Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.50 no.8 Texcoco Nov./Dez. 2016
Crop science
Maize (Zea mays L.) seed coating with chitosan and sodium alginate and its effect on root development
1 Edafología. Campus Montecillo. Colegio de Postgraduados. 56230. Montecillo, Estado de México, México.
2 Genética. Campus Montecillo. Colegio de Postgraduados. 56230. Montecillo, Estado de México, México. (hidalgo@colpos.mx).
Time between seed germination and an autotrophic plant is a critical stage. During this period the seedling is susceptible to competition with weeds and adverse soil and climate change conditions due to its reduced root system and scarce leaf area, as in the case of maize (Zea mays L.). The use of biochemical agents as coating on seeds that promote a greater root development in early stages favors a good initial development of the seedling. This study aimed to evaluate the effect of coatings with chitosan (30 g L-1), sodium alginate (50 g L-1), salicylic acid (1x10-3 M) and fertilizers equivalent to a dose of N-P2O5-K2O: 26.7-7.5-13.3 kg ha-1 in the emergence, root development, and absorption of water and nutrients in two maize genetic lines with contrasting root development. The hypothesis was that the coating of maizeseeds with one or more of these materials favors the water and nutritional status of the seedling, which stimulates an efficient emergence, increases the root biomass and the absorption of water and nutrients. The experimental design was completely randomized with a 2x6 factorial arrangement (2 maize lines, 6 coatings) with two controls (uncoated seed). The experimental units were pots and the comparison of means was performed with the LSD test (p≤0.05). Seeds were covered by the ionic gelation technique sowed in tezontle and irrigated with the Steiner nutrient solution without macronutrients in the greenhouse. The biomass and nutritional concentration of the seedlings were evaluated 30 and 50 d after sowing (DAS). The emergence of plants decreased as a result of the coatings: chitosan (40 %) and sodium alginate (14 %). Fertilizers and salicylic acid reduced biomass and root length (30 DAS). There was no consistent effect of coatings on nutrient concentration in maize plants.
Key words: Seeds coating; ionic gelling; root development; chitosan; sodium alginate
El tiempo entre la germinación de la semilla y una planta autótrofa es una etapa crítica. Durante este periodo la plántula es susceptible a la competencia con malezas y condiciones edafoclimáticas adversas debido a su reducido sistema radicular y escasa área foliar, como el caso del maíz (Zea mays L.). El uso de agentes bioquímicos como recubrimiento en semillas que promuevan un mayor desarrollo radical en etapas tempranas favorece un buen desarrollo inicial de plántula. Este estudio tuvo como objetivo evaluar el efecto de recubrimientos con quitosano (30 g L-1), alginato de sodio (50 g L-1), ácido salicílico (1x10-3 M) y fertilizantes equivalente a una dosis de N-P2O5-K2O: 26.7-7.5-13.3 kg ha-1, en la emergencia, desarrollo radical, y absorción de agua y nutrimentos, en dos líneas genéticas de maíz con desarrollo radical contrastante. La hipótesis fue que el recubrimiento de semillas con uno o más de estos materiales favorece el estatus hídrico y nutrimental de la plántula, que estimula una eficiente emergencia e incrementa la biomasa radical, la absorción de agua y nutrimentos en maíz. El diseño experimental fue completamente al azar con un arreglo factorial 2x6 (2 líneas genéticas de maíz, 6 recubrimientos) con dos testigos (semilla sin recubrimiento). Las unidades experimentales fueron macetas y la comparación de medias se realizó con la prueba LSD (p≤0.05). Las semillas fueron recubiertas mediante la técnica de gelificación iónica, sembradas en tezontle y regadas con solución nutritiva Steiner sin macronutrimentos en invernadero. La biomasa y concentración nutrimental de las plántulas se evaluaron 30 y 50 d después de la siembra (DDS). La emergencia de las plantas disminuyó por los recubrimientos: quitosano (40 %) y alginato de sodio (14 %). Los fertilizantes y ácido salicílico redujeron la biomasa y longitud radical (30 DDS). No hubo un efecto consistente de los recubrimientos sobre la concentración nutrimental en las plantas de maíz.
Palabras clave: recubrimiento de semillas; gelificación iónica; desarrollo radical; quitosano; alginato de sodio
Introduction
México is the largest maize (Zea mays L.) market in the world consuming 11 % of world production. From 2014 to 2015 maize imports into Mexico were 10 million Mg, and currently ranks second as an importing country in the world. Total maize production amounted to 37.1 million Mg, around 33 % of the total production value of the agricultural sector (SIAP-SAGARPA, 2015)2.
In maize cultivation weeds compete for water, soil nutrients and sunlight mainly in the early stages of development, when maize seedlings are more sensitive to competition due to their reduced root system and scarce leaf area. The early vigor of maize seedlings leads to the faster establishment of the crop and better possibilities in the face of stress caused by competition with weeds (Costar et al., 2013).
The root system of maize is divided into embryonic roots (primary root and seminal roots) and postembrionary roots (nodal roots and crown roots). In the first two weeks, the main root and the seminal roots make up most of the root system.
The nodal and crown roots that form the backbone of the maize root system appear after the embryonic roots. During the first phase of the root development, the lateral roots carry out most of the water and nutrient transport to the aerial parts of the maize plant because their branches have the ability to do so. The transition between the first and second stages of root development of the maize plant (early - late stage) begins 3 to 4 weeks after germination, along with the formation of crown nodal roots (Hochholdinger et al., 2004).
Soil nutrient uptake by the plant depends on: an adequate soil pore space (pores that can be penetrated by the roots) to store the water that forms the soil solution, sufficient amount of nutrient reserves, a healthy root system in the plant and the efficiency of nutrient uptake or proportion of nutrients absorbed by the plant from the total applied. It depends on factors such as the volume of the root system, the physical and chemical form of the applied fertilizer, and the reactions in the soil (Etchevers, 2008).
The germination process of the seed has three phases: hydration, sprouting and growth, which depend on the content of hydratable compounds and the permeability of the seed cover to water and oxygen. The growth phase comprises the mobilization of seed reserves and seedling development (Koornneef et al., 2002). The growth and development of the plant after the germination process is determined by the existing reserves in the seed and secondly by the environmental conditions.
Revilla et al. (1999) mention that three weeks after emergence, endosperm reserves are consumed and the seedling depends on its ability to generate assimilates, produce leaf area and continue its growth. The success of planting is largely determined by the physiological and biochemical characteristics of the seed, its reaction to the environment and the rate with which it uses its reserves to initiate and sustain seedling growth in the early stages of development, before being an autotrophic organism (Soltani et al., 2006).
Covered seeds established under different environmental conditions reach percentages and emergency rate close to 100 %, an adequate initial seedling development and high final yields (Tillmann and Miranda, 2006). The seed coating is based on the encapsulation process to make pellets, and aims to improve seedling growth and development characteristics and protect them from harmful external biotic aggressions (Lizárraga et al., 2011). The animal and plant polysaccharides frequently used in microencapsulation are alginate, arabic gum and chitosan (Lupo et al., 2012).
Chitosan is a biodegradable hydrophilic polysaccharide derived from the deacetylation of chitin which is used in the coating of seeds to preserve them during storage. Chitosan stimulates the growth of seedlings and has a bactericidal (Kong et al., 2010), fungicide and antiviral effect (Niquette et al., 2004; Lárez, 2006; Lárez, 2008). Alginate is a natural hydrophilic polysaccharide obtained from brown algae and is used in the microencapsulation of seeds for being biocompatible, non-toxic and degradable (Reddy and Reddy, 2010; Lupo et al., 2012). Spray drying (Parra, 2010) and ionic gelling are coating techniques of seeds practically and economically suited (Gouin, 2004; Lakkis, 2007; Guevara and Jiménez, 2008). Ionic gelling consists of the formation of three-dimensional networks by non-completely stable bonds (physical hydrogels) and weaker than covalent bonds (chemical hydrogels) (Lupo et al., 2012).
The objective of this study was to evaluate the effect of chitosan, sodium alginate, salicylic acid and nutrients on the emergence, radical development, and absorption of water and nutrients in two lines of maize with contrasting root development. The hypothesis was that the seed coating with one or more of the materials mentioned favors a hydric and nutritional status of the seedling, which stimulates an efficient emergence, an increase in the production of root biomass and a greater absorption of water and nutrients.
Materials and Methods
This study was conducted in greenhouses of the Colegio de Postgraduados, Montecillo, Mexico. Before evaluating corn seed coatings, we performed the following tests: 1) kinetics of the consumption of seed reserves to determine the time in which maize seedlings are autotrophic and no longer depend on seed reserves in a native maize race; 2) selection of two maize lines with contrasting root development; 3) comparison of techniques to coat corn seeds.
The time required by the plant to become independent of the seed reserves was determined with seeds of native white maize. All the seeds used in this experiment were obtained from the Seed Program of the Colegio de Postgraduados and sown in conic pots (25 cm in diameter, 25 cm height) filled with red tezontle (<5 mm in diameter) in greenhouse. The pots were watered with 0.5 L d-1 well water. The nutrient concentration (mg L-1) of the irrigation water was analyzed: NO30.003; NH4 + 0.001; P 0.025; K 9.334; Ca 29.210; Mg 9.334; Na 39.663; Fe 0.023; Cu 0.028; Zn 0.164; Mn 0.027.
Corn seeds were weighed and sown on January 27, 2014. On days 7, 15, 22, 29 and 36 after sowing (DAS) the dry matter (DM) of the remaining seed, above-ground and root were evaluated in six seedlings. The seedlings were manually extracted from the pot to obtain all the root biomass, washed with running water and dried 72 h in an oven at 70 °C to measure the dry matter and were weighed up on an analytical balance with an approximation of 1 x 10-4 g. Data were processed with Microsoft Office 2010 Excel and plotted with CurveExpert Professional version 2.0.4.
For selecting two maize lines with contrasting production of root biomass, eight lines or varieties (five replicates in each one) were used: CMS939083, C13x11, CS14x15, CPV-20, HS-2, HT-PRECOZ, PROMESA and VS22 . The seeds were weighed before planting and those weighing 0.21 to 0.3 g were selected to obtain homogeneous stock quantities and seeded in pots filled with fine red tezontle in greenhouse on March 25, 2014; and were irrigated with 0.3 L d-1 of water.
Seedlings were harvested 20 DAS and dried in an oven at 70 °C for 72 h. Dry weight per plant was measured on an analytical balance with an approximation of 1 x 10-4 g. Data were processed using Excel of Microsoft Office 2010.
The comparison of coating techniques comprised the solvent selection, concentration and coating methodology for each polymer to produce a solid, uniform and homogeneous layer on the seed surface. The evaluated materials were: corn seeds, low molecular weight chitosan (50-190 kDa) with a degree of deacetylation between 75 and 85 %, sodium alginate (Sigma Aldrich), salicylic acid, concentrated glacial acetic acid, calcium chloride and sodium hydroxide. The suspensions evaluated had concentrations of 1, 3 and 5 % from each polymer and were stirred for 60 min at medium speed on a magnetic stirrer.
From the proposed fertilization dose for field corn cultivation with a cycle of 180 d and a population density of 60 000 plants ha-1 that was 120-40-60 kg ha-1 N-P2O5-K2O, we calculated the dose 26.77.5-13.3 kg ha-1 for maize seedlings for 15 d. The nutrient amounts for each maize seedling during this period were: 0.44 g N, 0.05 g P and 0.18 g K per plant. The fertilizers used to cover the nutritional requirements of maize plants were: ammonium nitrate, potassium sulfate, monohydrate calcium phosphate, potassium nitrate and analytical grade monobasic potassium phosphate.
The suspensions were prepared by first solubilizing the polymers and then the fertilizers to avoid precipitates. Both were ground in a mortar to facilitate their integration. In the final suspensions, we added salicylic acid because it favors plant growth for being involved in physiological processes: thermogenesis, resistance to pathogens, induction of flowering, root growth and nutrient absorption (Larqué and Martín, 2007).
Chitosan is a polycation and forms complexes with molecules of opposite charge to obtain colloidal coatings (Rodríguez et al., 2010). The technique for gelling chitosan was proposed by Trasviña and Louvier (2010)4, which was modified to determine the appropriate concentration of NaOH because the alkalinity of this reagent can damage the seed surface. Maize seeds were immersed in the chitosan suspension, then in 0.5 and 1N NaOH solutions, for 5, 60 and 180 min for evaluation. Sodium alginate gelling was performed with 0.05 M calcium chloride by using the external gelation technique described by Lupo et al. (2012). The coated seeds were dried in the shade outdoors for 24 h.
We evaluated the effect of the proposed coatings with the emergence, biomass produced and nutrient concentration in the maize plants obtained. The maize seeds of the selected lines were weighed on an analytical balance and those weighing 0.21 to 0.30 g were selected. The interaction between the two maize lines and the six seed coat suspensions gave rise to the 12 treatments evaluated (Table 1), to which two control treatments (uncoated seeds) were attached.
Table 1 Final suspensions for the evaluation of coatings.
| Variedad | Materia fresca | Materia seca | ||||
| Parte aérea | Raíz | Parte aérea | Raíz | |||
| g planta-1 | ||||||
| CMS939083 | 1.518 | 1.476 | 0.157 | 0.098 | ||
| CS13x11 | 1.510 | 1.519 | 0.168 | 0.103 | ||
| CS14x15 | 1.77 | 2.027 | 0.183 | 0.122 | ||
| CPV-20 | 2.056 | 2.734 | 0.208 | 0.144 | ||
| HS-2 | 1.975 | 2.839 | 0.244 | 0.169 | ||
| HT-PRECOZ | 2.646 | 3.327 | 0.279 | 0.169 | ||
| PROMESA | 1.872 | 2.568 | 0.207 | 0.174 | ||
| VS22 | 1.806 | 2.192 | 0.166 | 0.140 | ||
Seeds of the two maize lines were coated with the final suspensions and dried in the shade outdoors for 24 h (Figure 1). For seed coating we used chitosan (30 g L-1), sodium alginate (50 g L-1), salicylic acid (1 x 10-3 M) and fertilizers (N-P2 O5K2O: 26.7-7.5-13.3) as promoters of root development.

Figure 1 Coated seeds. A) Seeds of variety HS-2 uncoated, B) Seeds of variety HS-2 coated with chitosan, C) Seeds of variety HS-2 coated with sodium alginate, D) Seeds of line CMS939083 uncoated, E) Seeds of line CMS939083 coated with chitosan and, F) Seeds of line CMS939083 coated with sodium alginate.
The coated seeds were sown in pots filled with fine red tezontle (<5 mm in diameter) in a greenhouse and watered daily with 0.3 L of modified Steiner solution (without macronutrients). Water for irrigation was analyzed to adjust the macro and micronutrient concentrations of the nutrient solution. The nutrient concentration of the nutrient solution according to Steiner (1961) without macronutrients was: 2 ppm Fe, 0.7 ppm Mn, 0.5 ppm B, 0.04 ppm Mo, 0.16 ppm Zn and 0.02 ppm Cu. This solution was prepared with: 0.50 mg L-1, H3 BO3, 1.98 mg L-1 chelate-Fe 13.2 %, 0.67 mg L-1 MnSO4 * H2O and 0.04 mg L-1 Na2 MoO4 * 2H2O.
Sowing was done on December 21, 2014 and two harvests were made 30 and 50 DAS, in which the entire plant was extracted and the aerial part of the root was removed. The variables measured in plants 30 DAS were above-ground biomass and root, and the root length; in plants 50 DAS we measured only the biomass of the above-ground and root biomass. The weight of the biomass was measured with an approximation of 1 ×10-4 g. The root length of the seedlings 30 DAS was evaluated with the modified Newman method (Böhm, 1979). In both crops chemical analyzes of the plant tissue were carried out using the methodology of the Walinga et al. (1995) manual , and N, P and K totals of composite samples of the aerial part and root were determined.
The experimental design was completely randomized with a 2x6 factorial arrangement (2 maize lines and 6 treatments) and two control treatments (seeds without coating) for the comparison test of means. The experimental units were pots with fine red tezontle (<5 mm in diameter). The treatments had 10 replicates, with a total of 140 experimental units. In the first harvest (30 DAS) we collected the above-ground and root parts of five plants per treatment. In the second crop the plants per treatment were variable, where treatment 3 was completely lost. Results were subjected to a normality test (Shapiro-Wilks) and a homoscedasticity test (Bartlett). The ANOVA of data were performed using the GLM procedure and the comparison of means with the LSD test (p ≤ 0.05). All results were processed with SAS 9.0 and plotted with Microsoft Office 2010 Excel.
Results and Discussion
After thedevelopment of the roots, leaves and stem, the seedlings began to photosynthesize and produce their own food or obtain it from the soil through their root system, which made the plant independent of the seed reserves. During this stage, the DM of the seed decreased due to the consumption of the reserves. With an average between 87 and 92 %, the consumption of seed reserves stabilized between 15 and 22 DAS (Figure 2). These results showed that the maize seedlings were independent of the seed reserves at approximately 20 DAS.
The evaluation of maize lines was done 20 DAS, when the plant was already independent of seed reserves. The maize varieties with higher fresh and dry root values were: HT-Precoz, PROMESA, HS-2 and CPV-20, compared to lines CMS939083, CS13x11 and CS14x15 (Table 2). The variety HS-2 and line CMS939083 were chosen to evaluate the coatings in seeds because they presented contrasting root development.
Table 2 Biomass produced by each race of maize plants in greenhouse.
| Variedad | Materia fresca | Materia seca | ||
| Parte aérea | Raíz | Parte aérea | Raíz | |
| g planta-1 | ||||
| CMS939083 | 1.518 | 1.476 | 0.157 | 0.098 |
| CS13x11 | 1.510 | 1.519 | 0.168 | 0.103 |
| CS14x15 | 1.77 | 2.027 | 0.183 | 0.122 |
| CPV-20 | 2.056 | 2.734 | 0.208 | 0.144 |
| HS-2 | 1.975 | 2.839 | 0.244 | 0.169 |
| HT-PRECOZ | 2.646 | 3.327 | 0.279 | 0.169 |
| PROMESA | 1.872 | 2.568 | 0.207 | 0.174 |
| VS22 | 1.806 | 2.192 | 0.166 | 0.140 |
Manual spray coating and ionic gelation techniques are common in seed treatment because they are low cost and methodogically easy to use. Two percent acetic acid was used to solubilize chitosan, and distilled water for sodium alginate. The concentrations leading to an adequate adhesion to the seed without interference in the emergence were 30 g L-1 of chitosan and 50 g L-1 of sodium alginate. Ammonium nitrate, potassium nitrate and monobasic potassium phosphate were the fertilizers chosen for the seed coating tests, since they did not generate precipitates in the suspension. The fertilizer concentrations were: 57 g ammonium nitrate (NH4NO3), 15.5 g potassium nitrate (KNO3) and 11 g monobasic potassium phosphate (KH2PO4) per liter of suspension. Final suspensions were prepared by adding 10 mL of salicylic acid 1x10-3 M per 100 mL of suspension before the fertilizers addition.
The retention of any product with the techniques of seed treatment depends on the adhesion of the products applied, the compatibility between the different formulations used and the characteristics of the seed cover (Avelar et al., 2012). The manual spray technique did not achieve a uniform coating on both sides of the seed. Ionic gelling was chosen for coating corn seeds since it caused no problems of adhesion or homogeneity on the seed surface. Chitosan was gelled in both 0.5 and 1N NaOH solutions with no notable physical differences in the coating. However, the seed surface was affected by 1N NaOH, whereby the 0.5N NaOH solution was chosen. The minimum time to obtain stable and homogeneous coatings of chitosan was 5 min.
Emergence
Bhaskara et al. (1999) reported that chitosan has positive effects on plant growth (roots, shoots and leaves) and seed germination. However, in our study, chitosan reduced the emergence of corn seeds, which was accentuated by the addition of fertilizers and salicylic acid. These results agree with those by Arias et al. (1998), who coated maize seeds with chitosan combined with Captan to control deterioration induced by Pythium spp. and observed that maize plants reduced their emergence significantly.
Coatings showed having an adverse effect on the emergence of all seeds, values of 13 to 67% lower than the control for chitosan, and 7 to 40% lower than the control in sodium alginate. However, treatments with sodium alginate only decreased by an average of 10% the emergence of the seeds of the variety HS-2, compared to the line CMS939083 whose emergence was reduced by 17% on average, compared to the control.
The negative effect of chitosan on the emergence of seeds can be explained by the high concentrations of polymer. Guan et al. (2009) observed an increase in the emergence of maize seeds coated with chitosan when subjected to temperature stress conditions. Lárez et al. (2012) showed that chitosan with high molecular weight stimulates a greater potential of emergence in zucchini seeds (Cucurbita pepo). This is repeated in American cotton (Gossypium hirsutum), maize (Zea mays L.) (Tingda et al., 1994), wheat (Triticum aestivum) (Bhaskara et al., 1999 and Reddy et al., 1999), cucumber (Cucumis sativus), pepper (Capsicum annuum), squash (Cucurbita sp) (Chandrkrachang, 2002) and artichoke (Cynara scolymus L.) (Ziani et al., 2010). The concentrations of chitosan evaluated in these studies range from 0.5 to 2% of the polymer (p/v), and the seeds were only immersed in the suspensions for a certain time and filtered, leaving a thin coating on the seed. These low coating conditions allowed the seeds to emerge without any physical barrier, but in our study it was the opposite because a greater amount of coating was generated on the seed, which was a quality required for the study. A greater coating on the seeds could reduce the rate of seed imbibition due to the polyionic properties of chitosan, which allow it to form waterinsoluble complexes (Argin et al., 2009), causing the seeds to take longer to emerge or not germinate.
Sodium alginate presents high permeability, does not repel water and swells with prolonged exposure to water (McHugh, 1987), allowing proper imbibition of the seed, which favors the germination process and generates emergence percentages similar to the control (uncoated seed). However, the combination of sodium alginate with fertilizers decreased germination in both lines evaluated, with a greater effect in the line CMS939083. These results are similar to those by Sarrocco et al. (2004), who coated wheat seeds with sodium alginate and emergence percentages were similar to those of untreated seeds.
Root development
In maize plants 30 DAS there was an effect (p≤0.05) of the fertilization dose on most response variables (Table 3). The 50 DAS maize plants showed significant effects (p≤0.05) only on the fresh root matter, therefore the information from ANDEVA was omitted.
Table 3 Statistical difference of factors and interactions in the biomass produced and root length of maize plants 30 days after sowing † .
| Fuente de variación | MFPA | MFR | MSPA | MSR | LR |
| Variedad (V) | ns | ** | ns | ** | * |
| Polímero (P) | ns | ns | ns | ns | ns |
| Dosis de fertilización (F) | ** | ** | ** | * | ** |
| Interacción V*P | ns | ns | ns | ns | ns |
| Interacción V*F | ns | ns | ns | ns | ns |
| Interacción P*F | ns | ns | ns | ns | ns |
| Interacción V*P*F | ns | ** | ns | ns | * |
ns: not significant; * p≤0.05; ** p≤0.01; †MFPA: fresh matter of the aerial part; MFR: fresh root matter; MSPA: shoot dry matter; MSR: root dry matter; LR: root length.
Brown and Scott (1984) mentioned that the soil or substrate where the seed is placed, as well as the climate and the own crop influence the physical qualities of the roots developed in the plants. The variety HS-2 evaluated 30 DAS showed an initial seed vigor greater than the line CMS939083, because it produced greater root biomass. However, the root length was higher in the line CMS939083, which can be explained by the use of seed reserves for root elongation instead of dry matter production (Figure 3).
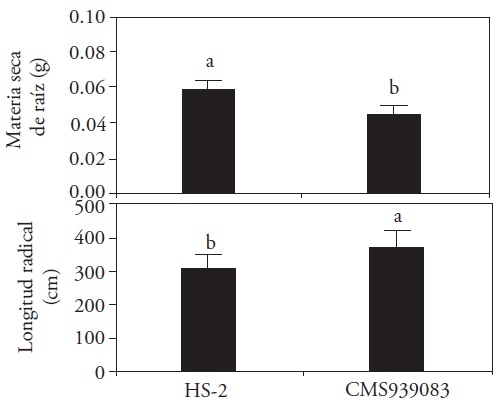
Figure 3 Effect of race on the root development of maize plants 30 days after sowing (LSD, p≤0.05, root dry matter, DMS=0.0057 and root length, DMS=50.756).
The variables measured in plants 30 DAS were susceptible to the fertilization dose, evidencing a marked negative effect of fertilizers and salicylic acid (Table 4). These results are similar to those by Gawade and Somawanshi (1979), who show that the application of fertilizers in insoluble forms does not affect the emergence, so they can be used in seeds for initial stages of cultivation. However, Scott et al. (1985) and Scott (1975) state that insoluble fertilizers are ineffective in promoting initial plant growth.
Table 4 Comparison of biomass means and root length in maize plants 30 days after sowing depending on the fertilization dose.
| Materia fresca | Materia seca | Longitud radical | |||||
| Tratamiento† | P. aérea | Raíz | P. aérea | Raíz | cm | ||
| g | |||||||
| Testigo | 1.772 a | 1.051 a | 0.155 a | 0.057 a | 370.4 a | ||
| D1 | 1.571 ab | 0.833 b | 0.132 b | 0.047 b | 359.9 a | ||
| D2 | 1.380 b | 0.755 b | 0.122 b | 0.049 b | 269.2 b | ||
| DMS¶ | 0.228 | 0.160 | 0.019 | 0.007 | 63.3 | ||
†Different letters indicate significant dif ferences (LSD, p≤0.05). Check: no fertilizer, D1=2.2 g N, 0.25 g P and 0.9 g K; D2=4.4 g N, 0.5 g P and 1.8 g K. ¶DMS = significant minimum difference.
The effect of fertilizers will depend on the type and amount of fertilizer added, crop species, and the texture and moisture of the substrate. The highest amount of fertilizer produced the lowest values in aerial and root biomass, and root length in plants with 30 days of development. However, 50 DAS plants did not show significant differences between treatments due to a likely decrease in the effect of fertilizers.
These results can be explained by what has been reported by Melaj and Daraio (2012), who found that chitosan releases fertilizer in short times since its swelling capacity is high, the previous phase for the release of fertilizer. Therefore, fertilizers probably generated saline conditions that affected the growth and development of the plant.
Nutritional concentration
When the needs of a crop (nutritional and others) are satisfied the concentration of nutrients throughout the plant reach levels that vary with age and the type of plant (Etchevers, 2008). This explains the differences in nutrient concentration between variety or lines and days of development of maize plants (Table 5), because in plants 30 DAS the concentration of N in leaf and P in the root of the line CMS939083 were higher than the variety HS -2, and in 50 DAS plants the leaf potassium concentration in the variety HS-2 was higher than in the line CMS939083 race (p≤0.05).
Table 5 Comparison of nutritional concentration means in the biomass of maize plants depending on the genetic line.
| Tratamiento | 30 DDS | 50 DDS | ||
| N-hoja | P-raíz | K-hoja | ||
| % | ||||
| HS-2 | 2.64 b | 0.16 b | 5.29 a | |
| CMS939083 | 2.83 a | 0.19 a | 4.87 b | |
| DMS† | 0.17 | 0.02 | 0.41 | |
†Different let ters indicate significant differences (LSD, p≤0.05). DAS: Days after sowing; N: nitrogen; P: phosphorus; K: potassium; DMS: minimum significant difference.
The internal requirements of N, P, K in the total above-ground biomass of maize plants that have reached the maximum production have been estimated at 1.0, 0.17 and 1.0 % (Rodríguez, 1993), and the concentration of some nutrients decreases in the tissue (MS) as the plant grows (N, P, K, Mg), while others increase (Ca, Mn) (Etchevers, 2008); this may explain that the nutrient concentration in plants 30 DAS only showed the significant effect (p≤0.05) of the polymers on the accumulation of K in the leaf of maize plants, while in plants 50 DAS, the polymers produced significant differences (p≤0.05) in the accumulation of N in the root (Table 6). In addition, chitosan is composed of two structural units: N-acetyl-D-glucosamine and D-glucosamine (Peniche, 2006), which contain nitrogen that during the chitosan degradation process was probably used by the plant, which explains the difference in nutrient concentration between 50 DAS plants.
Table 6 Comparison of nutrient concentration mean in maize plants biomass depending on the polymer.
| Tratamiento | K-hoja (30 DDS) | N-raíz (50 DDS) |
| % | ||
| Testigo | 4.97 a | 1.11 b |
| Quitosano | 4.32 b | 1.25 a |
| Alginato de sodio | 4.81 ab | 1.13 b |
| DMS | 0.56 | 0.11 |
Different letters indicate significant differences (LSD, p≤0.05). DAS: Days after sowing; N: nitrogen; P: phosphorus; K: potassium; † DMS: minimum significant difference.
Conclusions
Chitosan had lower average emergence percentages than the control in both maize genetic lines when applied to the seed alone or combined with fertilizers. Sodium alginate reduced the percentages of emergence to a lesser extent than chitosan in the variety HS-2 and the line CMS939083, although without fertilizers it obtained germination percentages similar to the control. The root development of plants was not stable in both genetic lines. The variety HS-2 race produced dryer root matter, but the line CMS939083 generated greater root length. The addition of fertilizers and salicylic acid to the seeds decreased biomass production and root length of maize plants. The concentration of nitrogen, phosphorus and potassium in maize plants was variable, so there is no conclusive effect of the coatings on the accumulation of nutrients in the plant.
REFERENCES
Arias R., B., C. McGee D., y S. Burris J. 1998. Evaluación del potencial de polímeros como agentes envolventes de fungicidas en el tratamiento de semillas de maíz para el control de Phythium spp. Agron. Trop. 48: 471-488. [ Links ]
Argin S., S., P. Kofinas, and Y. Martin L. 2009. Effect of complexation conditions on xanthan-chitosan polyelectrolyte complex gels. Food Hydrocoll. 23:202-209. [ Links ]
Avelar, S. A. G., F. V. De Sousa, G. Fiss, L. Baudet, and S. T. Peske. 2012. The use of film coating on the performance of treated corn seed. Rev. Bras. Sem. 34: 186-192. [ Links ]
Bhaskara, M., J. Arul, P. Angers, y L. Couture. 1999. Chitosan treatment of seeds induces resistance to Fusarium graminearum and improves seed quality. J. Agric. Food Chem. 47: 1208-1216. [ Links ]
Böhm, W. 1979. Methods of Studying Root Systems. Springer Verlag Berlin Heidelberg. Germany. 188 p. [ Links ]
Brown D., A., and D. Scott H. 1984. Dependence of crop growth and yield on root development and activity. In: Roots, Nutrient and Water Flux, and Plant Growth. Soil Sci. Soc. Am. and Am. Soc. Agron. 144 South Segoe Road, Madison. [ Links ]
Chandrkrachang, S. 2002. The application of chitin and chitosan in agriculture in Thailand. In: Suchiva, K., Chandrkrachang S., Methacanon P. y Peter M. (eds.). Advances in Chitin Science. 5(1):458-462. Bangkok, Thailand. [ Links ]
Costar, A., J. Peña A., M. Puig, A. Pérez, y A. Álvarez. 2013. Relaciones de competencia entre el maíz cultivado y las malas hierbas. Agricultura 1: 6-10. [ Links ]
Etchevers B., J. D. 2008. Nutrición en Maíz. In: De León C., y R. Rodríguez M. (eds). El Cultivo de Maíz. Temas Selectos Vol. 2. Colegio de Postgraduados, México. pp: 1-27. [ Links ]
Gawade J., R., and B. Somawanshi R. 1979. Presowing seed coating with P-Zn mixtures; their influence on available P and Zn in the soil and dry matter yield of wheat (Triticum aestivum L.). J. Maharashtra Agric. Univ. 4: 274-277. [ Links ]
Gouin, S. 2004. Microencapsulation: industrial appraisal of existing technologies and trends. Trends Food Sci. Tech. 15: 330-347. [ Links ]
Guan, Y. J., J. Hu, X. J. Wang, and C. X. Shao. 2009. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. B. 10: 427-433. [ Links ]
Guevara B., N. A., y T. Jiménez M. 2008. Encapsulación: Técnicas y aplicaciones en la industria alimentaria. Temas Sel. Ing. Alim. 2: 36-49. [ Links ]
Hochholdinger, F., F. Woll, M. Sauer, and D. Dembinsky. 2004. Genetic dissection of root formation in maize (Zea mays) reveals root-type specific developmental programmes. Ann. Bot. 93: 359-368. [ Links ]
Kong M., X. G. Chen, K. Xing, and H. J. Park. 2010. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbial. 144: 51-63. [ Links ]
Koornneef, M., L. Bentsink, and H. Hilhorst. 2002. Seed dormancy and germination. Current opinion. Plant Biol. 5: 33-36. [ Links ]
Lakkis J., M (ed). 2007. Encapsulation and Controlled Release Technologies in Food Systems. Blackwell Publishing. Oxford. 239 p. [ Links ]
Lárez V., C. 2006. Quitina y quitosano: materiales del pasado para el presente y futuro. Departamento de química. Universidad de Los Andes. Mérida, Venezuela. Avances en Quím. 1: 15-21. [ Links ]
Lárez V., C. 2008. Algunas potencialidades de la quitina y el quitosano para usos relacionados con la agricultura en Latinoamérica. Departamento de química. Universidad de Los Andes. Mérida, Venezuela. UDO Agrícola 8: 1-22. [ Links ]
Lárez V., C., A. Chirinos, M. Tacoronte, and A. Mora. 2012. Chitosan oligomers as bio-stimulants to Zucchini (Cucurbita pepo) sedd germination. Agriculture (Pol’nohospodárstvo) 58: 113-119. [ Links ]
Lupo P., B., C. González A., y A. Maestro G. 2012. Microencapsulación con alginato en alimentos. Técnicas y aplicaciones. Rev. Venezolana Ciencia Tecnol. Alim. 3: 130-151. [ Links ]
Larqué S., A., and R. Martín M. 2007. Effects of salicylic acid on the bioproductivity of plants. In: Hayat S. and A. Ahmad (eds). Salicylic Acid a Plant Hormone. Springer. Dordrecht, The Netherlands. pp: 15-24. [ Links ]
Lizárraga P., E. G., I. Torres P., E. Moreno M., y S. P. Miranda C. 2011. Protección contra estrés biótico inducida por quitosán en plántulas de maíz (Zea mays L). Rev. Mex. Ciencias Agríc. 2: 813-827. [ Links ]
McHugh D., J. 1987. Production, properties and uses of alginates. Department of Chemistry. University of New South Wales. Australian Defense Force Academy. Campbell, Australia. [ Links ]
Melaj M., A., y E. Daraio M. 2012. Matrices poliméricas sólidas basadas en quitosano y xantano para liberación controlada de fertilizantes. Avances Ciencias Ing. 3: 1-9. [ Links ]
Niquette, P., F. Monette, A. Azzouz, y R. Hausler. 2004. Impacts of substituting alumminium-based coagulants in drinking wáter treatment. Review article. Water Qual. Res. J. Can. 39: 303-310. [ Links ]
Parra H., R. A. 2010. Revisión: Microencapsulación de alimentos. Rev. Fac. Nal. Agr. Medellín 63: 5669-5684. [ Links ]
Peniche C., C. 2006. Estudios sobre quitina y quitosana. Tesis doctoral. Facultad de Química. Universidad de La Habana. Cuba. [ Links ]
Reddy K., R., and Reddy P., S. 2010. Effect of different co-polymers on sodium alginate microcapsules containing isoniazid. International J. Pharm. Tech. Res. 2: 2198-2203. [ Links ]
Revilla P., A., R. Butrón, A. Malvar, and A. Ordás. 1999. Relationships among Kernel weight, early vigor and growth in maize. Crop Sci. 39:654-658. [ Links ]
Rodríguez S., J. 1993. La Fertilización de Cultivos Segunda Edición. Pontificia Universidad Católica de Chile. Santiago, Chile. 291 p. [ Links ]
Rodríguez H., A., A. Valderrama, H. Alarcón, y A. López. 2010. Preparación de partículas de quitosano reticuladas con tripolifosfato y modificadas con polietilenglicol. Rev. Soc. Quím. Perú. 76: 336-354. [ Links ]
Sarroco, S., R. Raeta, and G. Vannacci. 2004. Seeds encapsulation in calcium alginate pellets. Seed Science and Technology 32: 649-661 [ Links ]
Scott, D. 1975. Effects of seed coating on establishment. NZ J. Agric. Res. 18: 59-67. [ Links ]
Scott J., M., J. Mitchell C., and J. Blair G. 1985. Effect of nutrient seed coating on the emergence and early growth of perennial ryegrass. Austr. J. Agric. Research. 36: 221-231. [ Links ]
Soltani, A., M. J Robertson, B. Torabi, M. Yousefi-Daz, and R. Sarparast. 2006. Modelling seedling emergence in chickpea as influenced by temperature and sowing depth. Agric. For. Meteorol. 138: 156-167. [ Links ]
Steiner A., A. 1961. A universal method for preparing nutrient solutions of a certain desired composition. Plant and Soil 15: 134-154. [ Links ]
Tillman M., A. A., e M. Miranda D. 2006. Análise de sementes. In: Peske S., T., O. A. Lucca F., e S. A. Barros A. C. (eds) Sementes: Fundamentos Científicos e Tecnológicos. Pelotas. UFPel. pp: 159-255. [ Links ]
Tingda, J., M. Ruixia, Q. Ruiming, and Z. Chunping. 1994. Seed treatment and inhibition of plant pathology of chitosan. Journal of Environmental Sciences 6:112-115. [ Links ]
Walinga, I., J. J. Van Der Lee, V. J. G. Houba, W. Van Vark, and I. Novozamsky. 1995. Plant Analysis Manual. Kluwer Academic Publishers. Dordrecht, Netherlands. 195 p. [ Links ]
Ziani, K., B. Ursúa, and J. Maté. 2010. Application of bioactive coatings based on chitosan for artichoke seed protection. Crop Protec. 29: 853-859. [ Links ]
Received: January 2016; Accepted: July 2016











 texto em
texto em 



