall production rate
The months with the highest production of leaves and branches -regardless of plantation age-, were April, May and June. Joint production during these months matched 37.9 % of the annual total production. The highest fruit production was recorded in February and March, mainly in over 5-years plantations (Figure 3).

Figure 3 Total and per component monthly litterfall: leaves, branches (A), and fruits (B), in Eucalyptus urophylla plantations in Huimanguillo, Tabasco, Mexico.
The monthly litterfall production was statistically different from month to month (p=0.0001). Thus, it was possible to differentiate the trees’ litterfall during the year, regardless of plantation age (Table 5). The largest production occurred during April, May, June, and October: the first three months match the end of the dry season, so litterfall is attributed to the plants’ water stress and the generation of new leaves. The greater litterfall accumulation in October could be the result of the season’s strong winds or the torrential rains.
Table 5 Above-ground biomass (Mg ha-1) and percentage per initial and final biomass component accumulated per plantation age for Eucalyptus urophylla in Huimanguillo, Tabasco, Mexico.

E1-2, E2-3, E3-4, E4-5, E5-6, E6-7: Plantation age at the beginning and end of the measurement; Bf: trunk biomass, Bh: leaf biomass, Br: branch biomass; %: percentage based on total biomass (Bt).
The behavior of the maximum production of each component, leaves and branches, was similar to TLP. The branches values were lower from July to September. The fruit fall was higher during October, February and March, with statistically higher values in older plantations, where the trees have a greater phenological maturation (Figure 3B).
Castañeda-Mendoza et al. (2012) asserted that the maximum litterfall in Bambusa aldhamii plantations, in Huatusco, Veracruz, Mexico, occurs from February to July, Gutiérrez et al. (2012) reported that the most important contributions of P. greggii and P. cembroides litterfall in plantations in Coahuila, Mexico, occurred during May and June. Di Stefano and Fournier (2005) concluded that the greatest litterfall in monoculture plantations of tropical species occurs during the dry season and during the strong winds period.
In the study site, there is less precipitation and higher temperature from February to June, which causes water stress in plants and leaf loss. Our results are similar to those obtained in natural pine-holm oak forests, with a larger production from March to May; additionally, during October and November, they presented a slightly higher accumulation, which was attributed to meteorological phenomena effects (Rocha and Ramírez, 2009).
Estimation of above-ground biomass
The trunk biomass proportion (Bf) in relation to Bt was higher in older trees (Table 6), due to biomass accumulation in its support structure; this is typical of young fast-growing plantations (Reed and Tomé, 1998). In contrast, the leaf (Bh) and branches (Br) biomass proportion decreased in older plantations. However, the production of these components was relatively constant across all ages; meanwhile, the productive capacity of the site affects this characteristic. In the initial and final measurements, the biomass average proportion per component was 85.8, 4.0, and 10.3%, for Bf, Bh, and Br respectively. At 7 years, the total biomass accumulated by E. urophylla was 148.0 Mg ha-1. The annual increment of the evaluation period represented 33.32, 25.55, 11.77, 14.31, 16.87, and 21.54 Mg ha-1 year-1 in the six plantations (Table 6) and an annual general average of 20.56 Mg ha-1 year-1. Binkley and Ryan (1998) reported similar values for total biomass (323 Mg ha-1) in 16-years E. saligna plantations; the average annual increment would be 20.3 Mg ha-1 year-1. Geldres et al. (2006) reported accumulated biomass values of 73.1, 111.8, and 159.5 Mg ha-1 in 4-, 5-, and 6- years E. nitens plantations, and annual biomass increments of 39.2 Mg ha-1 in 4 and 5 years plantations, and 50.1 Mg ha-1 in 5 and 6 years plantations. These estimates are higher to those of the present study. E. nitens’ high values are associated with weed control and fertilization during the first three years and thinning at five years (which matches the stage at which trees grow more). In contrast, in this study plantation management did not match any specific development stage.
Table 6 Above-ground net primary productivity (Mg ha-1 year-1) total and per component for Eucalyptus urophylla plantations in Huimanguillo, Tabasco, Mexico.

E1-2, E2-3, E3-4, E4-5, E5-6, E6-7: Plantation age at the beginning and at the end. Means with different letters in each row are statistically different.
Estimation of above-ground net primary productivity
The estimated average ANPP was 26.26 Mg ha-1 year-1, 84.0 % was the result of the increment in living biomass and 16.0 % to the increment in the biomass incorporated into the soil as fine litterfall. The average contribution to ANPP was: 20.23 Mg ha-1 year-1 (77.0 %) to Bf; 3.62 Mg ha-1 year-1 (13.8 %) to Bl; and 2.39 Mg ha-1 year-1 (9.1 %) to Br, for all ages (Figure 4). The variance analysis indicated significant differences (α=0.0001) in ANPP by plantation age (Table 5). The distribution of the ANPP data set at each age is variable, thus differences in their means are indicated.

Figure 4 Eucalyptus urophylla’s above-ground net primary productivity (ANPP) and: (A) contribution of trunk biomass (Bf), (B) leaf biomass (Bh), (C) branch biomass (Br), (D) average. Tabasco, Mexico.
The variability in ANPP with different ages was confirmed with the comparison of means, which indicated that the highest productivity of plantations was observed in the first years of growth, when the trees increase in size and their nutrient assimilation is more efficient. The ages with the highest ANPP increment were 1-2 and 2-3 years, with 37.33 and 30.37 Mg ha-1 year-1 values. A high value was observed during the 6-7 year, with 26.28 Mg ha-1 year-1, indicating that the ANPP declines slightly with age. Meanwhile, the low scores in the 3-4, 4-5, and 5-6 years may be due to the soil productive capacity, the competition for space and nutrients with herbaceous plants, the lack of fertilization or unrecorded differences between clones, and to the initial stage of the production of reproductive organs.
Young plantations presented the highest above-ground net primary productivity, which matches the observations of Tuner et al. (2009) and Ernst et al. (2000), who indicated that the growth rate is greater at a younger age and, consequently, an increase in carbon capture. Binkley and Ryan (1998) recorded the following ANPP in E. saligna plantations: 27.9 Mg ha-1 year-1 from 2 to 4 years, 22.4 Mg ha-1 year-1 at 6 years, and 10.9 Mg ha-1 year-1 at 10 and 16 years. Thus, the productivity pattern decreased as the plantation age increased. Ares and Fownes (2000) determined 15.8 and 23.8 Mg ha-1 year-1 ANPP in 28 to 36 years E. saligna plantations; these estimates were low due to age; also, ANPP decreased with altitude and in areas with low precipitation.
According to Ignacio et al. (2005), as the E. urophylla plantation grows older, the growth and yield of trees decreases, as a result of the competition for space or the change in the species’ phenological stage; besides, they indicated that diameter and volume growth become stable at 3 years, and productivity also decreases. The ANPP average value in our study (26.26 Mg ha-1 year-1) was higher than the value recorded for tropical ecosystems (21.60 Mg ha-1 year-1) (Murphy, 1975), which shows that the E. urophylla plantations analyzed present high biomass productivity.
Conclusions
The estimation of above-ground net primary productivity, by the evaluation of the total biomass increment and the litterfall loss in an established time interval, is a practical method to determine the biomass productivity of commercial forest plantations and the site of development.
The above-ground net primary productivity varies with age. Young plantations show greater productivity. In contrast, trees of this species produce more litterfall as they grow older and bigger. The production of new foliage occurs from June to August
Literatura Citada
Alice, F., F. Montagnini , y M. Montero. 2004. Productividad de plantaciones puras y mixtas de especies nativas en la estación biológica La Selva, Sarapiqui, Costa Rica. Agron. Costarricense 28: 61-71. [ Links ]
Ares, A., and J. H. Fownes . 2000. Productivity, nutrient and water-use efficiency of Eucalyptus saligna and Toona ciliate in Hawaii. For. Ecol. Manage. 139: 227-236. [ Links ]
Arriaza B., M. 2006. Guía Práctica de Análisis de Datos. Primera edición. Ideagonal diseño gráfico. IFAPA, Cordoba, España. 200 p. [ Links ]
Binkley, D., and M.G. Ryan . 1998. Net primary production and nutrient cycling in replicated stands of Eucalyptus saligna and Albizia facaltaria. For.Ecol.Manage. 112: 79-85. [ Links ]
Castañeda-Mendoza, A., J. J. Vargas-Hernández, and A. Gómez-Guerrero. 2012. Components of net aerial primary production in a Bambusa aldhamii plantation. Agrociencia 46: 63-74. [ Links ]
Clark, D.A., S. Brown , D.W. Kicklighter, J.Q. Chambers, J.R. Thomlinson, and J. Ni. 2001a. Measuring net primary production in forests: Concepts and field methods. Ecol. Appl. 11: 356-370. [ Links ]
Clark, D.A, S. Brown , D.W. Kicklighter, J.Q. Chambers, J.R. Thomlinson, J. Ni, and E.A. Holland . 2001b. Net primary production in tropical forests: an evaluation and synthesis of existing field data. Ecol. Appl . 11: 371-384. [ Links ]
Di Stefano, J.F., y L.A. Fournier . 2005. Caída de hojarasca y tasas de descomposición de las hojas de Vochysia guatemalesis en una plantación de 10 años, Tabarcia de Mora, Costa Rica. Agron. Costarricense 29: 9-16. [ Links ]
Ernst D., S.C. Wirth , and M. Heimann. 2000. Managing forests after Kyoto. Clim. Chang. 289: 2058-2059. [ Links ]
FAO (Food and Agriculture Organization). 2006. Tendencias y Perspectivas del Sector Forestal en América Latina y el Caribe. FAO: Dirección de productos y economía forestales, Departamento Forestal. FAO, Roma, Italia. 178 p. [ Links ]
FAO (Food and Agriculture Organization). 2015. Evaluación de los recursos forestales mundiales 2015: ¿cómo están cambiando los bosques del mundo? FAO, Roma, Italia. 49 p. [ Links ]
Gamarra R., J. 2001. Estimación de carbono en plantaciones de Eucalyptus globulus Labill, en Junin, Perú. Simposio internacional medición y monitoreo de la captura de carbono en ecosistemas forestales, Chile.. 21p. [ Links ]
Geldres, E., V.Gerding, and J. E. Schlatter. 2006. Biomasa de Eucalyptus nitens de 4-7 años de edad en un rodal de la X Región, Chile. Bosque 27: 223-230. [ Links ]
Gómez, A., y G.C. Gallopín . 1991. Estimación de la productividad primaria neta de ecosistemas terrestres del mundo en relación a factores ambientales. Ecolog.Aust. 1: 24-40. [ Links ]
González R., H., R.G. Ramírez-Lozano, I. Cantú-Silva , M.V. Gómez-Meza, M. Cotera-Correa, A. Carrillo-Parra, y J.J. Marroquín-Castillo. 2013. Producción de hojarasca y retorno de nutrientes vía foliar en un matorral desértico micrófilo en el noreste de México. Rev. Chapingo Ser. Ciencias For. Ambiente 19: 249-262. [ Links ]
Grier, C.C., K.M. Lee ., N.M. Nadkarni, G.O. Klock, and P.J. Edgerton. 1989. Productivity of forests of the United States and its relation to soil and site factors and management practices: a review. Forest Service: General Technical Report. Portland, United States 53 p. [ Links ]
Gutiérrez V., M.H., J.Méndez G ., C.Flores L., J. A.Ramírez D., y B. N. Gutiérrez V. 2012. Caída de hojarasca en plantaciones de Pinus greggi Engelm. y Pinus cembroides Zucc, en Coahuila, México. Rev. Fitotec. Mex. 35: 123-133. [ Links ]
Hanson, P.J., N.T. Edwards , T.J. Tschaplinski, S.D. Wullschleger, and J.D. Joslin. 2003. Estimating the net primary and net ecosystem production of a Southeastern Upland Quercus forest from an 8-year biometric record. In: Hanson, P.J. and S. D. Wullschleger. (eds.). North American Temperate Deciduous Forest Responses to Changing Precipitation Regimes. Springer, New York, United States. 472 p. [ Links ]
Huber J., A., y C.Oyarzún C . 1983. Producción de hojarasca y sus relaciones con factores meteorológicos en un bosque de Pinus radiata (D. Don). Bosque 5: 1-11. [ Links ]
Ignacio S., E., J.J.Vargas H ., J. López U., y A.Borja de la R. 2005. Parámetros genéticos del crecimiento y densidad de madera en edades juveniles de Eucalyptus urophylla S. T. Blake. Agrociencia 39: 469-479. [ Links ]
INEGI. 2005. Marco Geoestadístico Municipal 2005, versión 3.1. Disponible en http://www.beta.inegi.org.mx/app/biblioteca/ficha.html?upc=702825292850 [ Links ]
Klepac, D. 1983. Crecimiento e Incremento de Árboles y Masas Forestales. Segunda edición. Universidad Autónoma Chapingo. Chapingo, Texcoco, Edo. México 279 p. [ Links ]
Kotowska, M.M., C. Leuschner , T.Triadiati, S.Meriem, and D. Hertel. 2015. Quantifying above and belowground biomass carbon loss with forest conversion in tropical lowlands of Sumatra (Indonesia). Glob. Change Biol. 21: 3620-3620. [ Links ]
Li, S., S. Lu, Y. Zhang, Y. Liu, Y. Gao , and Y. Ao. 2015. The change of global terrestrial ecosystem net primary productivity (NPP) and its response to climate change in CMIP5. Theor. Appl. Climatol. 121: 319-335. [ Links ]
Marmolejo M., J. G., C. M. Cantú A., y M. A. Gutiérrez S. 2013. Degradación de la hojarasca en sitios con vegetación primaria y secundaria del matorral espinoso Tamaulipeco. Rev. Mex. Cien. For. 4: 174-181. [ Links ]
Martínez G., M., A. Sánchez V ., y J. Faulin F. 2006. Bioestadística Amigable. Segunda edición, Editorial Díaz de Santos, España. 920 p. [ Links ]
Miquelajauregui, Y. 2013. Modelos de simulación de la dinámica del carbono. In: Blanco, J. A. (ed). Aplicación de Modelos Ecológicos a la Gestión de Recursos Naturales. Barcelona, España. OmniaScience. pp: 15-38. [ Links ]
Murphy, P.G. 1975 . Net primary productivity in tropical terrestrial ecosystems. In: Lieth, H., and R.H. Whittaker (eds). Primary Productivity of the Biosphere. Springer. Berlin, Heidelberg, New York, United States. p: 217-231. [ Links ]
Navar C., J.J., y E. Jurado Y . 2009. Productividad foliar y radicular en ecosistemas forestales del noreste de México. Rev. Mex. Cien. For. 34: 89-106. [ Links ]
Pérez, C.A., J.F. Goya , F.Bianchini, J.L.Frangi, y R. Fernández. 2006. Productividad aérea y ciclo de nutrientes en plantaciones de Pinus taeda L. en el norte de la provincia de Misiones, Argentina. Interciencia 31: 794-801. [ Links ]
Quinto M., H., Y.A. Ramos P ., y D. Abadía B. 2007. Cuantificación de la caída de hojarasca como medida de la productividad primaria neta en un bosque pluvial tropical en Salero, Chocó, Colombia. Biodiversidad 26: 28-41. [ Links ]
Reed, D., and M. Tomé . 1998. Total aboveground biomass and net dry matter accumulation by plant component in young Eucalyptus globulus in response to irrigation. For. Ecol. Manage . 103: 21-32. [ Links ]
Rocha L., A.G., y N.Ramírez M . 2009. Producción y descomposición de hojarasca en diferentes condiciones sucesiones del bosque de pino-encino en Chiapas, México. Bol. Soc. Bot. México 84: 1-12. [ Links ]
Salas R., J., y A. Infante C . 2006. Producción primaria neta aérea en algunos ecosistemas y estimaciones de biomasa en plantaciones forestales. Rev. For. Lat. 40: 47-70. [ Links ]
Smith M., T., y R.L. Smith . 2007. Ecología. Sexta edición. Pearson Educación S. A., Madrid, España. 681 p. [ Links ]
Tuner G., M., E. A. H. Smithwick , D.B. Tinker, and W.H.Romme. 2009. Variation in foliar nitrogen and aboveground net primary production in young postfire lodgepole pine. Can. J. For. Res. 39: 1024-1035. [ Links ]
Vásquez G., I., M.A. López L., G. Ángeles P., A. Trinidad S., M.Jiménez C., y G. Aguilar B. 2015. Aclareo y fertilización química en la productividad primaria neta de plantaciones de Pinus patula Schiede ex Schltdl. et Cham. Rev. Mex. Cien. For . 6: 82-93. [ Links ]
Velázquez, A., J.F. Mas , R.Mayorga S., J.R.Díaz, C. Alcántara, R. Castro, T. Fernández, J.L. Palacio, G. Bocco, G.Gómez R., L. Luna G., I. Trejo, J.López G., M.Palma, A. Peralta, J. Prado M., y F. González M . 2002. Estado actual y dinámica de los recursos forestales de México. Biodiversitas 41: 8-15. [ Links ]
Received: May 2016; Accepted: August 2016











 text in
text in 






