Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.51 no.1 Texcoco ene./feb. 2017
Fitociencia
Seed and seedlings physical characteristics and seeds germination of wild and domesticated common bean (Phaseolus vulgaris L.) and their progeny
1Postgrado de Recursos Genéticos y Productividad, Fisiología Vegetal; Colegio de PostgraduadosMéxico.
2Postgrado en Botánica, Colegio de Postgraduados. 56230. Carretera México-Texcoco km 36.5, Montecillo, Texcoco, México.
3Instituto de Investigación de Zonas Desérticas, Universidad Autónoma de San Luis Potosí. 78377. Altair 200, Colonia del Llano, San Luis Potosí, Mexico.
The source of the tolerance to biotic and abiotic factors of currently consumed common bean (Phaseolus vulgaris L.) cultivars could be found in their wild counterparts. But they and their progeny should first be characterized. The aim of this study was to evaluate the physical characteristics of the seed, seed germination and seedling emergence of wild and domesticated common bean (P. vulgaris L.) and their progeny. The hypothesis was that one or more characteristics of the progeny are outstanding compared to cultivars. In random samples from three wild accessions (Typical Durango, Chihuahua and S13), two cultivars (Bayo Mecentral and Negro Tacaná) and five progenies (3.3, 11.1, 51b, 53b, and 118b) of the wild S13 and cv. Negro Tacaná: biomass, width, thickness, length, proportion of cotyledons, embryonic axis and seed coat, of the seeds, and percentage of seed germination and seedling emergence were evaluated. The experimental design was completely randomized, ten treatments (three wild accessions, two cultivars, and five progeny derived from crossing the cultivar Negro Tacaná and wild S13) with 100 replicates, each seed being an experimental unit. The results were analyzed with size frequency curves, ANOVA and multiple comparisons of means with the Tukey test (p≤0.05). Seed biomass (67 to 124 mg), width (4.36 to 5.72 mm), length (2.65 to 4.92 mm) and thickness (6.81 to 8.47 mm) showed a gradient from wild variants and progeny to domesticated variants. Wild seeds and progeny had higher proportion of embryonic axis (1.74%and 2.12%) than domesticated (1.34%). Seed germination and seedling emergence did not differ between the variants. Only some characteristics of the progeny, as the proportion of cotyledons and embryonic axis in seeds, were higher than in the cultivars.
Keywords: Seed biomass; seed size; Phaseolus vulgaris; domestication; wild legume
La fuente de tolerancia del frijol (Phaseolus vulgaris L.) a factores bióticos y abióticos de los cultivares que se consumen actualmente podría encontrarse en los congéneres silvestres, pero éstos y la progenie de sus cruzas deben caracterizarse primero. El objetivo de este estudio fue evaluar las características físicas y germinación de semillas y emergencia de plántulas de frijol (P. vulgaris L.) silvestre, domesticado y su progenie. La hipótesis fue que una o más características de la progenie sobresalen respecto a los cultivares. En muestras aleatorias de tres recolectas silvestres (Durango Típico, Chihuahua y S13), dos cultivares (Bayo Mecentral y Negro Tacaná) y cinco progenies (3.3, 11.1, 51b, 53b, y 118b) del silvestre S13 y cv. Negro Tacaná se evaluó: biomasa, anchura, grosor, longitud, proporción de cotiledones, eje embrionario y testa, porcentaje de germinación de las semillas y emergencia de plántulas. El diseño experimental fue completamente al azar, 10 tratamientos (tres recolectas silvestres, dos cultivares y cinco progenies derivadas de la cruza del cultivar Negro Tacaná y el silvestre S13) con 100 repeticiones, y una semilla como unidad experimental. Los resultados se analizaron con las curvas de frecuencia de tamaño, ANDEVA y comparación de medias con la prueba de Tukey (p≤0.05). La biomasa (67 a 124 mg), anchura (4.36 a 5.72 mm), longitud (2.65 a 4.92 mm) y el grosor (6.81 a 8.47 mm) de las semillas mostraron un gradiente entre variantes silvestres, progenie y domesticadas. Las semillas silvestres y de la progenie tuvieron proporción mayor de eje embrionario (1.74% y 2.12%) que las domesticadas (1.34%). La germinación de semillas y emergencia de plántulas no difirió entre las variantes. Solo algunas características de la progenie, como la proporción de cotiledones y de eje embrionario en las semillas, fueron superiores respecto a los cultivares.
Palabras clave: Biomasa de semillas; dimensiones de semillas; Phaseolus vulgaris; domesticación; leguminosa silvestre
Introduction
Common bean (Phaseolus vulgaris L.) is the most important food legume in the world. This crop is produced on diverse systems, regions and environments in Latin America, Africa, Middle East, China, Europe, USA and Canada. In 2013 the world production was of 23.1 million Mg (CEDRSSA, 2014). The bean crop grows in almost all world regions, soil conditions and climate in Mexico, from sea level up to 2700 m. Therefore, this crop is second most important regard its total sown area in Mexico, only after maize (Zea mays L.) (Secretaría de Economía, 2012).
Beans are an important source of protein (14-33%), starch, vitamins B, minerals (Ca, Cu, K, Mg, P, and Zn) and dietary fiber (15.5-21 g per 100 g of cooked grain). They are deficient in sulfur amino acids and tryptophan, but contain sufficient amounts of lysine (1.2-1.5 g per 100 g of grain) (Peña-Valdivia et al., 2011). The seeds can be eaten unripe, ripe, fresh or dried (OECD, 2016).
Cultivated beans are a result of the domestication of wild ancestors (Peña-Valdivia et al., 2012). Domesticated variants differ from the wild in their larger seeds, increased permeability of the seed coat, loss of latency, increased variability of color and decreased content of antiphysiological compounds (Peña-Valdivia et al., 2012; Delgado and Gama, 2015). The seed color, its brightness, size and shape may have been some of the characteristics responsible for the selection at the beginning of the domestication process (Peña-Valdivia et al., 2012).
The seed size and plant growth habit are related to the efficient biomass allocation to the seed in the growth environment but also depends on other seed characteristics, as the vigor (Celis-Velázquez et al., 2010; OECD, 2016). According to Gonzalez et al. (2008), that the weight of 100 bean seeds was altered by rainfed conditions and seed quality was more affected after accelerated aging. In contrast, the wild bean maintained its emergency capacity after accelerated aging (Peña-Valdivia et al., 1999). The loss of latency (Peña-Valdivia et al., 2002), the increased water impermeability and decreased hardness of bean seeds during domestication relate to characteristics of the seed coat (Peña-Valdivia et al. 1999 and 2011).
Latency is a physical condition that prevents the germination of mature seeds although there are environmental conditions that promote it (OECD, 2016). This condition is not static, as some seeds can go through latency cycles and without it, often induced by environmental conditions (Bewley, 1997). Mechanical scarification, which does not harm the embryonic axis, breaks dormancy in seeds of wild beans (López H. et al., 2001), but the seeds of non-domesticated beans do not show this phenomenon (Lépiz I.et al., 2005). Furthermore, storage for several months at room temperature, of about 25 °C, gradually eliminates dormancy of the wild seeds (Peña-Valdivia et al., 2005).
The germination capacity and vigor are the main characteristics involved in the physiological quality of the seed. Seed germination is the physiological process by which the essential structures emerge and develop, from the embryo, for the development of a normal plant (Delouche, 2002). This process begins with a variety of anabolic and catabolic activities such as respiration, protein synthesis and mobilization of reserves after water absorption (Desai, 2004). Seeds vigor is its biological potential for the fast and uniform establishment, even unfavorable conditions, plants in the field (González et al., 2008). External factors, such as temperature, water, oxygen and light, directly influence seed germination. The emergence of a seedling then depends on the physiological and biochemical seed characteristics, the response to external conditions and efficiency in using reserves during germination (Peña-Valdivia et al., 2013). In this regard, when assessing the efficiency of the use of seed reserves for germination and seedling emergence in improved cultivars, native cultivars and wild variants, Celis-Velázquez et al. (2010) found that improved cultivars were more efficient in the use of reserves than the other variants.
Seeds of wild bean are waterproof, which can prevent germination. In seeds, structures like the hilum, micropyle and chalaza pore regulate water absorption. In this regard, Pérez-Herrera and Acosta-Gallegos (2002) point out that the participation of the seed coat and micropile-hilum region in domesticated and wild variants participate differently in water absorption; in most wild variants and in some cultivars participation of the micropile and hilum was substantial in imbibition and its seed coat was waterproof. An exception was the cv. Flor de Mayo Bajío in which water absorption is carried out mainly through the seed coat.
The seed coat is between 8 and 10% of the total seeds (Celis-Velázquez et al., 2010), consists of 67% insoluble fiber and 4% soluble fiber, and is rich in phenolic compounds, which are susceptible to polymerization and contribute to the waterproofing of the seed coat (Shiga et al., 2011).
Storage under conditions, such as high temperature and humidity, which damage the seed, causes in domesticated beans, but not the wild, the phenomenon is known as hard-to-cook (Peña-Valdivia et al., 1999). Other intrinsic to the seed characteristics, as the thickness, composition and microstructure of the seed coat can affect the seed hardness; besides, changes during postharvest, such as lipid oxidation, the formation of insoluble pectates and changes of the cell wall components can also irreversibly alter the seeds. According to Velasco-González et al. (2013), bean cultivars with high ash content (3.60 to 4.63%) have higher water absorption, and the hardness of the seed was inversely correlated with water absorption. These authors confirm that the densest structures of the seed coat led less water absorption and hardness of the seeds. In this regard, Peña-Valdivia et al., (2012) agree on the inverse correlation of the seed hardness and water absorption between wild variants and cultivars of common bean, but the ash content in the wild seeds was greater or equal (4.3%) than in the cultivars.
In Mexico, there is a genetic potential with numerous wild variants of common bean, which can be used as a source of resistance to biotic and abiotic stress, with better agronomic and nutritional characteristics that domesticated types (Peña-Valdivia et al., 2011; Delgado and Gama, 2015). The biochemical and physiological response of wild plants to environmental factors that influence their growth and development, and wild seeds responses partially known (García H. et al., 1997; Celis-Velázquez et al., 2010; Peña-Valdivia et al., 2010; Peña-Valdivia et al., 2011; Porch et al., 2013), but the morphological and biochemical-physiological characteristics of progeny of domesticated and wild bean variants are virtually unknown. This is due to the fact that very few of these crosses have been carried out, because in breeding programs breeders prefer to use elite lines within the commercial classes and avoid using wild germplasm (Porch et al., 2013).
The aim of this study was to evaluate the physical seed characteristics, seed germination and seedling emergence of wild and domesticated common bean (P. vulgaris L.) and their progeny. The hypothesis was that one or more characteristics of the progeny stand out concerning the cultivars.
Materials and Methods
Biological material
In this study, three bean wild variants (Chihuahua, Durango Típico and S13), two domesticated (cv. Bayo Mecentral and Negro Tacaná) and five progeny derived from the cross of cv. Negro Tacaná and wild S13 (ID 3.3, 11.1, 51b, 53b and 118b) (Fernández et al., 1982; Toro et al., 1990; Garcia-Nava et al., 2014), were evaluated.
Variables evaluated
The characteristics evaluated were: seed width, thickness, and length, and biomass, proportion of cotyledons, embryonic axis and seed coat in the seed, and seed germination and seedling emergence.
Biomass. One hundred seeds of each variant were individually weighed on an analytical balance (Scentiech Model No. SA120, accuracy ±0.0001 g).
Dimensions. One hundred seeds of each variant were individually measured with a digital caliper (Truper, CALDI-6MP, 14388).
Seed structures. Seed coat, cotyledons and embryonic axis were separated with a scalpel, after soak seeds for 12 h at 5±2 °C; they were dehydrated at 75 °C for 72 h and biomass was determined on an analytical balance. The percentage of each structure was calculated on six replicates of three seeds each.
Germination. Seeds germination, at 25±1 °C and darkness (ISTA, 2009), was evaluated in Petri dishes using unscarified seeds. Ten seeds per Petri dish represented an experimental unit and five replications were evaluated. The number of germinated seeds was quantified every 12 h. The seed germination was considered when the radicle had emerged and had a length of 1 cm, this was measured using a digital, standard and millimetric caliper (Truper, CALDI-6MP, 14388).
Emergency. The emergency was quantified after sowing seeds in trays of plastic material, with volcanic rock as a substrate, with particles ≤0.5 cm, and 4 cm deep. The seeds were scarified with a cut with a scalpel in the seed coat, on the micropile opposite side. Fifteen seeds were sown per tray, five trays were obtained by variant, and each was a replication. The trays were kept moist by watering with water two or three times a week, from planting until the primary leaves were fully deployed (stage V2) (Fernández et al., 1982). The seedling emergence was evaluated every 12 h; emergency was considered successful when the cotyledons were observed at ground level (Fernández et al., 1982). The trays were placed in a polyethylene greenhouse at the Colegio de Postgraduados, in Montecillo, Texcoco, Estado de México (19° 31’ N, 98° 53’ O and 2353 m of altitude), during the autumn-winter cycle of 2014. In the greenhouse mean maximum and minimum temperature was 19.4 °C and 12.2 °C.
Experimental design and analysis of results
The study was developed with a completely randomized experimental design with five repetitions. The treatments were 10 variants of common bean (three wild, two domesticated, and five progeny derived from crossing the cultivar Negro Tacaná and wild S13). The results were analyzed with ANOVA and Tukey multiple comparisons (p≤0.05).
Results and Discussion
Seed biomass
The average seed biomass was different (p≤0.05) among the three groups, the wild variant had the lowest values, between 49.9 and 76.1 mg per seed on average. Of these, the S13 were the lightest and Chihuahua and Durango Typical were on average 52% heavier. Wild bean samples had some heterogeneity in the seminal biomass, as the coefficient of variation (CV) of the samples varied between 14.93% and 16.82% in S13 and Durango Típico, and up 27.27% in Chihuahua (Figure 1).

Figure 1 Seeds biomass (±standard error) of common bean (Phaseolus vulgaris L.) wild, domesticated and progeny of domesticated Negro Tacaná and wild S13.
Differences in biomass of wild seeds in each sample were confirmed with their frequency distribution (Figure 2). This was different among the three wild variants. For seeds of the Chihuahua it was asymmetrical and showed two maxima, one around 55 mg and another about 90 mg. The biomass of the Chihuahua seeds showed the widest distribution, from 30 to 120 mg, among wild samples, i.e. seeds with lower biomass sample were four times lighter than the heaviest (Figure 2A).
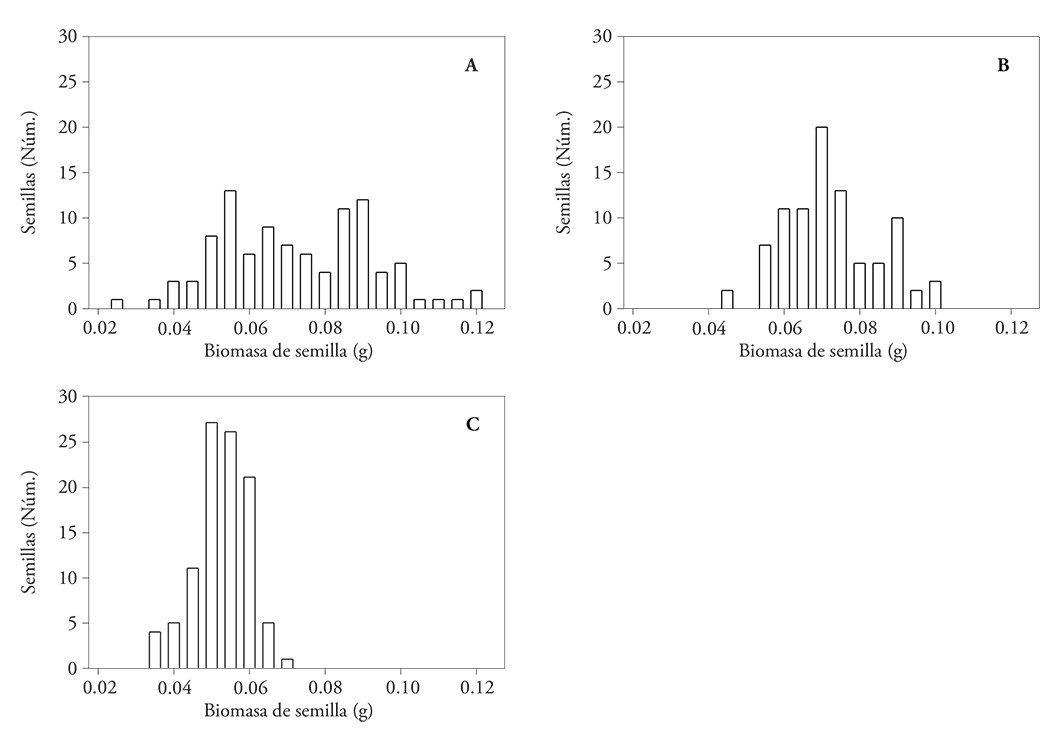
Figure 2 Seed biomass frequency distribution of wild common bean (Phaseolus vulgaris L.). (A) Chihuahua, (B) Durango Típico and (C) S13. Núm: Number.
The frequency distribution of seed biomass from the Durango Típico was nearly symmetric, with a bias towards smaller seeds than an average biomass, and mode around 70 mg. This distribution showed that the seeds of this sample had between 45 and 100 mg, and seeds with lower biomass were twice lighter than the heaviest (Figure 2B).
The frequency distribution of seed biomass of the S13 sample was symmetrical, with mode around 50 mg. This distribution showed that the S13 seeds weighed between 35 and 70 mg, but 73% of the seeds in the sample were concentrated around the 50 and 60 mg (Figure 2C).
Among the distinctive characteristics of wild bean populations, is the heterogeneity of their morphological, physiological and agronomic characters which contrast with the homogeneity of domesticated types (Harlan, 1992; Peña-Valdivia et al., 2012). Contrary to what might be expected, according to the CV the seed biomass heterogeneity of the cultivars was similar to that of wild samples, with the exception of the Chihuahua, which showed a CV about twice than the others.
Since wild seeds were multiplied several times, in field crop conditions, before using them in this study, it is possible that one effect of the repetitive cultivation of these seeds was a trend to homogenize its biomass. A similar effect was observed in some plants from an original wild population of Durango, which doubled their seminal biomass from one generation to another (Peña-Valdivia et al., 2012). Other outstanding changes in morphology and biomass of wild seeds due to repeated cultivation have already been documented (Garcia et al., 1997; Berrocal-Ibarra et al., 2002).
As expected, seeds of cultivars were the heaviest among all samples, as a domestication syndrome feature is the increased seed size (Harlan, 1992). Seed biomass was different (p≤0.001) among cultivars; on average seeds of Bayo Mecentral (311.6 mg; CV=15.21%) were 26% heavier than the Negro Tacaná (247.6 mg; CV=17.98%) and according to the CV homogeneity relatively similar in both samples. This was confirmed by the seed biomass frequency, which described a symmetrical data distribution with most of the results near the mean and the mode (47% of the Negro Tacaná and 29% of Bayo Mecentral) around the 250 and 325 mg (Figure 2B).
Several times lower wild seed biomass relative to the domesticated seeds had already been documented (Celis-Velázquez et al., 2010; Lépiz I. et al., 2010). But the frequency distribution of seed biomass showed an overlap between wild seeds, Chihuahua and Durango Típico, with the domesticated ones. Heavier seeds Biomass (between 150 and just over 250 mg) of the wild samples were similar to the lighter seeds of both cultivars (Figures 3A-B and 4A-B). These results were similar to those obtained by Peña-Valdivia et al. (1998) with other wild and domesticated samples.

Figure 3 Seeds biomass frequency distribution of domesticated common bean (Phaseolus vulgaris L.). (A) Bayo Mecentral and (B) Negro Tacaná. Núm: Number.

Figure 4 Biomass frequency distribution of progeny of common domesticated bean (Phaseolus vulgaris L.) (Black Tacaná) and wild (S13). (A) 3.3 (B) 1.11, (C) 51b, (D) 53b and (E) 118b.
Statistical differences were detected in the seminal biomass of the progeny (p≤0.05). Mean values ranged between 106.3 and 138.9 mg per seed; seeds from sample 53b had between 20 and 23% less biomass than those from samples 3.3, 11.1 and 51b (Figure 1). According to the CV, the heterogeneity of this characteristic in the progeny seeds of 51b (12.77%) and 53b (13.94%) samples was the lower and in 3.3 (19.94%), 11.1 (21.09%) and 118b (19.15%) was the higher. The samples from 51b and 53b selections were the most homogeneous among all evaluated, including wild and domesticated; it stands out that they were between 1 and 5% more homogeneous than their parental counterparts. These results indicate that the crossbreed seeds of domesticated and wild beans may present the exceptional feature of high biomass homogeneity.
The distribution of seed biomass frequency in the progeny showed differences among the tested samples (Figure 4). In the 3.3, 51b and 118b selections, seminal biomass showed a symmetrical distribution, with their mode around 130, 120 and 110 mg each. The seed biomass of sample 3.3 covered the widest range (35 to 70 mg) of the three samples and 33% of the seed biomass therein concentrated about 13 and 14 mg (Figure 4A) and 60% and 50% of the seeds from samples 51b and 118b were between 110 and 120 mg (Figures 4C and 4 E).
Increased biomass or size of the organs of the plant, particularly the seeds, is among the changes recognized as a result of the domestication process. Nevertheless, Peña-Valdivia et al. (1998) show that increasing seminal biomass, as part of domestication syndrome, does not represent a proportional increase in each of the plant structures, particularly seed. Those changes were related to the seedling emergence and vigor abilities (accumulated root biomass and leaflets and hypocotyl diameter) (Celis-Velázquez et al., 2008). Thus, homogeneous biomass or seed size is an agronomic quality and a consumer character (Celis-Velázquez et al., 2008).
Proportion of cotyledons, embryonic axis and seed coat in seeds
The proportion of cotyledons, embryonic axis and seed coat in seeds showed differences (p≤0.05) among wild, domesticated and crossbreeding variants and between groups (Table 1).
Table 1 Relative ratio (%) of seed structures in wild, domesticated common bean seeds (Phaseolus vulgaris L.) and crossbeeds of domesticated and wild Tacaná Black S13.

Values with different letters in a column show significant differences (p≤0.05).
Among the wild variants a gradient of the seminal structures content was observed; in it, S13 had the lowest cotyledon proportion (p≤0.05), and embryonic axis and seed coat proportion were both higher (twice and 46% more) than in the Chihuahua and Durango Típico (Table 1).
The proportion of cotyledons and seed coat showed no difference (p›0.05) among cultivars, but the embryonic axis presented significant and higher differences (p≤0.05) in Bayo Mecentral; the difference represented 13.6% more of this structure in seeds of this cultivar than in Negro Tacaná. Furthermore, the mean proportion of cotyledon in cultivars is significantly higher (22.4%) than in wild variants (Table 1).
The cotyledon proportion showed no differences (p›0.05) among the crossbreeding seeds and represent an average of 86.15% of its total biomass, but it significantly differed (p≤0.05) from both parents; it was 3.7% lower compared to the domesticated and 16% higher than the wild variants. These results indicated that the evaluated progeny selections tended to even the cotyledon ratio from the seeds of the domesticated parents (Table 1).
Germination is a coordinated response involving two-way interactions between the embryonic axis and cotyledons; therefore, the relationship of these two structures in common bean seed is physiologically relevant. According to Yan et al. (2014), tissues such as cotyledons in the seed have an important role in the embryonic growth due to their nutrient supply, protection of the embryonic axis and growth control, since they act as a mechanical barrier during germination and seedling development. The same authors indicated that a subset of cotyledon tissues is comprised of living cells even after seed maturation and are active in regulating germination; also transcriptome analysis has evident new regulatory functions of cotyledons during this process. Moreover, the embryonic axis releases signs, related to the degradation of seed reserve to the cotyledons. Advances in seed biology show that cotyledons detect environmental signals produce and releases signals regulating embryonic axis growth (Yan et al. (2014).
The appropriate content in the seed cotyledon can favor germination and seedling emergence because of the amount of energy reserves it represents; it has been noted that the embryonic axis is the most important seminal structure, as germination, emergence and plant establishment as an autotrophic organism depend on its metabolic activity (Peña Valdivia et al., 1998). These can be related to a higher proportion of embryonic axis and the ability of wild seeds to germinate and survive in the adverse environments where they remain. Thus, the same characteristic in the crossbreed seeds could favor germination success in restrictive agricultural environments.
The proportion of seed coat showed differences (p≤0.05) between and within the evaluated groups of variants. Seeds of wild S13 had the highest proportion of this structure and represented just over 30% than in Chihuahua and Durango Típico seeds (Table 1).
Seed coat content in domesticated beans on average was 7.79% of total seed. This proportion was lower (p≤0.05) to that corresponding to the wild group, as on average it represented a half. The difference between the S13 and cv. Negro Tacaná was one of the largest, as the seed coat of wild seeds was about three times more abundant than in the domesticated (Table 1). Peña-Valdivia et al., (1999) report similar results (9% and 14%, in each case) in samples of domesticated and wild beans.
The proportion of seed coat in the progeny formed two groups, based on their similarity; 3.3 and 51b formed a group with a mean content of 11.07% and it was lower (p≤0.05) than the group consisting of 53b and 118b, which had an average of 12.53%. Seed coat content in the progeny seeds had a decrease of 43 and 35.3%compared to the wild progenitor S13 and increased between 45.2 and 64.3% over their domesticated parent, the Negro Tacaná (Table 1). These results indicate that the progeny of wild and domesticated common beans tended to maintain an equivalent ratio to the average seed coat of the parents.
According to Peña-Valdivia et al. (2002) the largest seed coat proportion in wild common beans appears to be related to water input regulation to the seed during the imbibition and to the protection of their internal structures, as a result of adaptation to the natural environment where they live, for months or years, from their release from the parental plant until their germination. Therefore, the decrease in seed coat proportion in the crossbreed seeds, compared to the wild progenitor, is a desirable agronomic character.
The proportion of embryonic axis in the seeds showed differences (p≤0.05) among and within groups of variants. Wild variants formed two groups, according to their proportion of this seminal structure. The Chihuahua and Durango Típico formed a group with (1.53%) proportion about 30% less than S13 seeds (Table 1). These results showed that the significantly lower proportion of cotyledons in the S13 seeds was complemented with seed coat and embryonic axis.
The proportion of embryonic axis in the seeds did not differ among cultivars (1.33%), and contrasted with the largest in wild seeds; differences accounted for 12.2 to 38.2% less embryonic axis in the domesticated ones than in the wild seeds (Table 1). These results were similar to those of Peña-Valdivia et al. (1998) with wild samples from Durango and the Bayo Mecentral cultivar, multiplied in the same field and crop cycle; also, wild seeds and the cultivar had 2.18% and 1.6% of seed coat.
Statistical analysis showed that the proportion of embryonic axis in the seeds was not different (p›0.05) among the crossbreeding seeds (2.12% on average). The proportion of embryonic axis in these seeds was higher (p≤0.05) than in the domesticated parent, and the increment was 69%; in contrast, that ratio was lower (p≤0.05) compared to that of the wild progenitor although, on average accounted for only 2.3% (Table 1). Crossbreeding seeds in our study tended to maintain the same relative proportion of embryonic axis that wild showed.
Seeds dimensions
Width
The width of the wild seeds ranged from 4.02 mm, in S13, to 4.66 mm, in the Durango Típico, and showed significant differences (p≤0.05) among the variants. Although, on average seeds of Bayo Mecentral were only 2% wider than those of the Negro Tacaná, the differences was statistically significant and on average 65% wider than those of the wild variants; still, the difference between Negro Tacaná and S13 was lower, since they were 42% wider than the wild (Figure 5A).

Figure 5 Width and length (±standard error) of wild and domesticated common bean seeds (Phaseolus vulgaris L.) and progeny of domesticated Negro Tacaná and wild S13.
The seed width was somewhat a variable character, within and between wild and domesticated variants. In the first group the CV ranged from 6.47% (in S13) to 9.38% (in Durango Típico), in the domesticated was of less than 8% (7.67% in Bayo Mecentral and 5.63% in Negro Tacaná) (Figure 5A).
The width of the seeds in the crossbreeding selections ranged from 5.29 mm (in 53b) to 5.96 mm (in 11.1); significant differences (p≤0.05) were present only among some of them; and so, the 118b selection was not different (p›0.05) to 1.1 and 51b (Figure 5A). This seed characteristic was homogeneous among the crossbreed seeds, as shown by the CV less than 7.72% of most of the seeds, with the exception of 11.1 (CV 10%).
The width of the S13 seeds was 42% lower than that of the domesticated Negro Tacaná, and crossbreed seeds were on average 42% higher than their wild progenitor and 18% lower than that of the domesticated parent (Figure 5A). The results indicated that the width of the crossbreed seeds is an outstanding feature, as it is a stable character.
Length
There were statistical differences in seed length within and between groups of wild, domesticated and progeny (Figure 5B). In wild seeds, this characteristic varied between 6.48 mm (CV 9.35%), in S13, and 7.06 mm (CV 7.98%) in the Durango Típico. The seed length of the domesticated beans showed differences (p≤0.001) among them, those from the Negro Tacaná cultivar were 10% longer (CV 7.43%) than those from the Bayo Mecentral (CV 8.36%).
The length of the crossbreeding seeds also showed significant differences. Those of 53b were the smallest (7.67 mm; CV 10.42%). The other four had an average length of 8.67 mm (CV between 7.63 and 9.95%). The first were 15.5% longer than the wild parent and 25% shorter the domesticated; in contrast, the group with similar length, consisting of 3.3, 11.1, 51b, and 118b, showed 37.2% and 15% larger and shorter length than the wild and domesticated progenitors, respectively (Figure 5B). These results indicated that crossbreeding seeds tended to match the length of the domesticated parent.
Thickness
Significant differences in seed thickness were detected within groups and among variants. Among wild seeds samples S13 was the most homogeneous in this characteristic (CV 7.37%) and Durango Típico had the most heterogeneous (CV 13.07%) (Figure 6).

Figure 6 Seed thickness (±standard error) of wild and domesticated common bean (Phaseolus vulgaris L.) and crossbreeding seeds from domesticated Negro Tacaná and wild S13. Variants 1: Chihuahua, 2: Durango Típico, 3: S13, 4: Bayo Mecentral, 5: Negro Tacaná, 6: 3.3, 7: 11.1, 8: 51b, 9: 53b and 10: 118b.
Wild seed thickness was on average (2.6 mm) about a half of domesticated (4.9 mm). The proportions changed in the progeny, as its mean was 43% higher than the wild parent and 24.2% lower than the domesticated parent (Figure 6).
Germination and emergence
The percentage of germination in laboratory conditions showed no difference (p›0.05) among wild variants, domesticated or progeny, except for 51b (Figure 7A-C). Mean seed germination of wild (92%), cultivars (99%) and crossbreeding (93%) samples was an important character to assess the quality of the latter respect to the parents, because it was close to 100% in all cases. This characteristic was independent of differences in biomass and seed size (Figures 1 to 6).

Figure 7 Accumulated germination (25±1 °C and dark) of common bean seeds (Phaseolus vulgaris L.) (A) domesticated, (B) wild and (C) progeny from domesticated Negro Tacaná and wild S13; and progeny seedling emergence (25±3 °C) of (D) domesticated, (E) wild and (F) progeny from domesticated Negro Tacaná and wild S13. Variants: Bayo Mecentral ( ), Negro Tacaná (
), Negro Tacaná ( ), Chihuahua (
), Chihuahua ( ), Durango Típico (
), Durango Típico ( ), S13 (
), S13 ( ), 3.3 (
), 3.3 ( ), 11.1 (
), 11.1 ( ), 51b (
), 51b ( ), 53b (
), 53b ( ) and 118b (
) and 118b ( ).
).
The differences among and within groups of variants were detected throughout time until maximum germination was reached. Among the wild variants, germination time ranged between 84 h in the Durango Típico and 252 h in the Chihuahua. Among cultivars, values ranged from 72 h in Negro Tacaná to three times that in the Bayo Mecentral. Time for maximum germination among the progeny also widely varied, from 48 h in 53b to 648 h in 11.1. The time for maximum germination and the differences between variants did not show any direct relationship with the physical characteristics of the seeds. It should be noted that selection 51b was not considered in this analysis because 168 h were insufficient to assess their total germination (Figure 7A-C).
The emergence percentage in greenhouse showed no difference (p›0.05) among wild and domesticated variants or their progeny (Figure7 D-F). The mean emergence of wild seedlings (95%), cultivars (100%) and progeny (97%) is an important character to qualify the seeds quality, and specifically, in the case of the crossbreeding seeds, it should be noted that there were no differences regard its progenitors. So that this characteristic, as the germination, was independent of the seed biomass, structures and dimensions (Figures 1 to 6).
Differences among variants and within groups were also detected at the time seeds reached maximum emergence. Among the wild variants, time ranged between 15, 23 and 24 d in S13, Chihuahua and Durango Típico each. In the cultivars, the time for maximum seedling emergence in the Negro Tacaná seeds took (14 d) about half of that in the Bayo Mecentral (25 d). Among progeny selections, the time for maximum emergency also ranged from 14 d in 53b to 24 d in 3.3 and 11.1 (Figure 7D-E).
Much like germination, time for maximum emergency showed no direct relationship with the physical characteristics of the seeds in any of the variants. In addition, the maximum time for the emergence of wild variants and crossbreed seeds did not show a direct relationship with the time for maximum germination; as variants that germinated in less time did not emerge before or vice-versa (Figure 7). Cultivars were the exceptions because the Bayo Mecentral took 70% longer than the Negro Tacaná for its maximum germination and 79% more for their maximum emergence. Under this study conditions, the total emergence of the Negro Tacaná cultivar, wild S13 and three of the five crossbreeding seeds were of 14 and 15 d. This is an outstanding aspect, because with the improved modifications, seeds agronomic characters are expected, such as maximum and synchronous seed emergence; this reduces seed loss and promotes the homogeneous crop development (Celis-Velázquez et al., 2010).
Conclusions
The physical characteristics, such as seed biomass, width, thickness and length, of the wild and domesticated common bean, and their progeny, are typical of each group; and physiological characteristics, such as germination and emergence capacity are similar among groups in laboratory and greenhouse conditions.
Few characteristics of the crossbreeding seeds are superior regard their parental cultivars, including the proportion of cotyledons and embryonic axis in each seed
Literatura Citada
Berrocal-Ibarra, S., J. Ortíz C., and C. B. Peña-Valdivia. 2002. Yield components, harvest index and leaf area efficiency of a sample of wild population and a domesticated variant of the common bean Phaseolus vulgaris. S. Afr. J. Bot. 68: 205-211. [ Links ]
Bewley, J. D. 1997. Seed germination and dormancy. Plant Cell 9 (7) 1055-1066. [ Links ]
CEDRSSA (Centro de Estudios para el Desarrollo Rural Sustentable y la Soberanía Alimentaria). 2014. Evolución de los precios del maíz, frijol y sorgo. 24 p. [ Links ]
Celis-Velázquez, R., C.B.Peña-Valdivia, M. Luna C., J.R. Aguirre R., A. Carballo, y C. Trejo. 2008. Variabilidad morfológica seminal y del vigor inicial de germoplasma mejorado de frijol. Agron. Mesoamer. 19: 179-193. [ Links ]
Celis-Velázquez, R., C.B. Peña-Valdivia., M. Luna-Cabazos, y J. R. Aguirre R. 2010. Caracterización morfológica de las semillas y consumo de reservas durante la emergencia de plántulas de frijol (Phaseolus vulgaris L.) silvestre y domesticado. Rev. Fac. Agron. (LUZ). 27: 61-87. [ Links ]
Delgado, A., y S. Gama L. 2015. Diversidad y distribución de los frijoles silvestres en México. Revista Digital Universitaria 16: 1-11. http://www.revista.unam.mx/vol.16/num2/art10 / (Consulta: Junio 2016). [ Links ]
Delouche, J. C. 2002. Germinación, deterioro y vigor de semillas. Seed News 6(6). http://www.seednews.inf.br/espanhol/seed66/artigocapa66_esp.shtml (Consulta: Septiembre 2015). [ Links ]
Desai, B. B. 2004. Seed Handbook, Biology, Production, Processing, and Storage. Second edition. Marcel Dekker, INC. USA. pp. 787. [ Links ]
Fernández F., P. Gepts, y M. López. 1982. Etapas de Desarrollo de la Planta de Frijol Común; Guía de Estudio para ser Usada como Complemento de la Unidad Audiotutorial sobre el Mismo Tema. CIAT (Centro Internacional de Agricultura Tropical) Cali, Colombia. 26 p. [ Links ]
García H. et al. 1997. Morphological and agronomic traits of a wild population and an improved cultivar of common bean (Phaseolus vulgaris L.). Ann. Bot. 79: 207-213. [ Links ]
García-Nava, R. et al. 2014. Seed yield and its components of wild and cultivated Phaseolus vulgaris L. Annu. Rep. Bean Improv. Coop. 57: 303-304. [ Links ]
González, T. G. et al. 2008. Rendimiento y calidad de semilla de frijol en dos épocas de siembra en la región del Bajío. Agric. Téc. Méx. 34: 421-430. [ Links ]
Harlan, J. R. 1992. Crops and Man. 2nd ed. Am. Soc. Agronomy, Madison, WI. 283 p. [ Links ]
ISTA (International Seed Testing Association). 2009. International Rules for Seed Testing. Seed Sciences & Techonology. 27 p. [ Links ]
Lépiz I. et al. 2005. Latencia y escarificación química en semillas de frijol silvestre (Phaseolus vulgaris L., Fabaceae). Scientia-CUCBA 7: 105-113. [ Links ]
Lépiz I. et al. 2010. Características morfológicas de formas cultivadas, silvestres e intermedias de frijol común de hábito trepador. Rev. Fitotec. Mex. 33: 21-28. [ Links ]
López H. et al. 2001. Differences in seed germination of wild and domesticated common bean (Phaseolus vulgaris L.) in response to storage. S. Afr. J. Bot . 67: 620-628. [ Links ]
OECD. 2016. Common bean (Phaseolus vulgaris), in Safety Assessment of Transgenic Organisms in the Environment. Volume 6: OECD Consensus Documents, OECD Publishing. Paris. http://dx.doi.org/10.1787/9789264253421-7-en (Consulta: Junio 2016). [ Links ]
Peña-Valdivia, C. B. et al. 1998. Componentes del rendimiento de semilla de una población silvestre y un cultivar de frijol (Phaseolus vulgaris L.). Quad. Bot. Amb. Appl. 6: 181-187. [ Links ]
Peña-Valdivia, C. B., E. del R. García H, I. Bernal-Lugo, y J. R. Aguirre. 1999. Seed quality of wild and domesticated common bean (Phaseolus vulgaris L.) after storage. Interciencia 24: 8-13. [ Links ]
Peña-Valdivia, C. B., R. García N., J. R. Aguirre R., C. Trejo L. 2002. The effects of high temperature on dormancy and hypocotyl-root growth of wild common bean (Phaseolus vulgaris L.). Seed Sci. Technol. 30: 231-248. [ Links ]
Peña-Valdivia, C. B ., R. García N., A. B. Galicia J., y A. B.Sánchez-Urdaneta. 2005. Germinación, latencia y crecimiento de plántulas de frijol (Phaseolus vulgaris L.). SABER. Supl. 17: 258-260. [ Links ]
Peña-Valdivia, C. B ., A. B. Sánchez-Urdaneta., J. Meza R., J. Juárez M., R.García-Nava , y R. Celis V. 2010. Anatomical root variations in response to water deficit: wild and domesticated common bean (Phaseolus vulgaris L.). Biol. Res. 43: 417-427. [ Links ]
Peña-Valdivia, C. B ., J. R. García N., J. R. Aguirre R., Ma. C. Ybarra-Moncada, and M. López H. 2011. Variation in physical and chemical characteristics of common bean (Phaseolus vulgaris L.) grain along a domestication gradient. Chem. Biod. 8: 2211-2225. [ Links ]
Peña-Valdivia, C.B.,J.R.Aguirre R , y V.B.Arroyo-Peña . 2012. El Frijol Silvestre. Síndrome de Domesticación. Editorial COLPOS. México. 206 p. [ Links ]
Peña-Valdivia, C.B.,C. Trejo ,R. Celis-Velázquez, A.López O.2013. Reacción del frijol silvestre (Phaseolus vulgaris L.) a la profundidad de siembra. Rev. Mex. Cienc. Agríc. 4: 89-102. [ Links ]
Pérez-Herrera, P. y J. A. Acosta-Gallegos. 2002. Permeabilidad de la testa y la porción micrópilo-hilio en semilla de frijol silvestre y cultivado. Rev. Fitotec. Mex . 25: 57-63. [ Links ]
Porch, T. G., J. S. Beaver, D. G. Debouck, S. A. Jackson, J. D. Kelly, and H. Dempewolf. 2013. Use of wild relatives and closely related species to adapt common bean to climate change. Agronomy 3: 433-461. [ Links ]
Secretaría de Economía. 2012. Análisis de la Cadena de Valor del Frijol. Dirección General de Industrias Básicas. [ Links ]
Shiga, T. M, B. R. Cordenunsi, and F. M. Lajolo. 2011. The effect of storage on the solubilization pattern of bean hull non-starch polysaccharides. Carbohyd. Polym. 83: 362-367. [ Links ]
Toro, O., J. Thome, and D.G. Debouck. 1990. Wild Bean (Phaseolus vulgaris L.): Description and Distribution. IBPGR and CIAT, Cali, Colombia. 84 p. [ Links ]
Velasco-González, O., E.SanMartin-Martínez, M. Aguilar-Méndez., A. Pajarito-Ravelero, y R. Mora-Escobedo. 2013. Propiedades físicas y químicas del grano de diferentes variedades de frijol (Phaseolus vulgaris L.). Bioagro 25: 161-166. [ Links ]
Yan D., L. Duermeyer, C. Leoveanu, and E. Nambara. 2014. The functions of the endosperm during seed germination. Plant Cell Physiol. 55: 1521-1533. [ Links ]
Received: May 2016; Accepted: June 2016











 texto en
texto en 


