Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.51 no.3 Texcoco Abr./Mai. 2017
Plant Protection
Xanthomonas fragariae genetic variability and its severity on strawberry genotypes (Fragaria×ananassa Duch)
1Fitosanidad, Campus Montecillo, Colegio de Postgraduados. 56230. Km 36.5 Carretera México-Texcoco, Montecillo, Estado de México.
2Investigación Aplicada-Driscoll’s México. 45050. Avenida Moctezuma 144, Piso 1. Colonia Ciudad del Sol, Zapopan, Jalisco.
Xanthomonas fragariae is the causative agent of the angular leaf spot of strawberries (Fragaria×ananassa Duch). This microorganism is subject to quarantine for the mobilization and transport of propagation plant material in several countries. There is no information about the pathogen’s genetic diversity and its severity on commercial varieties in Mexico. The objective of this study was to find out X. fragariae’s genetic variability and evaluate its severity on different strawberry genetic materials. To achieve this, a phylogenetic analysis was carried out with partial sequences of the hrp and gyrB genes from 14 X. fragariae isolates collected from production locations in the Mexicans states of Michoacán, Jalisco, and Puebla. The phylogenetic tree showed 99 % similarity among the isolates analyzed. In order to obtain X. fragariae-resistant strawberry genotypes, for commercial use and in genetic improvement programs, a X. fragariae suspension was inoculated, injected and sprayed, in two commercial varieties and five genotypes. The symptom severity was evaluated using a diagrammatic scale. FragLa and FragMa strawberry genotypes showed the lowest severity values. This is the first study in Mexico about the X. fragariae’s genetic variability and its severity on strawberry genotypes.
Key words: Xanthomonas fragariae; Fragaria×ananassa; hrp; gyrB; diagrammatic severity scale
Xanthomonas fragariae es el agente causal de la mancha angular de la hoja en fresa (Fragaria×ananassa Duch) y es un microorganismo sujeto a cuarentena para la movilización y transporte de material vegetal de propagación en varios países. En México, la información sobre la diversidad genética del patógeno y su severidad en variedades comerciales es desconocida. Este estudio tuvo como objetivo conocer la variabilidad genética de X. fragariae y evaluar su severidad en diferentes materiales genéticos de fresa. Para ello se realizó un análisis filogenético con secuencias parciales de los genes hrp y gyrB de 14 aislamientos de X. fragariae recolectados en localidades productoras de los estados de Michoacán, Jalisco y Puebla, México. El árbol filogenético mostró similitud de 99 % entre los aislamientos analizados. Con el propósito de contar con genotipos de fresa, con resistencia a X. fragariae para uso comercial y en programas de mejoramiento genético, se inoculó por inyección y aspersión una suspensión de X. fragariae en dos variedades comerciales y cinco genotipos y se evaluó la severidad de los síntomas mediante una escala diagramática. Los genotipos de fresa FragLa y FragMa tuvieron los valores menores de severidad. Este es el primer estudio sobre la variabilidad genética de X. fragariae y de su severidad en genotipos de fresa en México.
Palabras clave: Xanthomonas fragariae; Fragaria×ananassa; hrp; gyrB; escala diagramática de severidad
Introduction
The angular leaf spot caused by Xanthomonas fragariae (Kennedy and King, 1962) is a disease that affects strawberry crops (Fragaria×ananassa Duch) reducing the yield, especially when the bacteria infects the calyx (Wyenandt and Nitzsche, 2013) causing discolouration, and the loss of its commercial value (Roberts et al., 1997). The bacterial infection is systemic and subject to quarantine in several countries, because it may be asymptomatic, which contributes to the long-distance movement of infected plant material (CABI/EPPO, 2015; Koike et al., 2005). In Mexico, the strawberry cultivation has socioeconomic importance; most of the production takes place in the states of Michoacán, Baja California, Guanajuato, and Jalisco (SIAP, 2014). Xanthomonas fragariae was first reported in Michoacán in 2014 (Fernández-Pavia et al., 2014). But there are no studies on the genetic variability among strains of this pathogen in strawberry production areas in Mexico. The knowledge of this bacteria’s genetic variability or its epidemiology can provide important tools to generate efficient disease management strategies.
The phylogenetic analysis of hrp and gyrB genes is used to find out the genetic variability in other species of the Xanthomonas genus (Yin et al., 2008; Young et al., 2008; Almeida et al., 2010). Therefore, studying these genes could provide relevant information about genetic variability among X. fragariae populations and its relation with the severity of the angular leaf spot disease on different strawberry genotypes. The knowledge of genetic variability is essential to determine the economic impact that different pathogen populations can have in specific environmental conditions in the country. Also, it can help to delimit production zones where strains with greater severity are detected (and where cultivating strawberries should be avoided) or to establish stricter management or mobilization measures when dealing with propagative material.
All cultivated strawberry varieties are susceptible to X. fragariae infection. However, some of them may possibly be less susceptible; therefore, searching for these materials is important to develop genetic improvement programs and to focus disease management with these materials, in areas that are major sources of the pathogen’s inoculum. Therefore, the objective of this research was to find out the genetic variability of 14 X. fragariae isolates from regions in central Mexico and to evaluate their severity in two strawberry commercial varieties and five genotypes.
Materials and Methods
Plant material and bacteria isolation
Strawberry leaf tissue was collected from commercial plantations in the states of Michoacán (Tupátaro, Tangancícuaro, Jacona, and Santiago de Tangamandapio), Jalisco (Ciudad Guzmán, Tapalpa), and Puebla (San Salvador del Seco) with watery lesions delimited by the nerves, characteristic symptoms of the angular leaf spot. Bacterial fluid was collected from diseased tissue and adjacent healthy tissue and seeded in Petri dishes with a Wilbrink culture medium (Koike, 1965, quoted by Vandroemme et al., 2008). The dishes were incubated at 25±1 °C until bacterial growth was observed. This growth was seeded in the same medium, until pure, small, silky white, shiny, mucoid, and viscous-looking colonies were obtained. Pure bacterial isolates were preserved in cryogenic vials with nutrient broth and 40 % glycerol at -20 °C.
Pathogenicity tests
The X. fragariae isolates were inoculated individually on two 4-weeks-old strawberry plants var. Monterrey grown in pots with sterile soil, according to the method proposed by Maas et al. (2000). The inoculated plants were kept in a growth chamber at 25-27 °C, with 50-65 % relative humidity, and a 16 h light photoperiod. Observations were made every 24 h, in order to record the incubation period. Lesion size in inoculated leaves was measured 15 days after inoculation.
Bacterial DNA extraction
Bacterial DNA was extracted from pure colonies isolated again from the inoculated plants with 72 h of growth in a Wilbrink culture medium, using the protocol described by Minas et al. (2011). The DNA obtained was again put in a sterile distilled water suspension and stored at -20 °C.
Phylogenetic analysis
Hrp and gyrB genes segments were amplified from the DNA obtained, by means of PCR primers and conditions employed by Roberts et al. (1996) and Young et al. (2008) who amplified 537 bp and 865 bp fragments, respectively. The amplicons obtained were purified and sent for sequencing at Macrogen Inc. (Seoul, Korea). The sequences obtained were edited with the BioEdit Sequence Alignment Editor program, generating consensus sequences that were then aligned with the CLUSTALW software included in the Molecular Evolutionary Genetics Analysis (MEGA 6) program (Tamura et al., 2011). The sequences were edited and 414 bp and 529 bp were obtained for the hrp and gyrB genes, respectively; subsequently, these results were concatenated and a 943-bp long segment was the result. A phylogenetic tree was developed with MEGA 6, using the neighbor-joining method. The evolutionary distances were obtained with the Tamura 3-parameter model using a 10 000-repetitions bootstrap. The sequences edited were deposited in the GenBank® database.
Diagrammatic severity scale
Two hundred trifoliate strawberry leaves with different levels of angular spot damage were collected. The leaves were scanned with a 200 dpi resolution, in an HP all-in-one (Officejet Pro 276dW®, USA). The resulting images were analyzed with Assess 2.0 software (The American Phytopathological Society, St Paul MN, USA), in order to determine the percentage of damaged leaf area. A diagrammatic symptom severity scale was developed according to Weber-Fechner’s law of visual acuity, considering the lower and upper disease severity limits detected in the field. Six levels of severity were established with the 2LOG v.1.0 program, starting from the highest damage percentage, taking into consideration the midpoint of each level, in order to locate representative images (Mora-Aguilera et al., 2000, quoted by Saucedo-Carabez et al., 2014).
Severity evaluation on the strawberry genotypes
The 12XfMxJal bacterial isolation was selected -because its incubation period was shorter in the pathogenicity tests described above- to evaluate the susceptibility of Fortuna and Festival strawberry commercial varieties, as well as of the Frag53, Frag40, FragLa, FragMa, and FragSp genotypes. A sterile water 3×108 CFU mL-1 bacterial suspension was prepared and two inoculation methods were used: 1) injection: 10 plants of each commercial variety and genotype were inoculated according to the methodology of Maas et al. (2000), and 2) spray: 10 plants per material were sprayed up to dripping point using the method of Bestfleisch et al. (2015). The experiment was set up in the field inside plastic tunnels.
The plants treated with both methods of inoculation were observed every 24 h for 11 d, in order to record the incubation period. Subsequently, they were observed every 4 d for four weeks, in order to evaluate the severity using the scale developed.
The experimental design was made up by fully random blocks. An ANOVA was carried out with the severity data, and means were compared using Tukey’s multiple comparison test (p≤0.05) in SAS (SAS System for Windows v9.0). Based on the incidence data, the area under the disease progress curve was obtained for each material evaluated (LANREF-Mora-Aguilera, COLPOS3 .
Results and Discussion
Pathogenicity tests
In the states of Michoacán, Jalisco, and Puebla, 14 X. fragariae isolates were obtained from leaves with angular spot symptoms (Table 1). All isolates were pathogenic in strawberry plants (var. Monterrey). They caused watery spots in the inoculation site (Figure 1), which are typical symptoms of a natural bacterial infection. The incubation period ranged between 3 and 5 d, and the shortest incubation period was recorded in the 2XfMxMich and 12XfMxJal isolates (Table 1).
Table 1 Xanthomonas fragariae isolates origin and identification, incubation period, and lesion size recorded in strawberry var. Monterrey plants.
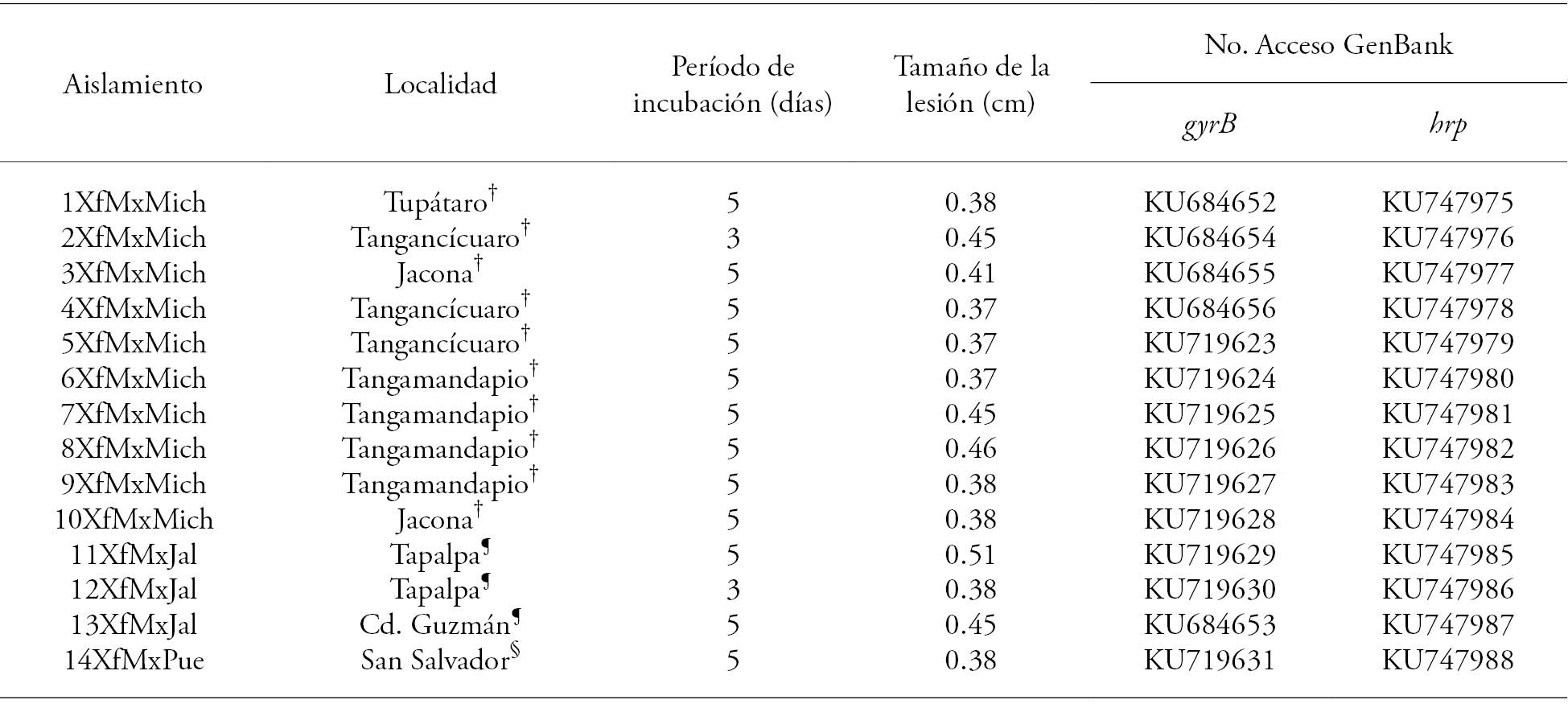
†Michoacán, ¶Jalisco, §Puebla.
Phylogenetic analysis
The sequence analysis of the hrp and gyrB genes segments grouped into a single branch the 14 isolates from our study with an Australian isolation (GenBank Accession HQ223085) (Figure 2), which indicates that they are very similar to each other. Xanthomonas fragariae isolates infected strawberry plants var. Monterrey with a 5 d incubation period, but the 2XfMxMich and 12XfMxMich isolates only took 3 d. These differences show the potential genetic variability inherent in this bacterial species. Although there were biological differences, it was not possible to find genetic differences in the regions of the genes examined. Stöger et al. (2008) studied the genetic variability of several X. fragariae isolates by means of Repetitive Sequence-based PCR (rep-PCR), Enterobacterial Repetitive Intergenic Consensus (ERIC), and Amplified Fragment Length Polymorphism (AFLP), but did not find any differences between them. However, studies that analyzed the hrp and gyrB genes for phytopathogenic bacterial species (including other genus Xanthomonas species) were consistent and showed genetic variability among them (Yin et al., 2008; Young et al., 2008; Almeida et al., (2010). In our research, the results of the analysis of the hrp and gyrB partial gene sequences of the 14 X. fragariae isolates showed no variability, despite, among other factors, the different environmental conditions, as well as crop varieties and management, in the regions where the samples were collected. This may indicate a low genetic diversity, probably because the isolates introduced in Mexico have a common origin or because there is a small number of hrp and gyrB gene sequences in the GenBank database for this species, making it impossible to detect differences in these genome regions. The complete genome sequencing of these isolates is currently being carried out, in order to obtain a greater understanding of the genotype/severity interaction of the induced symptoms, as well as possible phylo-geographic studies of these isolates.

Figure 2 Phylogenetic tree developed using the neighbor-joining method. The evolutionary distances were obtained with the Tamura 3-parameter model using 10 000-repetitions bootstrap, based on nucleotide sequences concatenated with partial hrp and gyrB gene regions of 14 Xanthomonas fragariae isolates. The bar indicates the number of substitutions per site. Only bootstrap values over 80 % are shown.
Diagrammatic scale
The diagrammatic scale was designed with six severity levels, which indicate the affected leaf area range. This scale was integrated with an image that shows the damage percentage calculated with the software, and that is representative of each level, in order to facilitate damage evaluation in the field (Figure 3).

Figure 3 Diagrammatic scale designed to evaluate the severity of Xanthomonas fragariae in strawberry genotypes.
The use of this type of scale reduces the subjectivity of the disease severity estimations and is an essential element in epidemiological studies (Menge et al., 2013). Bestfleisch et al. (2015) proposed a nine-level X. fragariae severity scale. It is not often used, perhaps due to the number of levels and because the damage is illustrated with black and white drawings. There are five- or six-level severity scales for other diseases, which have acceptable precision values and provide an accurate validation (Menge et al., 2013, Freitas et al., 2014). The scale proposed in our research shows a progressive sequence of the advance of the affected leaf area, with images that clearly show the damage level caused by this bacterium in the sites under study. (The figures are shown in grayscale and color -printed and electronic version, respectively).
Severity evaluation
During the evaluation period (September-October 2015), 16-28 °C daytime and 18-28 °C night-time temperatures were recorded, with 44-99 % relative humidity. The seven evaluated strawberry genotypes were susceptible to X. fragariae, after being inoculated by injection or spray.
Inoculation by injection
By means of this method, watery and necrotic lesions forming an angular spot delimited by nerves were observed. Eleven days after inoculation, 100 % plants of all genotypes showed symptoms; there was a 4, 6, 9, and 11 d incubation period variation. The area under the disease progress curve varied among different genotypes. The FragLa genotype had the lowest severity value (31.82) (Figure 4).

Figure 4 Area under the disease progress curve in seven strawberry genotypes inoculated with Xanthomonas fragariae by injection.
In all cases, the size of the lesions increased progressively during the experiment. Thirty-four days after inoculation (34 dai), the Frag53 genotype had the highest value, followed by the Festival variety; the other genotypes were statistically similar (p>0.05, Table 2). Eighteen days after inoculation (18 dai), the Frag53 and Fortuna genotypes were similar (p>0.05) and from 22 dai they behaved differently (p≤0.05) until the end of the evaluation.
Table 2 Lesion size (cm) caused by Xanthomonas fragariae in seven strawberry genotypes leaves inoculated by injection.
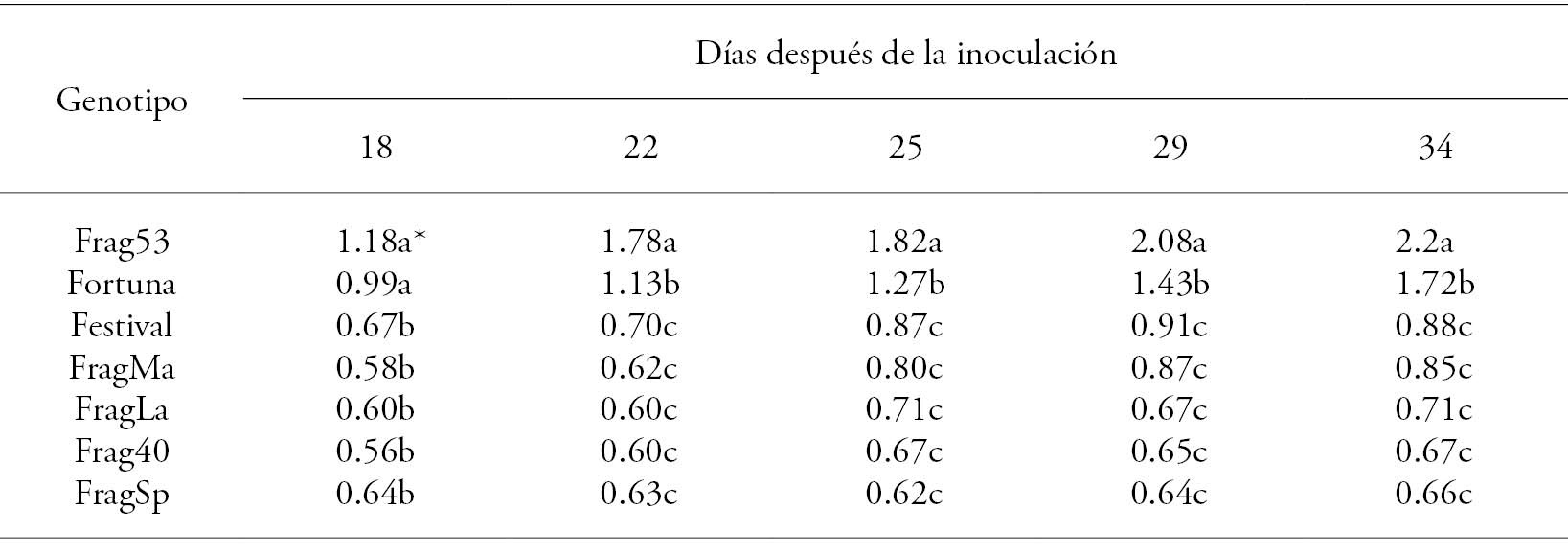
*Different-letter treatments are statistically significant (Tukey, p≤0.05).
The inoculated plants showed two types of foliar lesions: 1) irregular necrotic spots surrounded by a yellow halo that generally covered the entire leaf blade (Figure 5A); and 2) necrotic lesions bordered by watery tissue (Figure 5B). Only in the FragLa genotype, 65 % of the inoculation sites showed a third type of lesions: necrotic spots limited to the inoculation area (Figure 5C), similar to those that occur in a hypersensitive response (HR). HR is considered the highest expression of the plants’ resistance to pathogen attacks, and is defined as a rapid plant cell death associated with the restriction of the pathogen growth, which is recognized by the presence of one or more brown dead cells in the infection site (Greenberg and Yao, 2004; Sanzón and Zavaleta-Mejía, 2011). In addition, plants in which an HR is produced, have some degree of resistance to the pathogen that induced it (Vlot et al., 2008). Different species of phytopathogenic bacteria -such as Pseudomonas syringae, Erwinia amylovora, and Ralstonia solanacearum, among others- have elicitors that trigger HR (Grant and Mansfield, 1999). Bestfleisch et al. (2025) also report that, in the early stages of their development, strawberry plants are more susceptible to X. fragariae, and that, as they mature, they show some resistance that may become more evident over time.

Figure 5 Symptoms produced in seven strawberry genotypes inoculated with X. fragariae by injection. A) Irregular necrotic spots surrounded by a yellow halo that generally cover the leaf blade, B) Necrotic spot with watery tissue in the lesion’s advance area, C) Necrotic lesions that did not increase in size, D) Control plants inoculated with sterile distilled water, without apparent damage.
Spray inoculation
The same symptom types were consistently observed with this method: watery angular spots (Figure 6A) coalesced over time forming larger necrotic areas bordered by yellow-colored tissue (Figure 6B). This type of lesions were different from those observed in plants inoculated by injection. The first symptoms were observed 7 dai. Statistical analysis did not show significant differences (p>0.05) in severity among strawberry materials inoculated with X. fragariae.

Figure 6 Symptoms observed in seven strawberry genotypes inoculated spraying X. fragariae. A) Angular spot (initially watery) that suffered necrosis over time, B) Necrotic lesions with yellow halo with some areas in which the lesion has advanced.
The two inoculation methods used in this research efficiently infected strawberry genotypes. However, symptoms similar to those occurring in a natural environment were observed when spray inoculation was used.
Some bacteria control has been achieved, during experiments, with thermal treatments, surface sterilization by immersion of seedlings in chloro+ultraviolet radiation (UV-C), and removal of leaves and petioles in nursery plants (Turechek and Peres, 2009; Turechek et al., 2013). Genetic improvement as an additional disease management method could be a valuable tool that requires the constant selection of genotypes with desirable agronomic characteristics, as well as the evaluation of the resistance or tolerance to the bacteria. Therefore, it is necessary to continue evaluating the strawberry materials, in order to select the genotypes that are better adapted to this X. fragariae pathosystem, providing alternatives that contribute to an efficient disease management. In Mexico, the development of selection techniques for strawberry materials that can resist or which have some tolerance degree to angular leaf spot is incipient, due to its recent introduction in the country.
Two forms of X. fragariae inoculation were evaluated in our study -these forms are reported separately in other works. Although an infection occurred in both cases, the incubation period with inoculation by injection was shorter; therefore, using this inoculation method in selection programs may be more convenient, because it reduces the time needed to evaluate genetic materials.
Conclusions
The partial sequencing of hrp and gyrB genes showed a high genetic similarity between 14 Xanthomonas fragariae isolates from different localities in central Mexico.
All strawberry genotypes evaluated were susceptible to Xanthomonas fragariae. However, FragMa and FragLa showed the lowest symptom severity values, and may constitute gene sources that can potentially be used in genetic improvement programs for strawberry cultivation in Mexico.
The FragLa genotype showed evidence of hypersensitive response in 65 % of the inoculation sites and will be studied in greater depth, in order to find the genes involved in this reaction
REFERENCES
Almeida N. F, S. Yan, R. Cai, C. R. Clarke, C. E. Morris, N. W. Schaad, E. L. Schuenzel, G. H. Lacy, X. Sun, J. B. Jones, J. A. Castillo, C. T. Bull, S. Leman, D. S. Guttman, J. C. Setubal, and B. A. Vinatzer. 2010. PAMDB, a multilocus sequence typing and analysis database and website for plant-associated microbes. Phytopathology 100: 208-215. [ Links ]
Bestfleisch M., K. Richter, N.A. Wensing, J. Wünsche, M.V. Hanke, M. Höfer, E. Schulte, and H. Flachowsky. 2015. Resistance and systemic dispersal of Xanthomonas fragariae in strawberry germplasm (Fragaria L.). Plant Pathol. 64: 71-80. [ Links ]
CABI/EPPO (CAB International/European and Mediterranean Plant Protection). 2015. Invasive Species Compendium. Datasheets, maps, images, abstracts and full text on invasive species of the world. Xanthomonas fragariae (angular leaf spot). CAB International. Wallingford, UK. http://www.cabi.org/isc/datasheet/56934. [ Links ]
Fernández-Pavía S. P., G. Rodríguez-Alvarado, E. Garay-Serrano, and R. Cárdenas-Navarro. 2014. First report of Xanthomonas fragariae causing angular leaf spot on strawberry plants in Mexico. Plant Dis. 98: 682. [ Links ]
Freitas M.L.D.O ., E. A. Pozza, L. L. Belan, J. L. da Silva, and M. S. de Abreu. 2014. Diagrammatic scale for blister spot in leaves of coffee tree. Afr. J. Agric. Res.10: 2068-2075. [ Links ]
Grant M., and J. Mansfield. 1999. Early events in host-pathogen interactions. Curr. Opin. Plant Biol. 2: 312-319. [ Links ]
Greenberg J. T., and N. Yao. 2004. The role and regulation of programmed cell death in plant-pathogen interactions. Cell. Microbiol. 6: 201-211. [ Links ]
Kennedy B. W., and T. H. King. 1962. Angular leaf spot of strawberry caused by Xanthomonas fragariae sp. nov. Phytopathology 52: 873-875. [ Links ]
Koike H. 1965. The aluminium-cap method for testing sugarcane varieties against leaf scald disease. Phytopathology 55: 317-9. [ Links ]
Koike S. T., W. D. Gubler, U. C. Davis, and G. T. Browne. 2005. Guía para el manejo de las plagas: fresas. Universidad de California. Publicación 3473. UC Agricultura y Recursos Naturales. pp: 41-57. [ Links ]
Maas J. L., B. C. Gouin, J. S. Hartung, and S. C. Hokanson. 2000. Sources of resistance for two differentially pathogenic strains of Xanthomonas fragariae in Fragaria genotypes. Hortscience 35: 128-131. [ Links ]
Menge D., M. Makobe, S. Shomari, and V. A. Tiedemann. 2013. Development and validation of a diagrammatic scale for estimation of cashew blight for epidemiological studies. Int. J. Adv. Res. 1: 26-38. [ Links ]
Minas K., N. R. McEwan, C. J. Newbold and K. P. Scott. 2011. Optimization of a high-throughput CTAB-based protocol for the extraction of qPCR-grade DNA from rumen fluid, plant and bacterial pure cultures. FEMS Microbiol. Lett. 325: 162-169. [ Links ]
Mora-Aguilera G., P. Rivas-Valencia, C. Góngora-Canul, A. Tovar-Soto, J. Cristobal-Alejo, E. Loeza-Kuk, S. Michereff, A. Marinelli, y K. Osada-Velázquez. 2000. Sistemas computarizados en la epidemiología: 1. 2-LOG ver 1.0 y su aplicación en el diseño de escalas diagramáticas logarítmicas. In: XXIX Simposio Nacional de Parasitología Agrícola. Octubre 12-15, 2000. Puerto Vallarta, Jalisco, México. pp: 2-22. [ Links ]
Roberts P. D., J. B. Jones , and C. K. Chandler. 1997. Disease progress, yield loss, and control of Xanthomonas fragariae on strawberry plants. Plant Dis . 81: 917-921. [ Links ]
Roberts P. D., J. B. Jones , C. K. Chandler , R. E. Stall, and R. D. Berger. 1996. Survival of Xanthomonas fragariae on strawberry in summer nurseries in Florida detected by specific primers and nested polymerase chain reaction. Plant Dis . 80: 1283-1288. [ Links ]
Sanzón G. D., y E. Zavaleta-Mejía. 2011. Respuesta de hipersensibilidad, una muerte celular programada para defenderse del ataque por fitopatógenos. Rev. Mex. Fitopatol. 29: 154-164. [ Links ]
Saucedo-Carabez J. R., D. Téliz-Ortíz, S. Ochoa-Ascencio, D. Ochoa-Martínez, M. R. Vallejo-Pérez, and H. Beltrán-Peña. 2014. Effect of avocado sunblotch viroid (ASBVd) on avocado yield in Michoacan, Mexico. Eur. J. Plant Pathol . 138: 799-805. [ Links ]
SIAP (Servicio de Información Agroalimentaria y Pesquera). 2014. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. https://www.gob.mx/sagarpa (Consulta: Diciembre 2014). [ Links ]
Stöger A., D. Barionovi, A. Calzolari, R. Gozzi, W. Ruppitsch, and M. Scortichini. 2008. Genetic variability of Xanthomonas fragariae strains obtained from field outbreaks and culture collections as revealed by repetitive-sequence PCR and AFLP. J. Plant Pathol . 90: 469-473. [ Links ]
Tamura K., D. Peterson, N. Peterson, G. Stecher, M. Nei, and S. Kumar. 2011. Mega 5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731-2739. [ Links ]
Turechek W. W., and N. A. Peres. 2009. Heat Treatment effects on strawberry plant survival and angular leaf spot, caused by Xanthomonas fragariae, in nursery production. Plant Dis . 93: 299-308. [ Links ]
Turechek W. W., S. Wang, G. Tiwari, andN. A. Peres . 2013. Investigating alternative strategies for managing bacterial angular leaf spot in cranberry nursery production. Int. J. Fruit Sci. 13: 234-245. [ Links ]
VandroemmeJ., S.Baeyen, J.Van Vaerenbergh, P. De vos, and M. Maes. 2008. Sensitive real-time PCR detection of Xanthomonas fragariae in strawberry plants. Plant Pathol . 57: 438-444. [ Links ]
Vlot A. C., D. F. Klessig, and S. Park. 2008. Systemic acquired resistance the elusive signal (s). Curr. Opin. Plant Biol . 11: 436-422. [ Links ]
Wyenandt A. and P. Nitzsche. 2013. Angular leaf spot in fall transplanted-strawberries. Plant & Pest advisory. Rutgers University, New Jersey Agricultural Experiment Station. http://plant-pest-advisory.rutgers.edu/angular-leaf-spot-showing-up-in-fall-transplanted-strawberries/ . (Consulta: Octubre 2014) [ Links ]
Yin H.C., L. Cao, M. Xie, Q. Chen, G. Qiu, J. Zhou, L. Wu, D. Wang, and X. Liu. 2008. Bacterial diversity based on 16S rRNA and gyrB genes at Yinshan mine, China. Syst. Appl. Microbiol. 31: 302-311. [ Links ]
Young J. M., D. C. Park, H. M. Shearman, and E. Fargier. 2008. A multilocus sequence analysis of the genus Xanthomonas. Syst. Appl. Microbiol . 31: 366-377. [ Links ]
3Mora-Aguilera, G. Laboratorio Nacional de Referencia Epidemiológica Fitosanitaria (LANREF), Colegio de Postgraduados en Ciencias Agrícolas-DGSV SENASICA SAGARPA - Programa de Postgrado en Fitosanidad. https://www.lanref.org.mx
Received: May 2016; Accepted: October 2016











 texto em
texto em 



