Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.51 no.2 Texcoco Fev./Mar. 2017
Food Science
Extraction of Red Pitaya (Stenocereus stellatus) bioactive compounds applying microwave, ultrasound and enzymatic pretreatments
1Departamento de Ingeniería en Sistemas Ambientales, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Avenida Wilfrido Massieu, Unidad Profesional Adolfo López Mateos, D.F. 07738, México.
In recent years, the demand for natural food additives has increased. Although they can be used as a source of betalains and phenolic compounds, there is no information about the extraction of these compounds from red pitayas (Stenocereus stellatus). Therefore, the objective of this study was to evaluate the effect of microwave, ultrasound, and enzymatic pretreatments in the increase of betacyanins (Bc), betaxanthins (Bx), total betalains (TB), total phenolic compounds (TPC), and antioxidant capacity (AC) in the extracts of S. stellatus fruit pulp (with and without seeds). The experimental design was completely randomized and the results were analyzed using ANOVA and Principal Components Analysis (PCA). The highest concentration of bioactive compounds was obtained using seedless pulp. In this sample, 15 min ultrasound pretreatment increased (p≤0.05) the amount of Bc, Bx, and TB by 13.5, 12.7, and 13.1 %, respectively, in relation to control. The application of 0.5 % pectinase increased (p≤0.05) TPC and AC values by 109.7 %, and 102.6 %, respectively. The ultrasound pretreatment maximized the pigment content (480.3 mg TB 100 g-1 dry pulp) and with 0.5 % pectinase, 2 h, 40 °C, pH 4.0, the TPC content was maximized (804.5 mg equivalents of Gallic acid 100 g-1 dry pulp), as well as the AC (4925.7 mg equivalents of Trolox g-1 dry pulp). According to the PCA, there is a direct relation between the red pitaya S. stellatus TPC and AC content. Therefore, we conclude that the combined use of ultrasound and pectinase treatments maximizes the extraction of bioactive compounds from the pitaya.
Key words: extraction; Stenocereus stellatus; betalain; ultrasound; microwaves; enzymatic
La demanda de aditivos naturales en alimentos ha aumentado en años recientes. La pitaya roja (Stenocereus stellatus) se puede usar como fuente de betalaínas y compuestos fenólicos, pero no existen informes sobre la extracción de estos compuestos usando estos frutos. Por ello, el objetivo de este estudio fue evaluar el efecto de los pretratamientos por microondas, ultrasonido y enzimáticos en el aumento de betacianinas (Bc), betaxantinas (Bx), betalaínas totales (BT), compuestos fenólicos totales (CFT) y la capacidad antioxidante (CA) en los extractos de pulpa con y sin semillas del fruto de S. stellatus. El diseño experimental fue completamente al azar y los resultados se analizaron mediante un ANDEVA y un análisis de componentes principales (ACP). La mayor concentración de compuestos bioactivos se obtuvo utilizando la pulpa sin semillas y, para esta muestra, el pretratamiento con ultrasonido por 15 min aumentó (p≤0.05) la cantidad de Bc, Bx y BT 13.5, 12.7 y 13.1 % respecto al testigo. La aplicación de pectinasa al 0.5 % aumentó (p≤0.05) los valores de CFT en un 109.7 % y CA en 102.6 %. El pretratamiento con ultrasonido maximizó el contenido de pigmentos (480.3 mg BT 100 g-1 de pulpa seca) y con 0.5 % pectinasa, 2 h, 40 °C, pH 4.0 se maximizó el contenido de CFT (804.5 mg equivalentes de ácido gálico 100 g-1 de pulpa seca) y la CA (4925.7 mg equivalentes de Trolox g-1 de pulpa seca). De acuerdo con el ACP existe una relación directa entre el contenido de CFT y la CA de la pitaya roja S. stellatus. Por lo tanto, se concluye que el uso combinado de los tratamientos de ultrasonido y pectinasa maximiza la extracción de compuestos bioactivos de la pitaya.
Palabras clave: Extracción; Stenocereus stellatus; betalaínas; ultrasonido; microondas; enzimáticos
Introduction
The pitaya (Stenocereus stellatus) is consumed as a fresh product. No studies were found about its application in industrialized products that would enable its commercial exploitation. Obtaining pitaya extracts with high bioactive compounds concentrations may help to overcome this disadvantage by allowing its exploitation as a source of pigments and anti-oxidants for the food industry.
Research on Stenocereus genus fruits (pitayas) is limited. García-Cruz et al. (2012) and García-Cruz et al. (2013) evaluated the content of total phenolic compounds, antioxidant capacity, betalains, and minerals in S. griseus and S. pruinosus pitayas. Pérez-Loredo et al. (2016) classified the fruits of 30 cactaceous from different genera as poor, good, and excellent sources of betalains. According to this study, the S. stellatus red fruits from Puebla, Mexico, are a good source of pigments. Seedless Opuntia fruits are used to extract betalains, although seeds contain more total phenolic compounds and antioxidant capacity than the pulp (Morales et al., 2012). This suggests that it would be more convenient to use the whole fruit to obtain the largest possible number of bioactive compounds.
Pitaya seeds are small and fragile; they break easily during the fruit maceration, and give a dark color to the macerated pulp, which can affect pigment extraction. Weight-wise, the seeds represent 31 % of the pitaya’s edible fraction and, if they are separated from the pulp, some fruit bioactive compounds could be removed from the extract. Therefore, we need to determine the effect of the seeds’ presence on the extraction performance of the pigments and other compounds of interest.
Cactaceae fruits are the source of various phytochemicals (particularly pigments); for that reason, there are several studies about Cactaceae fruits in order to establish the best extraction and application conditions. The most studied fruits as a bioactive compounds source belong to the Hylocereus (Naderi et al., 2010; Woo et al., 2011) and Opuntia genera (Prakash and Manikandan, 2012; Cejudo-Bastante et al., 2015).
Conventional extraction methods (maceration, reflux, or Soxhlet extraction) require long extraction times, their performances are low, and their energy consumption is high, but they are popular because they are easy to operate and they are economical (Yang et al., 2011). Non-conventional extraction methods are more environmentally-friendly, because they reduce the use of chemicals and the extraction time, and they also improve the extract’s performance and quality. Methods such as ultrasound, electrical pulses, enzymatic digestion, extrusion, microwaves, and supercritical fluids are used to improve the performance and selectivity of bioactive compounds extraction (Azmir et al., 2013).
Ultrasound is a special kind of sound wave: its 20-100 MHz frequency is beyond human hearing, and -like other waves- it compresses and expands the exposed material. When ultrasound is used, the breaking down of the cells contributes to the diffusion through the cell wall and the cell content washing (Azmir et al., 2013). Other studies have considered the use of ultrasound to extract natural pigments from beetroot and other vegetal sources (Sivakumar et al., 2009).
Microwaves are electromagnetic fields with a frequency range of 300 MHz to 300 GHz. Alupului et al. (2012) proposed three sequential stages for microwave assisted extraction: 1) solutes separation from the matrix’ active sites, due to temperature and pressure increase; 2) solvent diffusion through the matrix and, 3) solutes release, from the matrix to the solvent. Microwave extraction has been studied to obtain polyphenols in citrus peels (Nayak et al., 2015), as pretreatment in the extracion of astaxanthin from Phaffia rhodozyma (Villalobos-Castillejos et al., 2013), and the extraction of betalains from beetroot (Cardoso-Ugarte et al., 2014).
The addition of cellulase, α-amylase, and pectinase improves the extraction of some bioactive compounds, because it breaks down cell walls and hydrolyzes the polysaccharide structures that connect them (Puri et al., 2012). Besides the enzyme composition, concentration, and kind, other factors -such as the particle size of the plant materials, the solid-water ratio, and the hydrolysis time- also affect the extraction process (Azmir et al., 2013). The enzymatic hydrolysis has been studied in order to extract phenolic compounds from grape residues (Gómez-García et al., 2012), raspberry solid residues (Laroze et al., 2010) and betalains in fruits of the Hylocereus genus (Naderi et al., 2010).
The demand for natural compounds has increased due to their beneficial health properties, but extraction processes with high temperatures or with certain solvents may be inadequate. The extraction of these compounds must be carried out under conditions that do not affect their bioactive properties. An ideal extraction method should be quick, quantitative, and non-destructive (Yang et al., 2011).
Therefore, the objective of this study was to apply microwaves, ultrasound, and enzymes (protease, cellulase, pectinase) pretreatments, in order to increase the extraction performance of the red pitaya (S. stellatus)’s bioactive compounds and to determine the effect of the seeds’ presence in the extraction of betacyanins, betaxanthins, total betalains, and total phenolic compounds, as well as their antioxidant capacity.
Materials and Methods
Reagents
All reagents used were analytical grade reagents. Gallic Acid, ABTS [2,2’-azino-bis (3-ethylbenzothiazoline 6-sulfonic acid)], and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were obtained from Sigma-Aldrich (Mexico). For the enzymatic treatments, food-grade commercial protease, cellulose, and pectinase preparations purchased in ENMEX (Mexico) were used.
Vegetal material
The red pitaya fruits were harvested during the harvest season (from August to September 2013), in Santiago Tonahuixtla, Puebla (18° 12’ 5.35” N, 97° 53’ 50.75” W). The fruits had the following physical characteristics: weight (45.1-103.1 g), length (4.6-5.6 cm), and diameter (3.9-5.8 cm). The selected fruits were healthy, without damage or spots. In order to store the pitayas, their spines were removed, the fruits were vacuum-packed in polyethylene bags, and stored at -20 °C until they were analyzed.
Preparation of samples
Before they were analyzed, samples were removed from the freezer and placed 24 h in cooling conditions (4-10 °C). The studies were carried out in pulp with and without seeds. For that purpose, the peel was separated from the fruits and the pulp was divided in two portions. One of them was sieved with a #25 mesh (710 µm) to remove the seeds and then homogenized with a food processor, in order to obtain the seedless pulp sample. The other part was homogenized as a whole and sieved to obtain the pulp with seeds.
Water content
The water content -in pulp with and without seeds- was determined by weight loss after samples had been oven-dried at 110 °C (AOAC method 942.05) (AOAC, 1990).
Determination of pH
The pH was measured using a potentiometer in a 10 % p v-1 aqueous solution of macerated pulp with and without seeds (Denver Instrument UB-10 Colorado, USA; AOAC official method 981.12).
Pretreatment of the sample
The three kinds of pretreatments evaluated were: microwave, ultrasound, and enzymatic hydrolysis. Sample portions of 6.2 g were placed in 20 mL test tubes with screw caps and underwent various pretreatments. In all cases, a sample was analyzed without pretreatment or control sample (C), in order to evaluate the pretreatment effect.
Microwaves
The sample was placed in the center of a domestic microwave oven (Acros AM1007Q, China, 1050 W) and the pretreatment was applied at 105 W (10 % of the maximum power) during 62 min (M1, M2). After the treatment, the temperature was 36±1 °C for M1 and 45±1 °C for M2.
Ultrasound
The samples were kept in an ultrasonic bath (Bransonic CPX5800H, USA 40 KHz), with temperature control at 20 °C, for 5 (U1), 10 (U2), 15 (U3), 20 (U4), 25 (U5), 30 (U6) and 35 (U7) minutes.
Enzymatic hydrolysis
Prior to the pretreatment, the pH of the samples was measured and the result was 4.0±0.5 (within the range recommended by the enzymes supplier). Samples of pulp (with and without seeds) were mixed separately with a 0.5 % p p-1 rate enzymatic preparation of protease (PT), pectinase (PC), cellulase (CL), and the three enzymes together (PPC). The mixtures were incubated in a stirring heating bath (Aquatherm G-86, New Brunswick Scientific, USA) for 2 h and 24 h, at 150 rpm, and 40±1 °C. At the end of the incubation, the enzymatic reaction was stopped, heating the reaction mixture in a water bath at 95 °C for 5 min and then cooled with an ice-water bath at 2 °C for 10 min (Naderi et al., 2010).
After the pretreatments were applied, the samples were extracted according to the procedure described in the “Preparation of the extracts” section below and the betacyanins, betaxanthins, total betalains, total phenolic compounds, and antioxidant capacity were measured.
Preparation of the extracts
Pulp samples of 6.2 g -with or without seeds, with or without pretreatment- were placed separately in a 50 mL tube with screw cap and 10 mL of distilled water were added. The mixtures were vortexed (GEMMY VM-300 Taipei, Taiwan) for 1 min at full speed (3200 rpm) and then centrifuged at 10576 xg for 20 min (Dynamica Velocity 14R, London, UK). The supernatant was removed and the remaining solids were used for a second extraction under the same conditions, adding 5 mL of water. The extracts obtained from the first and second extractions were placed in a 25 mL volumetric flask; the volume was adjusted with water and the solution was used to determine betacyanins, betaxanthins, total betalains, total phenolic compounds, and antioxidant capacity.
Quantification of pigments
The betaxanthins (Bx) and betacyanins (Bc) content was quantified by measuring absorbance at 483 and 538 nm, respectively (Castellanos-Santiago and Yahia, 2008), with a UV-Vis spectrophotometer (HACH DR5000, Mexico). Each pigment’s concentration was calculated using Equation 1. Total betalains were calculated by adding Bc and Bx.
where B: Bx or Bc were expressed in terms of indicaxanthin or betanin mg, respectively, per 100 g of sample in dry weight (dw); A: absorbance at 483 nm for Bx and at 538 nm for Bc; DF: dilution factor; MW: molecular weight (indicaxanthin 308 g mol-1 and betanin 550 g mol-1); V: Extract volume (mL); Ɛ: molar attenuation coefficient (indicaxanthin=48 000 L mol-1 cm-1 and betanin=60 000 L mol-1 cm-1; P: quantity of sample (g); L : cell length (1 cm).
Quantification of total phenolic compounds
For this quantification, 400 µL of the extract were mixed with 3 mL of distilled water, 200 µL of the Folin-Ciocalteu reagent, and 400 µL of 20 % sodium carbonate solution (Singleton et al., 1999). The mixture was vortexed for 30 s, kept in the dark for 30 min at 20±2 °C, and the absorbance measured at 765 nm. Absorbance values were interpolated in a gallic acid standard curve. The values of total phenolic compounds are shown as mg of gallic acid equivalent (GAE) 100 g-1 of dw simple.
Determination of antioxidant capacity by the ABTS test
The ABTS•+ radical was prepared mixing the ABTS solution (7 mM) with potassium persulfate (2.45 mM) (Re et al., 1999); the radical mixture was kept in the dark for 16 h before being used. One milliliter of the ABTS•+ radical was diluted with 100 mL of 0.01 M, pH 7.4 phosphate buffer saline (PBS), until an approximate absorbance of 0.7±0.02 at 734 nm was obtained. A 200 µL aliquot was taken from the extract, and 3.8 mL of the ABTS radical solution was added; afterwards, the mixture was vortexed for 10 s, kept in the dark for 7 min at 20±2 °C, and its absorbance was measured at 734 nm (Af). For each sample, a 200 µL of methanol (A0) blank was prepared. The percentage inhibition was calculated and interpolated in a 30-300 mg mL-1 Trolox calibration curve. The results are expressed as mg equivalents of Trolox (ET) 100 g-1 of dw sample.
Experimental design and statistical analysis of the results
The experimental design of pretreatments was completely randomized. Two factors were evaluated: 1) kind of sample (pulp with or without seeds) and 2) kind of pretreatment. The experimental unit was the 25 mL extract obtained under each experimental condition and all analysis were performed in triplicate. Results were reported as the average ± standard deviation (SD), an ANOVA was performed, and the means were compared using Fisher’s method (p≤0.05). To compare the effect of the kind of sample and pretreatment, a two-way analysis was performed, as well as a principal component analysis (PCA), in order to analyze the correlation between the variables evaluated and pretreatments. Data processing was carried out with the Minitab 16 (Minitab Inc., Pennsylvania, USA) and GraphPad Prism 6 (GraphPad Software, California, USA) softwares.
Results and Discussion
Effect of the pretreatments in the extraction of bioactive compounds in red pitaya pulp (with and without seeds)
Betalains extraction in red pitaya pulp (with and without seeds)
In all the extracts obtained from pulp (with and without seeds that received an enzymatic treatment), the total betalain content was reduced (p≤0.05) in relation to the control without pretreatment (Figure 1). When we compare the extracted pigments values from the controls without enzymatic treatment (CEA and CEB), as the incubation period increases from 2 h to 24 h, the total betalains decrease 28-42 % and 9-34 %, in pulp with and without seeds, respectively. This decrease may be the result of the samples heating during the incubation period with the different enzymes, which destroys thermosensitive pigments present in the pitaya pulp.
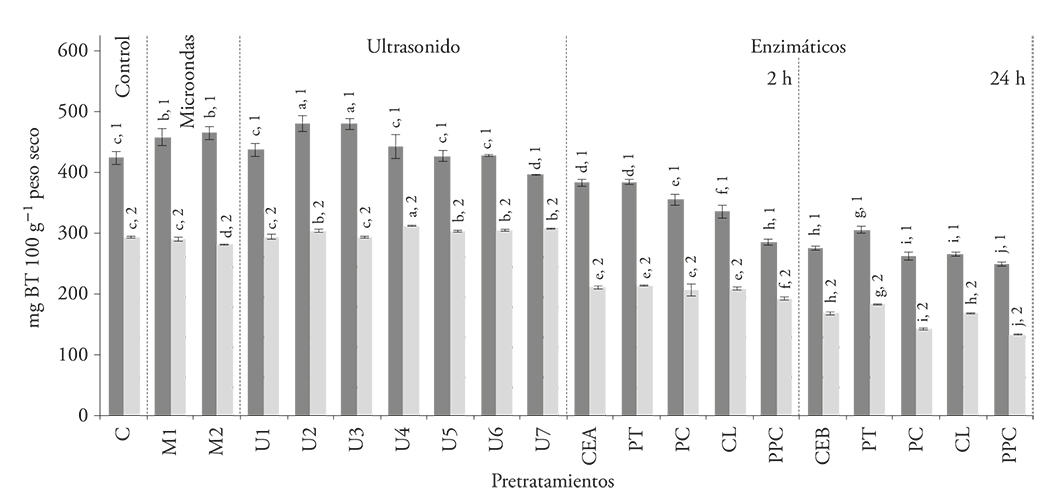
Figure 1 Total betalain content of the seedless pulp ( ) and pulp with seeds (
) and pulp with seeds ( ) extracts obtained after applying pretreatments: microwaves (M), ultrasound (U), protease (PT), pectinase (PC), cellulase (CL), and enzyme mixture (PPC). All compared with controls without treatment (C, CEA, CEB). Numbers indicate a significant difference between the samples; letters indicate a significant difference between pretreatments (p≤0.05).
) extracts obtained after applying pretreatments: microwaves (M), ultrasound (U), protease (PT), pectinase (PC), cellulase (CL), and enzyme mixture (PPC). All compared with controls without treatment (C, CEA, CEB). Numbers indicate a significant difference between the samples; letters indicate a significant difference between pretreatments (p≤0.05).
Herbach et al. (2007) observed a 24 % loss of total betalains, when they performed a partial hydrolysis of the pitaya mucilage (Hylocereus polyrhizus), with an enzymatic preparation at 40 °C for 2 h. The 9.4-32.7 % value for the extraction of these pigments in S. stellatus obtained in our study is similar to the value reported by those authors.
With regard to the ultrasound pretreatment, the total betalain content increased 13.1 % in the seedless pulp extracts after 15 min, but longer exposure to ultrasound did not increase the extraction efficiency (Figure 1). This effect may be due to cell walls alteration that facilitates the pigment extraction process (Panchev et al., 1988; Bagherian et al., 2011). The effect on the pulp with seeds was practically negligible in all exposure times (Figure 1). The increase obtained for seedless pulp was higher than the one observed by Sivakumar et al. (2009), who showed that the performance of the beet pigment extraction increased only by 8 % when the same pretreatment was applied.
The presence of pitaya seeds had a negative effect on pigment extraction: the content of total betalain in the pulp with seeds decreased by 22.3-46.7 %, in relation to seedless pulp.
Extraction of total phenolic compounds in red pitaya pulp (with and without seeds)
In contrast to the betalain results, the content of total phenolic compounds in both kinds of pulps extracts increased (p≤0.05) when enzymatic pretreatments were applied after 2 h, especially with PC application. As with betalain extraction, 24 h incubation had a negative effect on the total phenols extraction (Figure 2), also due to the heating effect (CEA vs CEB pretreatment).
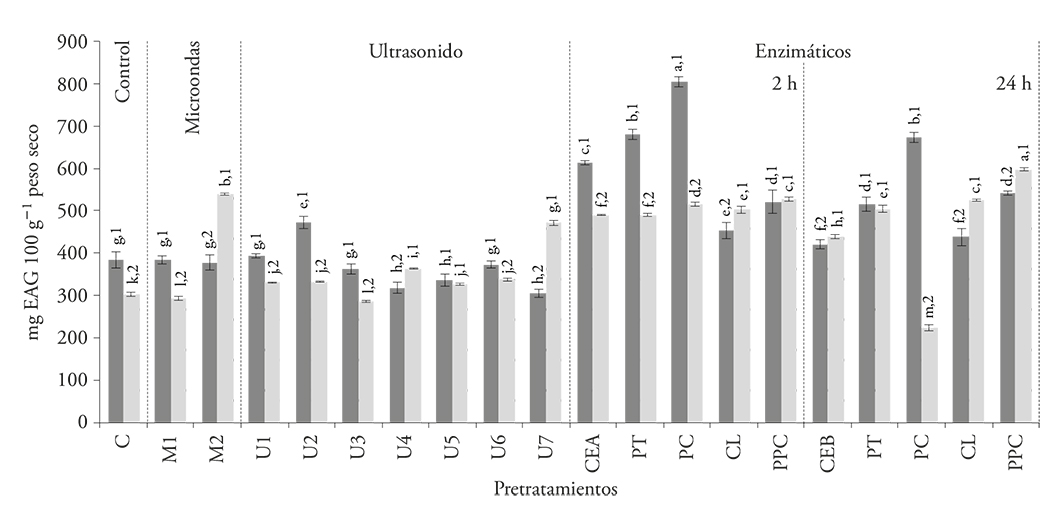
Figure 2 Total phenolic compounds of the seedless pulp ( ) and pulp with seeds (
) and pulp with seeds ( ) extracts obtained after applying pretreatments: microwaves (M), ultrasound (U), protease (PT), pectinase (PC), cellulase (CL), and enzyme mixture (PPC). All compared with controls without treatment (C, CEA, CEB). Numbers indicate a significant difference between the samples; letters indicate a significant difference between pretreatments (p≤0.05).
) extracts obtained after applying pretreatments: microwaves (M), ultrasound (U), protease (PT), pectinase (PC), cellulase (CL), and enzyme mixture (PPC). All compared with controls without treatment (C, CEA, CEB). Numbers indicate a significant difference between the samples; letters indicate a significant difference between pretreatments (p≤0.05).
Hydrolytic enzymes application increases the extraction of total phenolic compounds. In our study, the content of these compounds increased 70.4 % and 109.8 %, in pulp extracts with and without seeds, respectively, when we applied the pectinase pretreatment for 2 h (Figure 2). Gómez-García et al. (2012) reported 25 % increases in phenolic compounds in grape residues and 28-35 % in raspberry residues (Laroze et al., 2010); both figures are lower than the values in our study.
According to Puri et al. (2012), when enzymatic treatments are applied, the partial hydrolysis of the mucilage enables the extraction of the compounds bound to the polymeric matrix, which facilitates samples processing. Galacturonic acid is an abundant mucilage compound that can be released during enzymatic hydrolysis; meanwhile, functional groups exposure (hydroxyl and carboxyl) may increase the total phenols value (Combo et al., 2011).
With regard to microwave pretreatments, significant differences were observed between the pulp with and without seeds during the extraction of phenolic compounds. The application of microwave for 2 min (M2) resulted in a 78.1 % (p≤0.05) increase in the extraction of phenolic compounds from pulp with seeds, which may be the result of tissue rupture and greater interaction between the solvent and the matrix (Bagherian et al., 2011). However, this pretreatment had no effect in the seedless pulp (Figure 2).
In addition, exposure to ultrasonic waves did not result in significant increases (p≤0.05) in the total phenolic compounds content, in most of the conditions tested for both samples.
The extraction of phenolic compounds from pitayas using microwaves as pretreatment is more efficient than the one obtained by Nayak et al. (2015), who reported a 20.5 % increase in citrus peels, in comparison with conventional solvent extraction. This means that the effect of pretreatments over the performance of total phenolic compounds in extracts depends on which kind of vegetal material it is applied on. In pitaya, the extraction of phenolic compounds is greater with pectinase treatment, and better in seedless pulp.
Antioxidant capacity values in red pitaya pulp extracts (with and without seeds)
Enzymatic treatments in pulp (with and without seeds) increased the antioxidant capacity values in the extracts obtained from both samples (Figure 3), and the effect was greater due to the action of pectinase (PC) for 2 h. In a similar way to what was observed in phenolic compounds extraction, the increase in temperature had a positive effect, because it increased the extracts’ antioxidant capacity, but only in short incubation times.

Figure 3 Antioxidant capacity of the seedless pulp ( ) and pulp with seeds (
) and pulp with seeds ( ) extracts obtained after applying pretreatments: microwaves (M), ultrasound (U), protease (PT), pectinase (PC), cellulase (CL) and enzyme mixture (PPC). All compared with controls without treatment (C, CEA, CEB). Numbers indicate a significant difference between the samples; letters indicate a significant difference between pretreatments (p≤0.05).
) extracts obtained after applying pretreatments: microwaves (M), ultrasound (U), protease (PT), pectinase (PC), cellulase (CL) and enzyme mixture (PPC). All compared with controls without treatment (C, CEA, CEB). Numbers indicate a significant difference between the samples; letters indicate a significant difference between pretreatments (p≤0.05).
The antioxidant compounds extraction in pitaya improves with protease and pectinase treatments, but a higher extraction is obtained when the seeds are removed from the pulp (Figure 3). The use of pectinase increased the antioxidant capacity up to 74.8 % in seedless pulp extracts and 25.3 % in extracts of pulp with seeds. These values are higher than those obtained with microwave or ultrasound treatments (Figure 3).
The increase of the extracts’s antioxidant capacity -after applying the pectinase treatment- can be caused by the pectin hydrolysis or the pitaya pulp mucilage, which can release fragments with greater antioxidant capacity than the polymers from which they originate (Chaouch et al., 2015), or by the polymer networks break down, which releases antioxidant compounds, including phenolic compounds (Kunnika and Pranee, 2011).
In order to determine if the differences between the bioactive compounds concentrations in the extracts were caused by the presence of seeds in the pulp or a dilution effect, we had to calculate the corrected values of the pigments, antioxidant capacity, and total phenolic compounds per 100 g of pulp, considering the seeds proportion in the samples. Table 1 shows the results obtained for samples without pretreatment (control) and with ultrasound pretreatments for 15 min and pectinase after 2 h. According to the results, there would be a higher content of yellow pigments (Bx) than red pigments (Bc) in the studied pulps, although, according to Pérez-Loredo et al. (2016), the L*, a* and b* chromatic parameters of S. stellatus fruits confirm the pulp coloration in red pitayas. This discrepancy is also observed in betalains studies in red Opuntia ficus-indica prickly pears (Fernández-López et al., 2010), and it may be due to the overestimation of Bx values, as a result of the overlap of the bands of the Bx and Bc absorption spectrum, or the use of mean extinction coefficients.
Table 1 Pigments values (Bc, Bx, and TB), phenols (TPC), and antioxidant capacity (AC) from the aqueous extracts of pulp (with and without seeds) obtained with the best pretreatments.
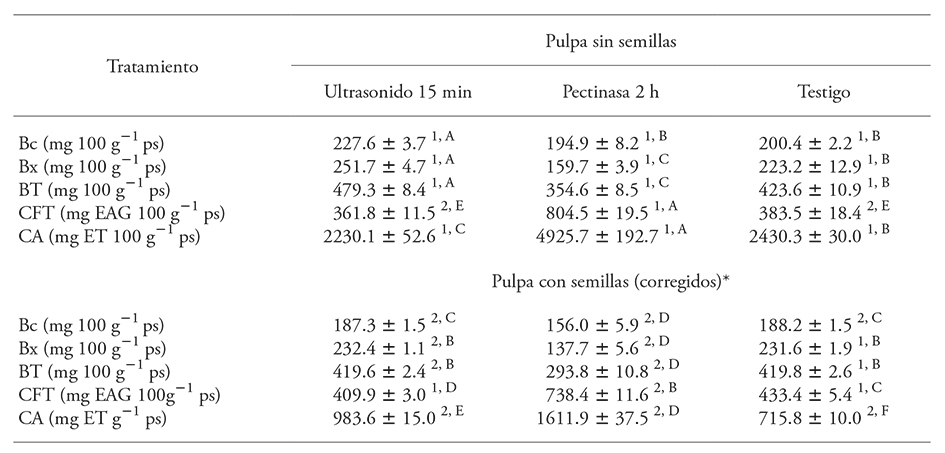
Mean ± standard deviation (SD), n=3. The samples’ moisture content was 88.6 % in seedless pulp and 87.5 % in pulp with seeds. Values expressed per 100 g dry weight (dw) of pulp. Superscripts indicate significant differences for each variable (p≤0.05); numbers indicate significant differences between samples; letters indicate significant differences between pretreatments. *The corrected values were calculated considering a 30.2 % seed content in the fruit pulp.
Our working group is carrying out a research about the number and kind of betalains present in pitayas of the Stenocereus genus using HPLC-DAD. Preliminary results indicate that there are four kinds of betaxanthins and five of betacyanins in the pulp extract (although the latter predominate). Despite a likely bias in the results, we can appreciate the level of betalains in cactaceae fruit extracts using spectrophotometric methods (Fernández-López et al., 2002). In our study, the total betalain values allowed us to evaluate and compare the effect of different pretreatments in red pitaya pigment extraction.
The comparison of the corrected data from Table 1 confirms that the presence of seeds has a negative impact in pigment extraction and antioxidant capacity. Only a slight increase in total phenolic compounds extraction is obtained (<13 %) that way; therefore, using seedless pulp is more advisable. In addition, we observed that the extract of pulp with seeds was darker, which may be the result of the oxidation or degradation of some extract compounds. The presence of seeds could favor non-enzymatic browning or ascorbic acid oxidation (Suh et al., 2003), both of which promote betalain decline.
In order to correlate the extraction efficiency of the pigments, phenolic compounds, and antioxidant capacity with the pretreatments used, we carried out a PCA. This statistical analysis allows to obtain graphs in which the grouping of data in quadrants helps to define the relations between variables.
Principal component analysis (PCA) of the bioactive compounds extraction with regard to the pretreatment used
PCA is used in food studies to classify variables by means of the data statistical reduction. The original data groups are transformed into new groups of uncorrelated variables called principal components (PC), which are used to develop graphs that enable the visual evaluation of similarities between the samples, as well as determining if these samples can be grouped (Perez-Loredo et al., 2016).
The analysis of the results for betacyanins (Bc), betaxanthins (Bx), total betalains (TB), total phenolic compounds (TPC), and antioxidant capacity (AC) through PCA showed that two PC explain 96.3 % of total result variations (PC1: 71.6 %, PC2: 24.7 %) in seedless pulp (Figure 4), and 97.3 % (PC1: 82.1 %; PC2: 15.2 %) in pulp with seeds (Figure 5).

Figure 4 Two-dimensional chart of PC1 and PC2 principal components for the PCA charges and scores for Bc, Bx, TB, TPC, and AC obtained in seedless pulp without (C) and with microwave (M), ultrasound (U) pretreatment and with protease (PT), pectinase (PC), cellulase (CL), protease, pectinase and cellulase (PPC) enzymes after 2(†) and 24 (††) h of incubation.
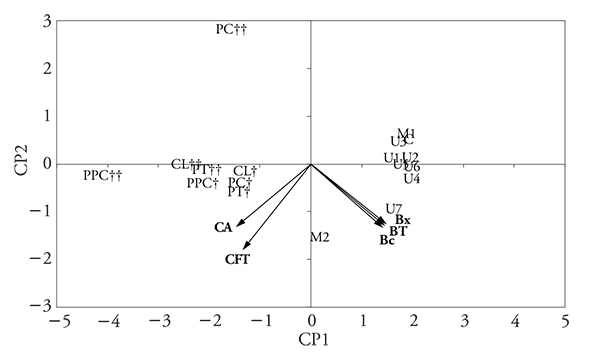
Figure 5 Two-dimensional chart of PC1 and PC2 principal components for the PCA charges and scores of Bc, Bx, TB, TPC, and AC obtained in pulp with seeds without (C) and with microwave (M), ultrasound (U) pretreatments and with protease (PT), pectinase (PC), cellulase (CL), protease, pectinase and cellulase (PPC) enzymes after 2(†) and 24 (††) h of incubation.
Bar charts are useful for quantitative analysis of differences between the treatments for each sample (Figure 1, 2 and 3). However, the PC chart allowed us to analyze all the results as a whole and to reach a conclusion on the basis of the groupings (Figures 4 and 5).
The PCA graphs analysis regarding the pulp with and without seeds (Figure 4, 5) shows that enzymatic treatments (PC and PT) provide a higher performance in TPC and AC extraction, whereas ultrasound (U) and microwaves (M) result in higher pigments performance. For seedless pulp (Figure 4), enzymatic pretreatments with long incubation (24 h, ††) or ultrasonic with longer exposure times (U4-U7) are far away from the TB, TPC, and AC variables, (in the PC2 positive quadrants): this means that longer times do not favor extraction. This effect is not so obvious in pulp with seeds (Figure 5), because the TB, TPC, and AC extraction performances are very similar under the different treatments and there are no significant differences (p>0.05) between most of them (Figure 1, 2 and 3).
It is important to highlight that antioxidant capacity has a higher correlation with the amount of phenolic compounds (both in PC1 negative) than with the amount of betalains (in PC1 positive).
Based on these results, we suggest that -in order to use of pitaya as a source of bioactive compounds- the pulp must be separated from the seeds, and -in order to maximize the extraction of pigments, phenolic compounds, and antioxidant capacity- the pulp must be treated previously with ultrasound for 15 min and pectinase at 0.5 % p p-1 for 2 h.
Conclusions
The application of microwave, ultrasound, and hydrolytic enzymes pretreatments affects the performance of betalains, phenols, and the antioxidant activity of the red pitaya (S. stellatus). Choosing the best sample pretreatment for the extraction of specific bioactive compounds will depend on the study’s matrix and purpose. The maximum total values of betacyanins, betaxanthins, and betalains were obtained applying ultrasound for 15 min. Total phenol content and maximum antioxidant capacity were obtained pretreating the pulp with pectinase
Literatura Citada
Alupului, A., I. Călinescu, and V. Lavric. 2012. Microwave extraction of active principles from medicinal plants. U P B. Sci. Bull., Series B. 74: 129-142. [ Links ]
AOAC, Association of Official Analytical Chemists. 1990. Official Methods of Analysis. (15th ed). Arlington, Virginia, USA. 1298 p. [ Links ]
Azmir, J. et al. 2013. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 117: 426-436. [ Links ]
Bagherian, H., F. Z. Ashtiani, A. Fouladitajar, and M. Mohtashamy. 2011. Comparisons between conventional, microwave -and ultrasound- assisted methods for extraction of pectin from grapefruit. Chem. Eng. Process. 50: 1237-1243. [ Links ]
Cardoso-Ugarte, G. A., M. E. Sosa-Morales, T. Ballard, M. F. San Martín-Gonzalez, and A. Liceaga. 2014. Microwave-assisted extraction of betalains from red beet (Beta vulgaris). LWT - Food Sci Technol. 59: 276-282. [ Links ]
Castellanos-Santiago, E., and E. M. Yahia. 2008. Identification and quantification of betalains from the fruits of 10 Mexican prickly pear cultivars by high-performance liquid chromatography and electrospray ionization mass spectrometry. J. Agric. Food Chem. 56: 5758-5764. [ Links ]
Cejudo-Bastante, M. J., N. Hurtado, and F. J. Heredia. 2015. Potential use of new Colombian sources of betalains. Colorimetric study of red prickly pear (Opuntia dillenii) extracts under different technological conditions. Food Res. Int. 71: 91-99. [ Links ]
Chaouch, M. A., J. Hafsa, C. Rihouey, D. Le Cerf, and H. Majdoub. 2015. Depolymerization of polysaccharides from Opuntia ficus indica: Antioxidant and antiglycated activities. Int. J. Biol. Macromol. 79: 779-786. [ Links ]
Combo, A., M. Aguedo, and M. Paquot. 2011. Pectic oligosaccharides: production and potential applications. Biotechno. Agron. Soc. Environ. 15: 153-164. [ Links ]
Fernández-López, J. A., R. Castellar, J. M. Obón, and L. Almela. 2002. Screening and mass-spectral confirmation of betalains in cactus pears. Chromatographia 56: 591-595. [ Links ]
Fernández-López, J. A., L. Almela, J. M. Obón, and R. Castellar. 2010. Determination of antioxidant constituents in cactus pear fruits. Plant Foods Hum. Nutr. 65: 253-259. [ Links ]
García-Cruz, L., Y. Salinas-Moreno, and S. Valle-Guadarrama. 2012. Betalaínas, compuestos fenólicos y actividad antioxidante en pitaya de mayo (Stenocereus griseus H.). Rev. Fitotec. Mex. 35: 1-5. [ Links ]
García-Cruz, L., S. Valle-Guadarrama, Y. Salinas-Moreno, and E. Joaquín-Cruz. 2013. Physical, chemical, and antioxidant activity characterization of pitaya (Stenocereus pruinosus) fruits. Plant Foods Hum. Nutr . 68: 403-410. [ Links ]
Gómez-García, R., G. C. G. Martínez-Ávila, and C. N. Aguilar. 2012. Enzyme-assisted extraction of antioxidative phenolics from grape (Vitis vinifera L.) residues. 3 Biotech. 2: 297-300. [ Links ]
Herbach, K. M., C. Maier, F. C. Stintzing, and R. Carle. 2007. Effects of processing and storage on juice color and betacyanin stability of purple pitaya (Hylocereus polyrhizus) juice. Eur. Food Res. Technol. 224: 649-658. [ Links ]
Kunnika, S., and A. Pranee. 2011. Influence of enzyme treatment on bioactive compounds and colour stability of betacyanin in flesh and peel of red dragon fruit Hylocereus polyrhizus (Weber) Britton and Rose. Inter. Food Res. J. 18: 1437-1448. [ Links ]
Laroze, L., C. Soto, and M. E. Zúñiga. 2010. Phenolic antioxidants extraction from raspberry wastes assisted by-enzymes. Electron. J. Biotechno. 13 DOI: 10.2225/vol13-issue6-fulltext-12. [ Links ]
Morales, P., E. Ramírez-Moreno, M. C. Sanchez-Mata, A. M. Carvalho, and I. Ferreira. 2012. Nutritional and antioxidant properties of pulp and seeds of two xoconostle cultivars (Opuntia joconostle F. A. C. Weber ex Diguet and Opuntia matudae Scheinvar) of high consumption in Mexico. Food Res. Int. 46: 279-285. [ Links ]
Naderi, N., F. C. Stintzing, H. M. Ghazali, Y. A. Manap, and S. D. Jazayeri. 2010. Betalain extraction from Hylocereus polyrhizus for natural food coloring purposes. J. Prof. Assoc. Cactus 12: 143-154. [ Links ]
Nayak, B., F. Dahmoune, K. Moussi, H. Remini, S. Dairi, O. Aoun, and M. Khodir. 2015. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 187: 507-516. [ Links ]
Panchev, I., N. Kirchev, and C. Kratchanov. 1988. Improving pectin technology: II. Extraction using ultrasonic treatment. Int. J. Food Sci. Tech. 23: 337-341. [ Links ]
Pérez-Loredo, M. G., F. García-Ochoa, and B. E. Barragán-Huerta. 2016. Comparative analysis of betalain content in Stenocereus stellatus fruits and other cactus fruits using principal component analysis. Int. J. Food Prop. 19: 326-338. [ Links ]
Prakash M. J., and S. Manikandan. 2012. Response surface modeling and optimization of process parameters for aqueous extraction of pigments from prickly pear (Opuntia ficus-indica) fruit. Dyes Pigments 95: 465-472. [ Links ]
Puri, M., D. Sharma, and C. J. Barrow. 2012. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 30: 37-44. [ Links ]
Re, R., N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, and C. Rice-Evans. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio. Med. 26: 1231-1237. [ Links ]
Singleton, V. L., R. Orthofer, and R. M. Lamuela-Raventos. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method. Enzymol. 299: 152-178. [ Links ]
Sivakumar V., Lakshmi J. A., Vijayeeswarri J., and Swaminathan G. 2009. Ultrasound assisted enhancement in natural dye extraction from beetroot for industrial applications and natural dyeing of leather. Ultrason Sonochem. 16: 782-789. [ Links ]
Suh, H. J., D. O. Noh, C. S. Kang, J. M. Kim, and S. W. Lee. 2003. Thermal kinetics of color degradation of mulberry fruit extract. Nahrung 47: 132-135. [ Links ]
Villalobos-Castillejos, F., P. Cerezal-Mezquita, M. L. Hernández-De Jesús, and B. E. Barragán-Huerta. 2013. Production and stability of water-dispersible astaxanthin oleoresin from Phaffia rhodozyma. Int. J. Food Sci. Tech. 48: 1243-1251. [ Links ]
Woo, K. K., F. H. Ngou, L. S. Ngo, W. K. Soong, and P. Y. Tang. 2011. Stability of betalain pigment from red dragon fruit (Hylocereus polyrhizus). Am. J. Food Tech. 6: 140-148. [ Links ]
Yang, B., Y. Jiang, J. Shi, F. Chen, and M. Ashraf. 2011. Extraction and pharmacological properties of bioactive compounds from longan (Dimocarpus longan Lour.) fruit -A review. Food Res. Int . 44: 1837-1842. [ Links ]
Received: April 2016; Accepted: December 2016











 texto em
texto em 


