Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.50 no.8 Texcoco nov./dic. 2016
Biotechnology
Differential expression of biochemical markers during vegetative development and reproductive stages of the aztec lily (Sprekelia formosissima (L.) Herbert)
1 Laboratorio de Biología Molecular Vegetal, Facultad de Ciencias Agrícolas, Universidad Autónoma del Estado de México. 50200. Carretera Toluca-Ixtlahuaca Km 11.5, entronque al Cerrillo, Campus Universitario “El Cerrillo”, Toluca, Estado de México, México. (amaury1963@yahoo.com.mx).
2 Centro Universitario Tenancingo, Universidad Autónoma del Estado de México. 52400. Carretera Tenancingo-Villa Guerrero Km 1.5, Ex Hacienda de Santa Ana, Tenancingo, Estado de México, México.
Some isozymes are involved in processes of interest to geneticists, such as flowering, plant development, height, pest and disease resistance, to name some. Therefore, it is important to determine, using biochemical markers, if there is differential expression during the vegetative and reproductive phases in the Aztec lily (Sprekelia formosissima (L.) Herbert). To do so, during those stages we collected tissue from the leaves, tepals gynoecium and stamens. These were then macerated, an extraction buffer was added and the total liquid extracts were used to determine isozymatic patterns via electrophoresis in starch gels. The differential enzymatic activity was evaluated using eleven isozymatic systems, from which only nine enzyme forms or isozymatic band patterns (IBP) were observed: two bands with peroxidase (POX; EC 1.11.1.7), three bands with acid phosphatase (ACP; EC 13.1.3.2), one with phosphoglucose isomerase (PGI; EC 5.3.1.9), two for phosphoglucomutase (PGM; EC 2.7.5.1) and one for malate dehydrogenase (MDH, EC 1.1.1.37), which showed the same pattern of isozymatic banding at all stages of the plant development. For the purpose of this research we only considered here the isoenzymatic banding patterns that were different.
Keywords: Sprekelia formosissima; isozymes; differential expression
Algunas isoenzimas intervienen en procesos de interés para los genetistas, tales como la floración, desarrollo de la planta, altura, resistencia a plagas y enfermedades, entre otras. Por lo tanto, es importante determinar si existe expresión diferencial en lirio azteca (Sprekelia formosissima (L.) Herbert durante las fases vegetativa y reproductiva utilizando marcadores bioquímicos. Por ello, durante estas fases se recolectó tejido de hojas, tépalos, gineceo y estambres. Estos tejidos se maceraron adicionando un amortiguador de extracción y los extractos líquidos totales se usaron para determinar patrones isoenzimáticos mediante electroforesis en geles de almidón. La actividad enzimática diferencial se evaluó utilizando once sistemas isoenzimáticos, de los cuales sólo se observaron nueve formas enzimáticas o patrones de bandeo isoenzimáticos (PBI): dos bandas con peroxidasa (POX; EC 1.11.1.7), tres bandas con fosfatasa ácida (ACP; EC 13.1.3.2), una con fosfoglucosa isomerasa (PGI; EC 5.3.1.9), dos para fosfoglucomutasa (PGM; EC 2.7.5.1) y una para malato deshidrogenasa (MDH; EC 1.1.1.37), la cual mostró el mismo patrón de bandeo isoenzimático en todas las etapas de desarrollo de la planta. Para el propósito de esta investigación únicamente se consideraron los patrones de bandeo isoenzimáticos diferentes.
Palabras clave: Sprekelia formosissima; isoenzimas; expresión diferencial
Introduction
A primary goal of molecular biology is to understand how the genetic information is organized and how the genetic expression is regulated. Thus, extracting DNA from any tissue of an organism at any stage of development does not change their DNA sequence; meaning it is stable and identical to the original sequence. Active zones of this macromolecule with coding capacity, are called exons, and they stimulate the synthesis of specific enzymes in the different stages of development of an organism. The catalytic property of these enzymes reveal its presence by histochemical methods. Since 1957 geneticists have used electrophoresis to identify thousands of proteins contained in a raw cellular extract. Biomolecules that have the same catalytic property are called isozymes.
With isozymes or biochemical markers it is possible to perform genetic variability studies, where tissue variability (TV) means that various tissues or organs from the same individual show different molecular forms or different relative amounts of the same isozyme. In addition, the ontogenetic variability (OV) refers that in the same organ at certain stages of development (phenological stages) different molecular forms or different relative amounts of the same isozyme are shown. Thus, we can infer that both the TV and the OV are the result of differential regulation. So, every tissue of an organism has different genes expressions and in the same tissue at different points of the phenological cycle diverse groups of genes are expressed. In an electrophoresis gel each band that appears on the support matrix corresponds to a different protein whose synthesis may be controlled by one or more genes. Due to the fact that the order and nature of the amino acids determine the primary structure of the protein, this sequence depends only on the information contained in the DNA, which encodes a particular polypeptide. Thus, the phenotypes observed on the support matrix can be interpreted in terms of genotypes, in other words, in terms of genes and their alleles.
For geneticists, some of the most important isozyme’s characteristics are: 1) A proportion of these genes are polymorphic, this means that they are in the form of one or more alleles; and 2) these alleles are codominant, in other words, both alleles are expressed in heterozygous organisms; this phenomenon allows to relate the observed phenotypes with a given genotype. When interpreting isozymatic phenotypes in a support matrix, it should be considered its quaternary structure, depending on the number of polypeptide subunits (one, two, three or four). This allows us to classify them as monomers, dimers, trimers or tetramers, respectively, and the banding pattern for homozygous is represented, in all cases, by a single allele.
Nevertheless, in heterozygotes the transcripts encode for proteins associated with two alleles (monomers), three alleles (dimers), four alleles (trimers) and five alleles (tetramers). Hence, a study using isozymes can detect the presence of different molecular forms of these proteins at specific stages of development of an organism, from which it is inferred that the presence of a new or different isozyme in a specific state or stage of development might be controlling a process by the isozymes themselves. Therefore, to increase the efficiency of the technique, the developmental stages of the plant could be identified when the protein is expressed (Kephart, 1990).
Electrophoresis in polyacrylamide or starch gels is used to calculate and detect gene products. The isozyme analysis by electrophoresis is a strong, reproducible and powerful technique with numerous applications because isozymes present co-dominant expression (Kephart, 1990) and because they do not exhibit ambiental effects (Marquard and Chan, 1995). Besides the method’s reliability, they are relatively cheaper than those of DNA analysis and allows us to obtain enzymatic information from different tissues (Vincent and Fulton, 2003). The isozyme’s analysis have been performed successfully in several plant species: Nicotiana tabacum L. (Stafford and Galston, 1970), Zea mays L. (Scandalios, 1964, Efron, 1970; Rao et al., 1997), Hordeum vulgare L. (Simonsen and Pedersen, 1987), Cereus peruvianus (Machado et al., 1993), Cucurbita pepo L. (Carpin et al., 1999), Brassica oleraceae (Bellani et al., 2002), plants belonging to the family Asteraceae (Ros-Barcelo and AznarAsensio, 2002) Mammillaria gracillis Pfeiff. (Balen et al., 2003), Tigridia pavonia (Arzate-Fernández et al., 2005a) and Lilium spp. (Arzate-Fernández et al., 2005b).
Sprekelia formosissima (L.) Herbert, also known as Aztec lily, is a species native to Mexico that is still considered wild, it belongs to the Amaryllidaceae family and is characterized by being bulbous, annual and of short of cycle. It grows in grasslands and stony fields in the states of Durango, Jalisco, Guanajuato, Hidalgo, Estado de Mexico, Mexico City, Puebla, Veracruz, Guerrero and Oaxaca (Lopez-Ferrari and Espejo-Serna, 2002). The ornamental value of this species is given by its scarlet flowers; therefore, in Mexico it is frequently seen in gardens and used as a medicinal plant (Vázquez-García et al., 1998). Despite the ornamental potential of the species, no studies have been carried out using isozymes for S. formosissima. Therefore, the objective of this study was to evaluate the differential expression (tissue variation and ontogenetic variation) in this wild plant with biochemical markers, to determine the different enzyme forms expressed in the vegetative and reproductive stages.
Materials and methods
In this study we used Aztec lily plants (S. formosissima (L.) Herbert cv. Terciopelo, obtained from cloned bulbs and grown in a substrate composed of: leaf litter, sand and bovine manure in a 1:1:1 ratio for 78 days, in a rustic greenhouse at the Faculty of Agricultural Sciences at the Universidad Autonoma del Estado de Mexico (UAEMex).
The S. formosissima cultivation cycle was divided into two phenological stages to evaluate their differential expression: vegetative stage and reproductive stage (flowering). During the vegetative stage only tissue samples from the leaves (H) were collected. In the reproductive stage, which initiated after the first sprouting of leaves, and continued for approximately 40 d, the tissue samples were obtained from tepals (T), stigma-style (G), stamens (E) and leaves (H).
Once the seedlings emerged, the tissue sampling took place every 8 days, in each sampling all available tissues were collected. During the reproductive stage three samplings were carried out: the first was only H, the second of T, G and E, and the third H, T, G and E. In the vegetative stage seven samples were taken, all of H. Each sample consisted in collecting tissue samples of at least three plants, the samples were placed into Ziploc bags, and placed in a cooler and then kept at -20 °C in a domestic freezer. All samples were processed at the Laboratory of Plant Molecular Biology, in the Faculty of Agricultural Sciences of the UAEMex.
Electrophoretic procedure
The extraction of total proteins was performed using approximately 50 mg of fresh tissue, each sample was homogenized with a plastic crowbar in 2 ml Eppendorf tubes with 50 L extraction buffer Tris-HCl (hydroxymethyl aminomethane) 0.1M, EDTA 2Na-(ethylene diamino tetraacetic) 2 %, glycerol 50.4 %, tween-80 3.2 % and DTT (dithiothreitol) 0.8 %, pH 7.5 (Arzate-Fernández et al., 2005a). Once the proteins were extracted, all the samples were stored at -20° C until they were analyzed. Two buffer systems were used: histidine-citric acid, pH 5.7 y pH 6.5 (H-AC) and Tris-citrate/Tris-histidine, pH 7.8 (T-C/T-H) were used according to the procedures of Glaszmann et al. (1988) and Stuber et al. (1988). The five isozyme systems evaluated were: peroxidase (POX; EC 1.11.1.7), acid phosphatase (ACP; EC 13.1.3.2), phosphoglucose isomerase (PGI; EC 5.3.1.9), phosphoglucomutase (PGM; EC 2.7.5.1) and malate dehydrogenase (MDH, EC 1.1.1.37). The supernatant of the samples was loaded into a hydrolyzed potato starch gel 12 %, the electrophoretic shift was made at a temperature of 4 °C, with a mean duration of 3 h, 40-50 mA and 100-150 v. Each sample was tested at least three times to check reproducibility. Histochemical staining procedures were performed according to the techniques described by Torres et al. (1978) in citrus, Vallejos (1983), Stuber et al. (1988) used extracts of Zea mays L., and Wendel and Weeden (1990), Glaszmann et al. (1988) and Ishikawa (1994) in rice (Oryza sativa L.).
For differential expression it was considered the presence or absence of each band in every phenological stage (vegetative and reproductive) of the sampled plants.
Results and discussion
Bands with enough sharpness and resolution for registration and further analysis were observed in five electrophoretic systems (Table 1). The PGI, PGM and MDH isozymes showed better resolution in the citric acid-histidine system at pH 5.7; whereas the ACP isozyme showed improved resolution in the same buffer, but at pH 6.5. In the POX case, better resolution was provided by the Tris citrate/Trishistidine system at pH 7.8.
Table 1 Isozyme banding patterns (IBP) observed in the different phenological development stages (reproductive and vegetative) in Sprekelia fromosissima (L) Herbert.
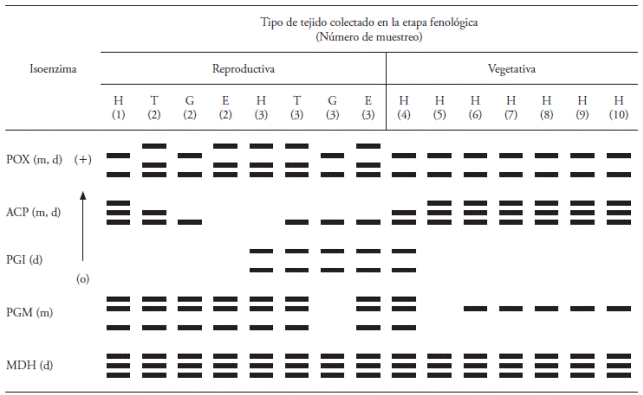
Numbers (1) to (10) means the number of sampling; “o”: Origin of electrophoresis; H: Leaf; T: Tepal; G: Stigma-Style; E: Stamen; m: monomer; d: dimer.
The five isozymes produced 15 bands and nine isozyme banding patterns (IBP) (Figure 1): two for POX, three for ACP, one for PGI, two for PGM and one for MDH. The IBP obtained with each isozyme in both phenological stages is described below.

Figure 1 Schematic representation of isozyme banding patterns (IBP) in Sprekelia formosissima using biochemical markers:(A) POX in Tris-citrate system, pH 7.8; (B) ACP in Histidine-citric acid system, pH 6.5; (C) PGI in Histidine-citric acid system, pH 5.7; (D) PGM in Histidine-citric acid system, pH 5.7; (E) MDH in Histidine-citric acid system, pH 5.7. Numbers figure footer represent the number of isozyme banding pattern (IBP), while “o” represents the origin of the electrophoresis.
Peroxidase (POX)
Plants have numerous POX enzymes that differ in location within the plant and between species. POX enzymes have an important function in lignin biosynthesis (Conroy et al., 1982), hormone generation and detoxification of hydrogen peroxide. They are also associated with physical and biochemical processes such as growth, cell formation, development of fruits, ethylene synthesis and response mechanisms to different forms of stress (Matamoros et al., 2003). The expression of this enzyme is in a wide range of plant tissue types during the reproductive stage (Passardi et al., 2004). In our study, the POX system showed four active bands with different migration rates; the bands were grouped into two IBP, one with two bands (1) and one with three bands (2) (Figure 1A). In pattern 1, the side closer to the origin was stronger compared to the second one. Both IBP were observed in the reproductive stage while in the vegetative stage only pattern 1 was observed. During the reproductive stage only two samples were collected from H; in the first sample, pattern 1 was observed and in the third sample extract pattern 2 was observed, which is also expressed in tissues T and E in the same stage, whereas in the two extracts of G, pattern 1 was observed, as shown in Table 1. These results are consistent with those reported by McInnis et al. (2006) who pointed out that in Senecio squalidus during the reproductive stage of this species specific isoforms of peroxidases were identified, which are probably involved in complex process of cellular recognition during the pollination period.
Acid phosphatase (ACP)
In plants, the ACPs are described in several species, tissues and cell compartments, and its main function is the collection and recycling of phosphate (Li et al., 2002; Bozzo et al., 2006). Furthermore, the ACPs exhibit activity during different stages of development such as flowering and germination (Duff et al., 1994; Bozzo et al., 2002). In our study, when using ACP three active bands were detected and three IBPs, the first with three bands, the second with two and the third with a single band (Figure 1B). The existence of differential expression was found through the ACP system, because during the reproductive stage all three IBPs were observed. At this stage, the first sampling of H tissue exhibited the first pattern and in the third sampling we could not detect any band (Table 1). In the tissue sample of T, patterns 2 and 3 were observed (Table 1), while pattern 3 was expressed in tissue samples from G and in the third sample of E. In the second sampling of E we did not observe any band.
During the vegetative stage, the ACP system generated the IBP 1 and 2. The H tissue of the fourth sample exhibited pattern 2 and all remaining tissue samples of H showed pattern 1. These results are similar to those reported in a monocot by Pedersen and Simonsen (1987), who evaluated leaf tissues in different positions of the plant.
Phosphoglucose isomerase (PGI)
The PGI is an enzyme that is actively involved in the metabolism of sugars in most eukaryotic organisms; also it is located in plastids and in the cytosol of angiosperms. In our study the PGI showed two active bands and a single IBP (Figure 1C). This indicates the existence of differential expression throughout the PGI system, in other words, this isozyme showed no tissue specificity but it showed ontogenetic specificity in its expression, hence in the reproductive stage it was observed only in the third sampling of tissues of H, T, G and E and in H tissue only at the beginning of the vegetative stage (fourth sampling) (Table 1). Therefore, this isozyme may have a major role during the flowering process of S. formosissima.
Phosphoglucomutase (PGM)
PGM is actively involved in the metabolism of sugars, it catalyzes the conversion of glucose-1 and glucose-6 phosphate in the synthesis and consumption of sucrose. Mühlbach and Schnarrenberger (1978) indicate that PGM is present in plants and that it is possible to obtain a pattern with two active bands often located in the cytosol and chloroplasts. In our study, in the PGM system we detected differential expression due to the observation of three active bands and two IBP; the first with three bands and the second with one band (Figure 1D). PGM showed very little variability in the enzymatic activity during both phenological stages of development. During the reproductive stage only pattern 1 was obtained in the sampled tissues, but in the third sampling of G tissues, no bands that prove enzymatic activity were observed. In the vegetative stage, pattern 1 was only observed in tissues of the fourth sampling, whereas in H tissues, pattern 2 was observed from the sixth up to the tenth sampling, probably this is due to the increased number of chloroplasts in tissue H (Table 1).
Malate dehydrogenase (MDH)
MDH is an enzyme that, according its subcellular localization, actively participates in the Krebs cycle, photorespiration, and other catabolic and anabolic pathways (Machado et al., 1993). In our study, MDH presented three active bands with a single IBP (Figure 1E), all present in both development stages and all sampled tissues (Table 1). MDH was the only isozyme system in which no differential expression was observed in both phenological stages.
In living organisms DNA is the most important molecule, because it encodes the hereditary information that determines the proteins structure, which, for its expression during development and cell differentiation, they involve factors such as hormones, light type, day length, temperature, etc., This factors are crucial in the regulation of gene expression or in their differential expression (Grierson and Covey, 1991). Isozymes can be used as genetic improvement tools (Arzate-Fernández et al., 2005b) and the process of differential expression has been studied in maize (Scandalios, 1964, Efron, 1970. Rao et al., 1997), tobacco (Stanford and Galston, 1970), barley (Pedersen and Simonsen, 1987) and Brassicca oleraceae (Bellani et al., 2002), using electrophoresis in starch gels with samples of different parts of the plant such as leafs, stems, roots, young and mature ears, sheath, pollen grains, tissue scutellum, and in vitro plant lets.
Conclusions
With the horizontal electrophoresis technique on starch gels, differential isozyme banding patterns were identified throughout two phenological stages of Sprekelia formosissima. This technique has advantages over molecular DNA markers, given that several gels can be obtained at the same time and the gels can be dyed with up to seven enzymes, reducing times and costs.
The IBP obtained in our study with the following biochemical markers: peroxidase, acid phosphatase, glucose phosphate isomerase and phosphoglucomutase indicate that this technique is useful in investigations of differential expression in vegetative and reproductive stages of S. formosissima as well as a diagnosis tool in breeding programs or in a genetic variability or diversity studies.
Literatura citada
Arzate-Fernández, A. M., A. Hoyos-Basurto, L. M. VázquezGarcía, and M. G. Gutiérrez-Martínez. 2005a. Isozyme characterization of nine botanical varieties of Tigridia pavonia (L.f.) DC. Agrociencia 42: 519-528. [ Links ]
Arzate-Fernández, A. M., O. Mejía-González, T. Nakazaki, Y. Okumoto, and T. Tanisaka. 2005b. Isozyme electrophoretic characterization of 29 related cultivars of lily (Lilium spp.). Plant Breeding 124: 71-78. [ Links ]
Balen B., M. Krsnik-Rasol, and V. Simeon-Rudolf. 2003. Isoenzymes of peroxidase and esterase related to morphogenesis in Mammillaria gracillis Pfeiff. tissue culture. J. Plant Physiol. 160: 1401-1406. [ Links ]
Bellani, L. M., M Guarnieri, and A. Scialabba. 2002. Differences in the activity and distribution of peroxidases from three different portions of germinating Brassica oleraceae seeds. Physiol. Plantarum. 114: 102-108. [ Links ]
Bozzo, G. G., K. G. Raghothama, and W. C. Plaxton. 2002. Purification and characterization of two secreted purple acid phosphatase isozymes from phosphate-starved tomato (Lycopersicon esculentum) cell cultures. Eur. J. Biochem. 269: 6278-86. [ Links ]
Bozzo, G. G., E. L. Dunn, and W. C. Plaxton. 2006. Differential synthesis of phosphate-starvation inducible purple acid phosphatase isozymes in tomato (Lycopersicon esculentum) suspension cells and seedlings. Plant Cell Environ. 29: 303-13. [ Links ]
Carpin, S., M, Crevecoeur, H, Greppin, and C. Penel, C. 1999. Molecular cloning and tissue-specific expression of an anionic peroxidase in zucchini. Plant Physiol. 120: 799-810. [ Links ]
Conroy, J. M., D. C. Borzelleca, and L. A. McDonell. 1982. Homology of plant peroxidases on immunochemical approach. Plant Physiol. 69: 28-31. [ Links ]
Duff, S. M. G., G, Sarath, and W. C. Plaxton. 1994. The role of acid phosphatases in plant phosphorus metabolism. Physiol. Plant. 90: 791-800. [ Links ]
Efron, Y. 1970. Tissue specific variation in the isozymes pattern of the AP1 acid phosphatase in maize. Genetics 65: 575-583. [ Links ]
Glaszmann, J. C., B. G. De los Reyes, and G. S. Khush. 1988. Electrophoretic variation or isoenzymes in plumules of rice (Oriza sativa L.) a key to the identification of 76 alleles at 24 loci. IRR Research Paper Series. No. 134. 14 p. [ Links ]
Grierson, D., y S. N. Covey. 1991. Biología Molecular de las Plantas. Ed. Acribia. S.A. Zaragoza, España. pp: 103-127. [ Links ]
Ishikawa, R. 1994. Genetical studies on isozyme genes in rice (In Japanese with English summary). Bulletin Faculty of Agriculture Hirosaki University. 57: 105-180. [ Links ]
Kephart, S. R. 1990. Starch gel electrophoresis of plant isozymes: a comparative analysis of techniques. Am. J. Bot. 77: 693-712. [ Links ]
Li, D. P., H. F. Zhu, K. F. Liu, X. Liu, G. Leggewie, M. Udvardi, and D. W. Wang, 2002. Purple acid Phosphatases of Arabidopsis thaliana. Comparative analysis and differential regulation by phosphate deprivation. J. Biol. Chem. 27: 277-281. [ Links ]
López-Ferrari A. R, y A. Espejo-Serna. 2002. Amaryllidaceae. In: Sosa, V., L. C. Rodríguez, T. Duncan, M. T. Mejía-Saulés, N. P. Moreno, M. Nee, L. I. Nevling, J. Rzedowski J., y B. G. Schubert (eds). Flora de Veracruz. Instituto de Ecología. Xalapa, Veracruz, México. 32 p. [ Links ]
Machado, M. F., A. J. Prioli, and C. A. Mangolin. 1993. Malate dehydrogenase (MDH; EC 1.1.1.37) isozymes in tissues and callus cultures of Cereus peruvianus (Cactaceae). Biochem. Genet. 31: 167-172. [ Links ]
Marquard, R. D., and Ch. R. Chan. 1995. Identifying Crabapple cultivars by isozymes. J. Am. Soc. Hort. Sci. 120: 706-709. [ Links ]
Matamoros, M. A., D. A Dalton, J. Ramos, M. R. Clemente, M. C. Rubio, and M. Becana. 2003. Biochemestry and molecular biology of antioxidants in the rizobia-legume simbiosis. Plant Physiol. 133: 499-509. [ Links ]
McInnis, S. M., D. C. Emery, R. Porter, R. Desikan, J. T. Hancock, and S. J. Hiscock. 2006. The role of stigma peroxidases in flowering plants: insights from further characterization of a stigma-specific peroxidase (SSP) from Senecio squalidus (Asteraceae). J. Exp. Bot. 57: 1835-1846. [ Links ]
Mühlbach, H., and C. Schnarrenberger. 1978. Properties and intracellulardistribution of 2 phosphoglucomutases from spinach leaves. Planta 141: 65-70. [ Links ]
Passardi, F., C. Penel, and C. Dunand. 2004. Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci. 9: 534-540. [ Links ]
Pedersen, S., and V. Simonsen. 1987. Tissue specific and developmental expression of isozymes in barley (Hordeum vulgare L.). Hereditas 106: 59-66. [ Links ]
Rao, K.V., P. Suprasanna, and G. M. Reddy. 1997. Differential expression of esterase and MDH isozymes during in vitro culture in maize (Zea mays L.). Act. Physiol. Plantarum 19: 29-32. [ Links ]
Ros-Barceló, A., and G. J. Aznar-Asensio. 2002. Basic peroxidases in cell walls of plants belonging to the Asteraceae family. J. Plant Physiol. 159: 339-345. [ Links ]
Scandalios, J. G. 1964. Tissuespecific isozymes variation in maize. The J. Heredity: 281-285. [ Links ]
Stafford, H. A., and A. W. Galston. 1970. Ontogeny and hormonal control of polyphenoloxidase isozymes in tobacco pith. Plant Physiol. 46: 763-767. [ Links ]
Stuber, C. W., J. Wendel, M. Goodman, and J. Smith. 1988. Techniques and scoring procedures for starch gel electroforesis of enzymes from maize (Zea mays L.) Tech. Bull. 286. North Carolina State University. Raleigh, North Carolina. 217 p. [ Links ]
Torres, A. M., R. K. Soost, and U. Diedenhofen. 1978. Leaf isozymes as genetic markers in citrus. Am. J. Bot. 65: 869-881. [ Links ]
Vallejos, E. 1983. Enzyme activity staining. In: Tanksley, S. D., and T. J. Orton (eds). Isozymes in Plant Genetics and Breeding, Part A. Elsevier, Amsterdam, Netherlands. pp: 416-459. [ Links ]
Vázquez-García, L. M., T. H. Norman-Mondragón, y M. del C. Corona-Rodríguez. 1998. El Lirio Azteca. Colección: Ciencias Naturales y Exactas, Serie: Ciencias Agrícolas. Universidad Autónoma del Estado de México. Toluca, Estado de México. 29 p. [ Links ]
Vicente, M. C., y T. Fulton. 2003. Tecnologías de Marcadores Moleculares para Estudios de Diversidad Genética de Plantas: Módulo de Aprendizaje. Illus. Nelly Giraldo. Instituto Internacional de Recursos Fitogenéticos (IPGRI), Roma, Italia. 1: 1-52. [ Links ]
Wendel, J. F., and N. F. Weeden. 1990. Visualization and interpretation of plant isozymes. In: Soltis D. E., and P. S. Soltis (eds). Isozymes in Plant Biology. Dioscorides Press. Portland, Oregon. pp: 5-45. [ Links ]
Received: February 2016; Accepted: August 2016











 texto en
texto en 


