Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.50 no.8 Texcoco Nov./Dez. 2016
Crop science
Led light quality and in vitro development of Oncidium tigrinum and Laelia autumnalis (orchidaceae)
1 Universidad Michoacana de San Nicolás de Hidalgo. Avenida Francisco J. Múgica S/N Ciudad Universitaria. 58030. Morelia, Michoacán, México. (marelpesa@yahoo.com.mx).
2 Campus Puebla, Colegio de Postgraduados. Km. 125.5 carretera federal México-Puebla. 72760. Puebla, Puebla, México. (grangeln@yahoo.com.mx).
3 Campus Montecillo, Colegio de Postgraduados. Km.36.5 carretera México-Texcoco. 56230. Montecillo, Texcoco, Estado de México. (marinie@colpos.mx)
Quality, intensity and duration of light affect plant development because they promote different physiological responses. The aim of this study was to evaluate the influence of different lighting spectra on in vitro development of Oncidium tigrinum La Llave and Lex. and Laelia autumnalis (Lex.) Lindl. Three-month-old seedlings were grown in a Murashige and Skoog medium without plant hormones plus thiamine (0.4 mg L-1), inositol (100 mg L-1), saccharose (30 g L-1), and agar (6 g L-1) with pH of 5.7. The experimental design was completely randomized and the experimental unit consisted of 30 seedlings. Treatments were red and blue LEDs in a 3:1, 2:2, and 1:3 ratios, white LEDs and fluorescent white light (control), with six repetitions per treatment. Wave lengths were: 590-670 nm for red, 430-480 nm for blue, and 400-700 nm for white; photosynthetically active radiation was adjusted to 31±2 µmol m-2 s-1. The dependent variables were seedling and root length, number of leaves and roots, fresh and dry matter, and a, b and total chlorophyll content. An ANOVA was performed with this data and means were compared with a Tukey test (p(0.05). Results showed that white LED lighting was similar to fluorescent lighting in terms of in vitro seedlings development of L. autumnalis. Seedlings showed twice as much a, b, and total chlorophyll and up to 2 % more biomass accumulation compared with the other treatments. For O. tigrinum, white LED light induced the development of vigorous bright green plants with no photooxidation damage. LEDs lights were confirmed as an effective alternative for in vitro seedling growth and development of O. tigrinum and L. autumnalis.
Key words: Laelia autumnalis; Oncidium tigrinum; in vitro development; in vitro directed radiation; LEDs
La calidad, intensidad y duración de la luz afectan el desarrollo vegetal porque promueven diferentes respuestas fisiológicas. El objetivo de esta investigación fue evaluar la influencia de distintos espectros de iluminación sobre el desarrollo in vitro de Oncidium tigrinum La Llave y Lex. y Laelia autumnalis (Lex.) Lindl. Plántulas de tres meses de edad se cultivaron en medio Murashige y Skoog sin fitohormonas más tiamina (0.4 mg L-1), inositol (100 mg L-1), sacarosa (30 g L-1), agar (6 g L-1) con pH de 5.7. El diseño experimental fue completamente al azar y la unidad experimental consistió de 30 plántulas. Los tratamientos fueron LEDs rojo y azul en proporción 3:1, 2:2 y 1:3, LEDs blancos y luz blanca fluorescente (testigo), con seis repeticiones por tratamiento. Las longitudes de onda fueron: rojo de 590 a 670 nm, azul de 430 a 480 nm y blanca de 400 a 700 nm; el flujo de fotones fotosintéticos se ajustó a 31±2 µmol m-2 s-1. Las variables evaluadas fueron longitud de plántula y raíces, número de hojas y raíces, materia fresca y seca, y contenido de clorofila a, b y total. Con los datos se realizó un ANDEVA y las medias se compararon con la prueba de Tukey (p(0.05). Los resultados mostraron que la iluminación con LEDs blancos fue similar a la iluminación con luz fluorescente en el desarrollo in vitro de plántulas de L. autumnalis con valores del doble o más de clorofila a, b y total, y hasta 2 % más acumulación de biomasa, en comparación con los demás tratamientos. En O. tigrinum la luz LED blanca indujo el desarrollo de plantas vigorosas color verde intenso, sin daño por fotoxidación. Esto confirma que los LEDs son una alternativa eficaz para el crecimiento y desarrollo in vitro de plántulas de O. tigrinum y L. autumnalis.
Palabras clave: Laelia autumnalis; Oncidium tigrinum; desarrollo in vitro; irradiación dirigida in vitro; LEDs
Introduction
There are more than 1200 known orchid species and subspecies in Mexico, located south of the Tropic of Cancer, from the Pacific Ocean to the Gulf of Mexico’s coasts, in areas up to 3500 amsl (Espejo and López Ferrari, 1998). Oncidium and Laelia are two orchid genera which are distributed across Mexico and have high commercial value. The beauty of their flowers makes them very attractive, which is why specimens are illegally extracted from wild populations to satisfy growing market demands. Together with the deforestation of their habitat and land use changes, this demand is one of the three main causes of its population decline in the wild (Szeszko, 2011). In vitro reproduction or plant micropropagation is a feasible technique for massive plant multiplication and is used for the propagation and conservation of important native orchids, such as Laelia and Oncidium (Lee et al., 2007; Mengxi et al., 2011).
In a commercial micropropagation laboratory, controlling physical-chemical, nutritional, and environmental conditions (temperature, moisture, and light) is crucial (Loberant and Altman, 2010), because these factors determine vegetal tissue growth and development. Plants use photosynthetically active radiation (PAR) for carbon fixation, which match the blue (400-500 nm) and red (over 600 nm) areas of the visible spectrum (400-700 nm). The rest of the spectrum is reflected and it is responsible for the green color of leaves. Therefore, improving light quality and intensity accelerates plant photosynthesis, especially when they are lighted with the red and blue radiation wavelengths of the spectrum (Casierra Posada et al., 2011).
Light sources usually used for in vitro plant tissue culture are fluorescent tube lamps (FTLs) (Lin et al., 2011) that emit a wide spectrum, which produces unspecific physiological effects in plants (Da Rocha et al., 2010). Its use in plant tissue culture laboratories represents 65 % of the total electricity costs (Jao and Fang, 2004) and it provides wavelengths which are unnecessary for plants. For this reason, alternative energy sources and more efficient ways to illuminate crops must be sought (Loberant and Altman, 2010).
Light-emitting diodes (LEDs) have high potential to be used as a light source in micropropagation (Loberant and Altman, 2010). They provide a more efficient energy conversion, small volume, longer life, specific wavelength radiation that make photosynthesis more efficient (Araujo et al., 2009), and other advantages such as the possibility to adjust light intensity/quality; furthermore, their thermic radiation and maintenance costs are low and they protect the environment with reduced CO2 emissions (Lee et al., 2010). In micropropagation, red and blue LED light, separated or combined, have a significant influence on plant growth (Dutta and Jatothu, 2013). Red LED light is associated with plant growth (internode lengthening), as was observed in Oncidium (Mengxi et al., 2011), and in low concentrations of photosynthetic pigments proved for Dendrobium officinale (Lin et al., 2011). However, there is little information about the effect of blue light over seed plants physiology; it is usually associated with vigorous growth, differentiation, and high chlorophyll and carotenoid contents on in vitro plants (Lin et al., 2011; Mengxi et al., 2011).
Red LED light increases wet and dry mass of the roots of Paphiopedilum seedling, compared to fluorescent white light and blue light (Lee et al., 2011), and increases wet and dry weight of the sprouts of Dendrobium officinale (Lin et al., 2011). Meanwhile, combined with blue light, biomass accumulation of Oncidium PLBs was increased (Mengxi et al., 2011). This indicates that plant response to LED light quality varies among species and their developmental stage. Its effect on each species needs to be studied separately in order to achieve certain goals: to promote or inhibit sprouts, roots and bulbs, and to control flowering (Kim et al., 2004; Poudel et al., 2008). On the basis of such researches, it might be possible to substitute fluorescent light with LED light during the micropropagation of O. tigrinum and L. autumnalis; also, the plants’ morphogenic responses to light quality can be used to control the development of in vitro tissues. For this reason, the aim of this study was to determine favorable light quality, out of different combinations of LED light, required for optimum in vitro development of O. tigrinum and L. autumnalis orchid seedlings.
Materials and methods
Plant material
For this study, in vitro cultured seedlings -approximately 1.0 cm tall and three month oldwere used. Seedlings were obtained from the germination of seeds of O. tigrinum and L. autumnalis plants that belong to Mexico’s National Phyto Genetic Resources System (SINAREFI), held by President Juárez Agrobiology School of the Universidad Michoacana de San Nicolás de Hidalgo, in Uruapan, Michoacán.
Culture medium
A Murashige and Skoog (MS) medium with no plant hormones, plus thiamine (0.4 mg L-1), inositol (100 mg L-1), saccharose (30 g L-1), agar (6 g L-1), and with pH adjusted at 5.7 was used to grow seedlings. This medium was sterilized in an autoclave, during 15 minutes, at 121 °C.
Establishment and experimental design
For this study, 100 mL glass jars with 20 ml of MS medium were used and 30 seedlings were placed on each one. The procedure was performed in a laminar flow hood. The labeled jars were incubated in 0.10125 m3 plywood boxes painted in white on the inside. On the top of each box an aluminum base with four SiLed® high-efficiency 1 W LED lights was placed. LEDs were red, blue or white, coupled in series to an external 12 V source that was connected to the 110 V AC.
Experimental design was completely random and treatments were: red and blue LEDs in a 3:1, 2:2, and 1:3 ratios, and white LEDs (Table 1). Seedlings in the control group were incubated with a Phillips white fluorescent 39 W lamp. Each treatment was repeated six times and the experimental unit was a jar with 30 seedlings.
Table 1 Treatments to evaluate the effect of LED light quality on the in vitro development of Oncidium tigrinum and Laelia autumnalis seedlings.
| Tratamiento | Descripción |
| 1 | LEDs rojo y azul (3:1) |
| 2 | LEDs rojo y azul (2:2) |
| 3 | LEDs rojo y azul (1:3) |
| 4 | LEDs blancos |
| 5 (testigo) | Luz blanca fluorescente |
Wavelengths emitted by the light sources were measured with an ASD® (Analytical Spectral Devices) field spectrum radiometer (Field Spec Pro). Blue and red LEDs emitted spectrums featuring a single emission peak centered at 458 and 636 nm respectively, of Gaussian appearance and with a standard deviation of 13 nm. On the one hand, white LEDs had a 444 nm emission peak of (also with a 13 nm standard deviation) and a wide area of emission centered around 553 nm (but with a 50 nm dispersion). On the other hand, fluorescent lamps had a series of close peaks at 436, 490, 545, 586, and 612 nm (Figure 1). Light intensity was determined with the radiation sensor of a portable photosynthesis system (IRGA6400, LI-COR®); the resulting intensity was 31±2 µmol m-2 s-1 in all treatments, with a photoperiod of 16 h of light and 8 h of dark and an average temperature of 25 °C.
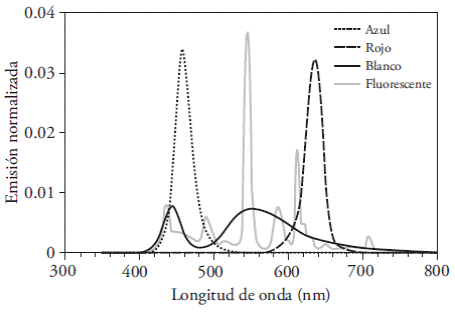
Figure 1 Characteristics of the wavelengths emitted by LED lights (blue, red, and white) and fluorescent lamp, measured with an ASD ® (Analytical Spectral Devices) field spectrum radiometer (Field Spec Pro). Normalized emission is equivalent to the percentage of total visible energy found on each 1 nm band.
Measured variables
Seedling and root length (cm) was measured from the base and the apex of the plantlet or the root, respectively, using graph paper, on 10 seedlings for each treatment and repetition.
The number of leaves and roots was also registered in 10 seedlings per treatment and repetition.
Seedlings’ fresh weight (mg), 10 seedlings of each treatment were selected and weighted in an OHAUS® analytical electronic balance with a 0.01 mg readability.
Seedlings’ dry matter (%). This variable was measured in a 100 mg sample of seedling’s fresh matter, one for each treatment and repetition, using the gravimetric method. Drying was performed in a FELISA oven, at 70 °C during 72 h. Dry samples were weighted in an OHAUS® analytical electronic balance and results are shown as percentage of dry matter with respect to its fresh weight.
Seedlings’ pigment content (mg g-1). a, b, and total chlorophyll concentration was determined in 100 mg samples of each treatment and repetition, extracting the leaves and measuring them through spectrophotometry (Porra et al., 1989). Leaves were placed in glass jars with 3 mL of N-dimethylformamide covered with aluminum foil. The jars were stored at 4 °C during 48 h. Afterwards, a 1 mL sample was placed in a cell to testmeasure absorbance in a JENWAY 6320D spectrophotometer; standard deviation of 13 nm. On the one hand, white LEDs had a 444 nm emission peak of (also with a 13 nm standard deviation) and a wide area of emission centered around 553 nm (but with a 50 nm dispersion). On the other hand, fluorescent lamps had a series of close peaks at 436, 490, 545, 586, and 612 nm (Figure 1). Light intensity was determined with the radiation sensor of a portable photosynthesis system (IRGA6400, LI-COR®); the resulting intensity was 31±2 µmol m-2 s-1 in all treatments, with a photoperiod of 16 h of light and 8 h of dark and an average temperature of 25 °C.
Measured variables
Seedling and root length (cm) was measured from the base and the apex of the plantlet or the root, respectively, using graph paper, on 10 seedlings for each treatment and repetition.
The number of leaves and roots was also registered in 10 seedlings per treatment and repetition.
Seedlings’ fresh weight (mg), 10 seedlings of each treatment were selected and weighted in an OHAUS® analytical electronic balance with a 0.01 mg readability.
Seedlings’ dry matter (%). This variable was measured in a 100 mg sample of seedling’s fresh matter, one for each treatment and repetition, using the gravimetric method. Drying was performed in a FELISA oven, at 70 °C during 72 h. Dry samples were weighted in an OHAUS® analytical electronic balance and results are shown as percentage of dry matter with respect to its fresh weight.
Seedlings’ pigment content (mg g-1). a, b, and total chlorophyll concentration was determined in 100 mg samples of each treatment and repetition, extracting the leaves and measuring them through spectrophotometry (Porra et al., 1989). Leaves were placed in glass jars with 3 mL of N-dimethylformamide covered with aluminum foil. The jars were stored at 4 °C during 48 h. Afterwards, a 1 mL sample was placed in a cell to testmeasure absorbance in a JENWAY 6320D spectrophotometer; measurement was performed with the following wavelengths: 664 nm for chlorophyll a and 647 nm for chlorophyll b. Absorbance data was used to calculate the concentration of each chlorophyll through the following equations: chlorophyll a=12.70 (A664)-2.97 (A647); chlorophyll b=20.70 (A647)-4.62 (A664) and total chlorophyll=17.90 (A647)-8.08 (A664).
Percentage of necrotic and albino plants was only measured for O. tigrinum. Each experimental unit (jar) was divided into quadrants to facilitate counting. Then, normal, necrotic or albino plants were counted by quadrant and added to obtain the total number of each condition per jar. With the total sum of normal, necrotic, and albino plants per experimental unit, the total number of plants per experimental unit was obtained. This number was then used as denominator to calculate the percentage of necrotic or albino plants with the formula: percentage of necrotic or albino plants per experimental unit=(Number of necrotic or albino plants per jar/Total number of plants in the jar)×100.
Variable recordings for O. tigrinum were performed 30 d after treatments started, while variable recordings for L. autumnalis were performed after 90 d.
Data analysis
With the data a variance analysis and a Tukey test (p≤0.05) were performed with SAS 9.0. To better illustrate mean comparisons between treatments, R software 3.1.1 was used, which provides a graphic representation of the differences. The Shapiro test was used to verify distribution normality in the data (R Core Team, 2014).
Results and discussion
Oncidium tigrinum development
Oncidium tigrinum seedlings did not have significant differences between treatments in terms of fresh matter weight, total leaf number, or a, b or total chlorophyll. The exposure to the combination of red and blue spectrums was expected to increase the concentration of pigments and fresh weight, given that: 1) a and b chlorophylls reach their maximum absorbance in the blue and red range (Pimentel et al., 2007); 2) light has a strong influence in cellular multiplication and in in vitro cultured plant tissue growth (Araujo et al., 2009); 3) an increase in biomass and photosynthetically active pigment concentration (a, b, and total chlorophyll) as a result of the exposure to the combination of red and blue light (4:1 and 7:3) had already been documented on seedlings of another species of Oncidium (Mengxi et al., 2011).
In our study significant differences in seedling length, root number, and size were found, as well as differences in dry matter, albino, and necrotic plant percentages. The longest seedlings (1.64 cm) were obtained with the combination of red and blue light (1:3); however, they were statistically similar to other treatments except T2, where the size of seedlings was reduced by 28 % (Figure 2). T2 also inhibited root production and length (Figure 3A). T1 only reduced root length (Figure 3B).
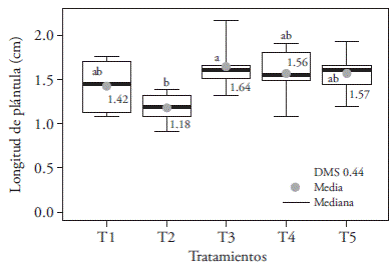
Figure 2 Effects of T1 (3:1 red and blue LEDs), T2 (2:2 red and blue LEDs), T3 (1:3 red and blue LEDs), T4 (white LEDs), and T5 (white fluorescent light) light treatments on in vitro cultured Oncidium tigrinum seedlings’ length. Different letters on each figure indicate significant differences (Tukey; p≤0.05).
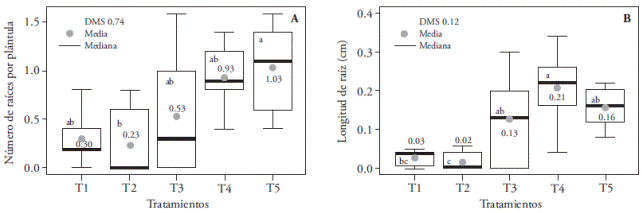
Figure 3 Effect of T1 (3:1 red and blue LEDs), T2 (2:2 red and blue LEDs), T3 (1:3 red and blue LEDs), T4 (white LEDs), and T5 (white fluorescent light) on root number (A) and length (B) of Oncidium tigrinum in vitro cultured seedlings. Different letters on each figure indicate significant differences (p≤0.05).
The reduced root length on T1 and T2 (larger and equal ratio of red versus blue light, respectively) could be attributed to what was noted by Correll and Kiss (2005). They claim that red light receptors (A and B phytochrome) have an inhibitory effect on root elongation. White light -which has less radiation ratio in the red region of the spectrumstimulates rooting in Cattleya loddigessi seedlings (Araujo et al., 2009) and, together with green light, boosts bud rooting of Dendranthema×grandiflora ‘Lilac Wonder’ (Miler and Zalewska, 2006). Our study confirms that white light from LEDs and fluorescent lamps promoted rooting of in vitro O. tigrinum seedlings and that these light sources emitted the least amount of radiation in the red region, compared to all other studied spectrums.
Micropropagated seedlings had good quality in all lighting treatments and degenerative changes in leaves and pseudobulbs were not found (Figure 4), apart from some cases with total chlorophyll absence. The highest incidence of albino seedlings (33.7 %) was found on T5 (fluorescent light) and was similar to that of T4 (15.8 %), but significantly different from the other treatments, which showed an average of 2.16 % of albino seedlings (Figure 5). These findings seem to indicate that fluorescent lamps and white LEDs light emission on the red and blue regions of the spectrum are not enough to ensure that some O. tigrinum seedlings can perform normal photosynthesis.

Figure 4 In vitro Oncidium tigrinum seedlings lighted during 30 days with different light combinations T1 (3:1 red and blue LEDs), T2 (2:2 red and blue LEDs), T3 (1:3 red and blue LEDs), T4 (white LEDs), and T5 (white fluorescent light).
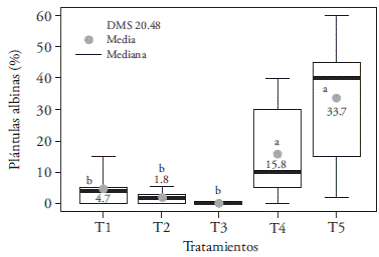
Figure5. Effecto flight treatments T1 (3:1 redand blue LEDs), T2 (2:2 red and blue LEDs), T3 (1:3 red and blue LEDs), T4 (white LEDs) and T5 (white fluorescent light) on the incidence of albino seedlings of in vitro cultured Oncidium tigrinum. LSD: Least significant difference. In the figure, different letters indicate significant differences (Tukey; p≤0.05).
Albinism is an evolutionary genetic characteristic that has not been documented on the Oncidium genus, although it occurs in some individuals of orchid populations of the Epipactis (Stöckel et al., 2011) and Cephalanthera (Roy et al., 2013) genera, which grow under canopies. In any other green plants the photosynthetic process is decreased due to the reduced ambient light or the low efficiency of their photosynthesis apparatus or both (Roy et al., 2013). Furthermore, during the first stages (protocorm formation), Oncidium sphacelatum seedlings are completely myco-heterotrophic, i.e., they need to establish a symbiotic relationship with a mycorrhizial fungus that provides them with a carbon source (Valadares et al., 2014).
The largest percentage of dry matter (12.2 %) was obtained with T1; it was statistically larger than T3 -the latter had 4% less biomass-, but it was similar to the other treatments (Figure 6A).
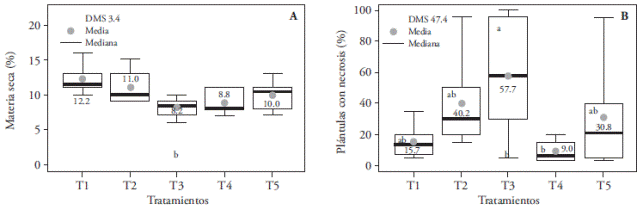
Figure 6 Effect of light treatments T1 (3:1 red and blue LEDs), T2 (2:2 red and blue LEDs), T3 (1:3 red and blue LEDs), T4 (white LEDs), and T5 (white fluorescent light) on dry matter accumulation (A) and in the incidence of necrotic seedlings (B) of in vitro cultured Oncidium tigrinum. Different letters on each figure indicate significant differences (p≤0.05).
Similar responses have been found in in vitro conditions with light intensities of 70 µmol m-2s-1. In Lilium oriental hybrid ‘Pesaro’, blue+red (1:1) LED lighting and fluorescent light increased bulbils’ dry matter (Lian et al., 2002) and, in Dendrobium officinale, red+blue (1:1) light improved biomass concentration in protocorms (Lin et al., 2011). Also, blue-only lighting reduces dry matter (Lian et al., 2002); which is why in our study, the lowest registered value from T3 might be the result of the highest proportion of blue light versus red light. T3 had the largest percentage of necrotized seedlings (57.7 %), and it may be possible that tissue necrosis is due to photoinhibition or photooxidation related damage, as Araujo et al. (2009) and Casierra-Posada et al. (2011) suggest. However, this percentage was similar (p>0.05) to the rest of the treatments, except T4 that significantly decreased the incidence of necrotized seedlings. i.e., with 100 % white LED light there was almost no observable damage in the seedlings (Figure 6B).
Laelia autumnalis development
Significant differences on seedling and root length, number of leaves and roots, in seedling’s fresh and dry weight, and in a, b, and total chlorophyll content were found in this species. The longest seedlings were obtained in treatments with the highest emissions of light in the blue region: T3 (3.26 cm), T1 (3.04 cm), and T2 (2.93 cm), statistically similar, but superior to T4 (2.65 cm), and T5 (1.88 cm) (Figure 7A). However, this elongated plants presented degenerative morphological changes, with thinner stems and leaves than the other treatments (Figure 8). This could be explained partly because blue light increases plants’ water consumption to their maximum cellular capacity, which can produce increased tissue length (Ribeiro et al., 2009). Contrary to our study’s results, Ribeiro et al. (2009) did not find differences in Zantedeschia aethiopica seedlings length as a result of the quality of white, red, blue, and green light; however, in Paphiopedilum sprouts, Lee et al. (2011) observed apical dominance loss (small plants) as a result of blue light.
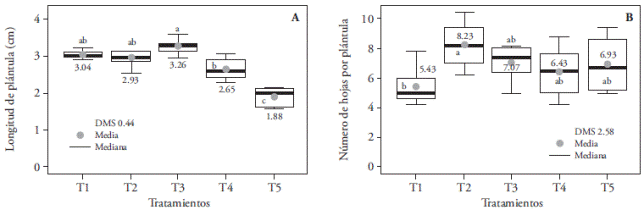
Figure 7 Effect of light treatments T1 (3:1 red and blue LEDs), T2 (2:2 red and blue LEDs), T3 (1:3 red and blue LEDs), T4 (white LEDs), and T5 (white fluorescent light) on leaf length (A) and number of leaves (B) of in vitro cultured Laelia autumnalis seedlings. Different letters on each figure indicate significant differences (p≤0.05).

Figure 8 In vitro Laelia autumnalis seedlings irradiated with different combinations of light during 90 days: T1 (3:1 red and blue LEDs), T2 (2:2 red and blue LEDs), T3 (1:3 red and blue LEDs), T4 (white LEDs), and T5 (white fluorescent light).
Average leaf number in all treatments was over six, except in T1 where the average was just five, although only T2 was statistically different with 8.23 leaves per plant (Figure 7B). This could be a reflection of the low proportion of blue light versus red light in T1 (1:3), compared with T2 (2:2). Lateral bud and leaf imposition in Anthurium seedlings (Budiarto, 2010) and Dendrobium officinale PLBs (Lin et al., 2011) are stimulated when exposed to more blue light than red light. However, this is not a viable option to increase leaf production in L. autumnalis seedlings, given the degenerative morphological changes produced by excessive blue light.
The greatest fresh matter weight was obtained with T3 (red and blue 1:3) and T2 (red and blue 2:2), which were superior to the other treatments (Figure 9A). These results are similar to those obtained by Wu and Lin (2012) who reported that red and blue light (1:1) at 50 µmol m-2s-1 intensity increases the accumulation of Protea cynaroides seedling’s fresh weight; but this finding differs from what was reported in Cymbidium, where PLBs’ fresh weight increase is attributed to green light (Nahar et al., 2012). Red and blue light affect stomatal opening, but Squeo and León (2007) consider that only blue light is responsible for this mechanism, and therefore plants exposed to this light spectrum consume more water, which could help to explain our results (larger fresh weight in T3).
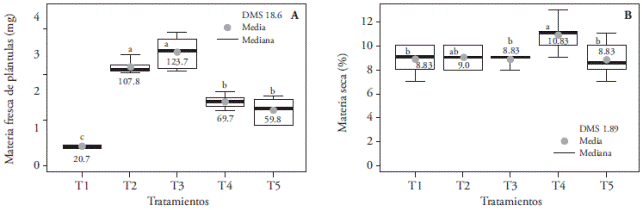
Figure 9 Effect of light treatments T1 (3:1 red and blue LEDs), T2 (2:2 red and blue LEDs), T3 (1:3 red and blue LEDs), T4 (white LEDs) and T5 (white fluorescent light) on fresh (A) and dry (B) weight of in vitro cultured Laelia autumnalis seedlings. Different letters on each figure indicate significant differences (p≤0.05).
The largest percentage of dry matter (10.83) was registered with T4, larger than T1, T3, and T5 (8.83) (Figure 9B). These results differ from those of Lin et al. (2011), who point out that dry weight accumulation in Dendrobium officinale seedlings was favored with 70 µmol m-2s-1 of red and blue light (1:2) and is halved when the proportion is even (1.1).
White LED light (T4) and control (T5) increased the accumulation of a chlorophyll (Figure 10A), b chlorophyll (Figure 10B), and total chlorophyll (Figure 11) compared with the other treatments, with up to 16.6 mg g-1 more total chlorophyll (in T1). This could be the result of the spectral composition of white light (400-700 nm) that includes blue (430480 nm), green (495-570 nm), and red (590-670 nm) wavelengths that absorb photosystems II (≤680 nm) and I (≤700 nm), because maximum efficiency requires both photosystems to be engaged (Solarte et al., 2010). The fact that leaves mainly absorb photons of the blue and red spectrums -and that they absorb a lesser amount of green photonsmust be taken into consideration, although the plants reflect most of those photons as diffuse radiation (Lazo and Ascencio, 2010). Lee et al. (2011) observed a decrease in total chlorophyll content in Paphiopedilum leaves, under blue, red, and red plus blue (9:1) light conditions, which matches the results of our study; while Lin et al. (2011) found that in Dendrobium officinale the blue spectrum increased more than twofold the concentration of photosynthetically active pigments.
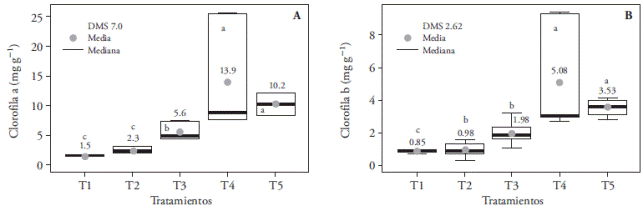
Figure 10 Effect of light treatments T1 (3:1 red and blue LEDs), T2 (2:2 red and blue LEDs), T3 (1:3 red and blue LEDs), T4 (white LEDs), and T5 (white fluorescent light) on the accumulation of a chlorophyll (A) and b chlorophyll (B) of in vitro cultured Laelia autumnalis seedlings. Different letters on each figure indicate significant differences (p≤0.05).
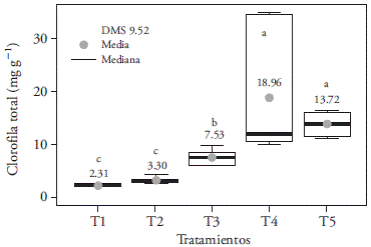
Figure 11 Effect of light treatments T1 (3:1 red and blue LEDs), T2 (2:2 red and blue LEDs), T3 (1:3 red and blue LEDs), T4 (white LEDs), and T5 (white fluorescent light) on the accumulation of total chlorophyll of in vitro cultured Laelia autumnalis seedlings. Different letters on each figure indicate significant differences (p≤0.05).
The highest number of roots per seedling (0.90) was found in the fluorescent light treatment (T5) and was similar to LED white light (T4); whereas that combinations of red and blue light (3:1, 2:2, and 1:3) did not induce root formation (Figure 12). This rhizogenesis inhibition could be related to an increase in the blue spectrum radiation in T1 (3:1), T2 (1:1), and T3 (1:3). Results of Chrysanthemun seedlings (Kurilčik et al., 2008) match this tendency, where root formation is inhibited when blue light increases with respect to red and far red light; they, however, differ with observations in Paphiopedilum, where root development was brought about with red and blue light, and where fresh and dry weights reached up to 387.7 and 31.2 mg, respectively, more than with white light (Lee et al., 2011).
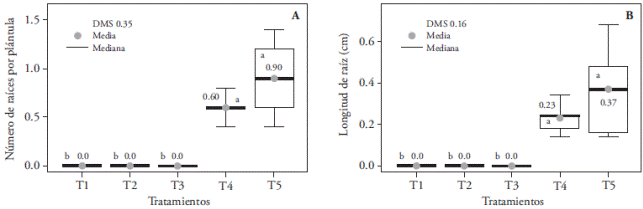
Figure 12 Effect of light treatments T1 (3:1 red and blue LEDs), T2 (2:2 red and blue LEDs), T3 (1:3 red and blue LEDs), T4 (white LEDs) and T5 (white fluorescent light) on the number (A) and length (B) of in vitro cultured Laelia autumnalis seedlings’ roots. Different letters on each figure indicate significant differences (p≤0.05).
Blue light stimulates phenolic compound biosynthesis, and therefore reduces rooting of in vitro sprouts of Protea cynaroides; furthermore, combined with red light, it increases the accumulation of 3,4-dihydroxybenzoic acid, malic acid, and ferulic acid. Conversely, root formation in seedlings lighted with red LEDs is the result of a low endogenous concentration of such phenols (Wu and Lin, 2012). During simultaneous lighting with blue and red lights, a synergic interaction between cryptochromes and phytochromes (blue and red photoreceptors, respectively) takes place, and those pigments could be responsible for the rhizogenesis process perception and activation. Phytochromes could possibly regulate the rhizogenesis process via phytohormone systems (Kurilčik et al., 2008).
Based on our results, it is feasible to light in vitro cultures of O. tigrinum and L. autumnalis with white LED light, because it produces similar developmental effects than those produced by white fluorescent light. Furthermore, LED lights produce little heat and can be placed directly over the plants (Schroeter-Zakrzewska and Kleiber, 2014). This solution allows installing several levels in a shelf, increasing the number of seedlings per area unit, and reducing energy expenses due to lighting and cooling. However, it is still necessary to study the effect of photoperiod and photosynthetic photon flux density, which vary among species, and represent a particular ecologic adaptation (Kurilčik et al., 2008). For instance, strawberry seedling development requires 60 µmol m-2 s-1 (Nhut et al., 2003), while chrysanthemum explants only need 40 µmol m-2 s-1 (Kurilčik et al., 2008).
Conclusions
The light spectrum emitted by white LEDs was the most favorable for O. tigrinum and L. autumnalis in vitro seedlings development. Therefore, it is possible to replace white fluorescent lamps with white LEDs in an orchid micropropagation laboratory, given that both kinds of light are highly efficient as photosynthetically active radiation sources and they promote similar photomorphic responses in plants. White light reduces length and fresh matter in L. autumnalis’ seedlings, but increases photosynthetic pigments content and promotes rooting. While lighting O. tigrinum seedlings with a greater proportion of red light versus blue light inhibits rooting, it diminishes the formation of albino plants.
Literatura citada
Araujo, A. G. D., M. Pasqual, L. Y. Miyata, E. M. D Castro, e H. S. Rocha. 2009. Qualidade de luz na biometria e anatomia foliar de plântulas de Cattleya loddigessi L. (Orchidaceae) micropropagadas. Cienc. Rural 39: 2506-2511. [ Links ]
Budiarto, K. 2010. Spectral quality affects morphogenesis on Anthurium plantlet during in vitro culture. Agrivita 32:234-240. [ Links ]
Casierra-Posada, F., J. E. Peña-Olmos, y C. Ulrichs. 2011. Crecimiento y eficiencia fotoquímica del fotosistema II en plantas de fresa (Fragaria sp.) afectadas por la calidad de la luz: implicaciones agronómicas. Rev. UDCA Act. & Div. Cient. 14: 43-53. [ Links ]
Correll, M. J., and J. Z. Kiss. 2005. The roles of phytochromes in elongation and gravitropism of roots. Plant Cell Physiol. 46: 317-323. [ Links ]
Da Rocha, P. S. G., R. P. de Oliveira, W. B. Scivittaro, e U. L. dos Santos. 2010. Diodos emissores de luz e concentrações de BAP na multiplicação in vitro de morangueiro. Cienc. Rural 40:1922-1928. [ Links ]
Dutta, G. S., and B. Jatothu. 2013. Fundamentals and applications of light emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 7: 211-220. [ Links ]
Espejo S., A., y A. R. López-Ferrari. 1998. Las Monocotiledóneas Mexicanas una Sinopsis Florística 1. Consejo Nacional para el Conocimiento y Uso de la Biodiversidad. Universidad Autónoma Metropolitana-Ixtapalapa. México, DF. 90 p. [ Links ]
Jao, R. C., and W. Fang. 2004. Growth of potato plantlets in vitro is different when provided concurrent vs alternating blue and red light photoperiods. Hort Science 39: 380-382. [ Links ]
Kim, S. J., E. J. Hahn, J. W. Heo, and K. Y. Paek. 2004. Effects of LED on net photosynthetic rate, growth and leaf stomata of Chrysanthemum plantlets in vitro. Sci. Hort. 101: 143-151. [ Links ]
Kurilčik, A., R. Miklušytė-Čanova, S. Dapkūnienė, S. Žilinskaitė, G. Kurilčik, G. Tamulaitis, P. Duchovskis, and A. Žukauskas. 2008. In vitro culture of Chrysanthemum plantlets using light-emitting diodes. Cent. Eur. J. Biol. 3(2): 161-167. [ Links ]
Lazo J., V., y J. Ascencio. 2010. Efecto de diferentes calidades de luz sobre el crecimiento de Cyperus rotundus. Bioagro 22: 153-158. [ Links ]
Lee E., H. E., A. Laguna C., J. Murguía G., P. Elorza M., L. Iglesias A., B. García R., F. A. Barredo P., y N. Santana B. 2007. Regeneración in vitro de Laelia anceps ssp. dawsonii. Revista Científica UDO Agrícola 7: 59-67. [ Links ]
Lee, N. Y., M. J. Lee, Y. K. Kim, J. C. Park, H. K. Park, J. S. Choi, J. N. Hyun, K. J. Kim, K. H. Park, J. K. Ko, and J. G. Kim. 2010. Effect of light emitting diode radiation on antioxidant activity of barley leaf. J. Korean Soc. Appl. Biol. Chem. 53: 685-590. [ Links ]
Lee Y., I., W. Fang, and C. C. Chen. 2011. Effect of six different LED light qualities on the seedling growth of Paphiopedilum Orchid in vitro. Acta Hort. 907: 389-391. [ Links ]
Lian, M. L., H. N. Murthy, and K. Y. Paek. 2002. Effect of light emitting diodes (LEDs) on the in vitro induction and growth of bulblets of Lilium oriental hybrid ́Pesaro ́. Sci. Hort. 94: 365-370. [ Links ]
Lin Y., J. Li, B. Li, T. He, and Z. Chun. 2011. Effects of light quality on growth and development of protocorm-like bodies of Dendrobium officinale in vitro. Plant Cell Tiss. Organ. Cult. 105: 329-335. [ Links ]
Loberant B., and A. Altman. 2010. Micropropagation of plants. In: Flickinger, M. C. (ed). Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology. J. Wiley & Sons Inc. New, York. pp: 1-16. [ Links ]
Mengxi L., X. Zhigang, Y. Yang, and F. Yijie. 2011. Effects of different spectral lights on Oncidium PLBs induction, proliferation, and plant regeneration. Plant Cell Tiss. Organ. Cult. 106: 1-10. [ Links ]
Miler, N., and M. Zalewska. 2006. The influence of light colour on micropropagation of chrysanthemum. Acta Hort. 725: 347-350. [ Links ]
Nahar, S. J., K. Shimasaki, and S. M. Haque. 2012. Effect of different light and two polisaccharides on the proliferation of protocorm-like-bodies of Cymbidium cultured in vitro. Acta Hort. 956: 307-131. [ Links ]
Nhut D.T., T. Takamura, H. Watanabe, K. Okamoto, and M. Tanaka. 2003. Responses of strawberry plantlets cultured in vitro under superbright red and blue lightemitting diodes (LEDs). Plant Cell Tiss. Org. 73: 43-52. [ Links ]
Pimentel V., C., R. Machado K., e C. L. Salgueiro L. 2007. Qualidade de luz e producao de pigmentos fotossintéticos em plantas in vitro de Phyllanthus tenellus Roxb. Rev. Bras. Biociencias 5: 213-215. [ Links ]
Porra R., J., A. Thompson, and P. Kreidemann. 1989. Determination of accurate extinctions coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standars by atomic absorption spectroscopy. BBA-Bioenergetics 957: 384-394. [ Links ]
Poudel, P. R., I. Kataoka, and R. Mochioka. 2008. Effect of redand blue-light-emitting diodes on growth and morphogenesis of grapes. Plant Cell Tiss. Organ Cult. 92: 147-153. [ Links ]
R Core Team. 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org (Consulta: Noviembre 2014). [ Links ]
Ribeiro, M. D. N. O., M. Pasqual, A. B. da Silva, y V. A. Rodrigues. 2009. Multiplicão in vitro de copo-de-leite: espectros de luz e sacarose. Ciencia Rural 39: 2388-2393. [ Links ]
Roy, M., C. Gonneau, A. Rocheteau, D. Berveiller, J.-C. Thomas, C. Damesin, and M.-A. Selosse. 2013. Why do mixotrophic plants stay green? A comparison between green and chlorophyllous orchid individuals in situ. Ecol. Monogr. 83: 95-117. [ Links ]
Schroeter-Zakrzewska, A., and T. Kleiber. 2014. The effect of light colour and type of lamps on rooting and nutrient status in cuttings of michaelmas daisy. Bulg. J. Agric. Sci. 20: 1426-1434. [ Links ]
Solarte M., E., L. Moreno, y L. M. Melgarejo. 2010. VI. Fotosíntesis y pigmentos vegetales. In: Melgarejo L. M. (ed). Experimentos en Fisiología Vegetal. Universidad Nacional de Colombia. pp: 107-122. [ Links ]
Squeo F., A., y M. F. León. 2007. III. Transpiración. In: Squeo F. A., y L. Cardemil (eds). Fisiología Vegetal. Universidad de la Serena. La Serena, Chile. pp: 67-84. [ Links ]
Stöckel, M., C. Meyer, and G. Gebauer. 2011. The degree of mycoheterotrophic carbon gain in green, variegated and vegetative albino individuals of Cephalanthera damasonium is related to leaf chlorophyll concentrations. New Phytol. 189: 790-796. [ Links ]
Szeszko F., D. R. 2011. La Orquideoflora Mexiquense. Primera edición. Consejo Editorial de la Administración Pública Estatal. México. 362 p. [ Links ]
Valadares, R.B.S., S. Perotto, E. C. Santos, and M. R. Lambais. 2014. Proteome changes in Oncidium sphacelatum (Orchidaceae) at different trophic stages of symbiotic germination. Mycorrhiza 24: 349-360. [ Links ]
Wu, H. C., and C. C. Lin. 2012. Red light-emitting diode light irradiation improves root and leaf formation in difficult to propagate Protea cynaroides L. plantlets in vitro. HortScience 47: 1490-1494. [ Links ]











 texto em
texto em 


