Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.50 no.7 Texcoco Out./Nov. 2016
Plant protection
Cacarizo of nopal in Opuntia ficus-indica L. Miller, Milpa Alta, México City
1Posgrado en Fitosanidad, Entomología y Acarología, Colegio de Postgraduados, Carretera México-Texcoco Km 36.5, Montecillo. 56230. Texcoco, Estado de México, México (mpalomares@colpos.mx)
2 Departamento de Parasitología Agrícola, Universidad Autónoma Chapingo. 56230. Chapingo, Estado de México, México.
Milpa Alta, in the south of Mexico City, is the most important production zone of nopal (Opuntia ficus-indica) in the world, and is consumed as a vegetable. In this location, there are symptoms known as nopal cacarizo, related to the presence of red chinch bug (Hesperolabops nigriceps), and despite the importance of the crop, there are no studies about the distribution of nopal cacarizo. To determine the distribution, incidence and severity of the disease, samplings were made in vegetable nopal plantations in different geographic points of Milpa Alta. A logarithmic diagram scale was constructed to evaluate its severity, and correlated to nopal cacarizo with H. nigreceps. Aggregation and dispersal indices of Morisita and Lloyd were also estimated. Nopal cacarizo is distributed throughout Milpa Alta with some aggregation in the west (Morisita=1.768; Lloyd=2.535), as is H. nigriceps (Morisita=1.003; Lloyd=1.006). Furthermore, there was a close relationship between nopal cacarizo and H. nigriceps (r=0.765). Damage to individual plants is concentrated in the basal, second and third cladodes. Using the logarithmic diagrammatic scale, with acceptable precision (r2=0.988) and exactness (b1=1.012), an incidence of 56.4±18.5 % was established along with a severity of 10.5±6.4 % of nopal cacarizo in Milpa Alta. This is evidence of the importance of the symptoms in plants of Opuntia, the need to know the phenomenon and the conditions that favor its dispersion.
Key words: Hesperolabops nigriceps; vegetable nopal; symptoms
Milpa Alta, al sur de la Ciudad de México, es la zona productora de nopal (Opuntia ficus-indica) más importante en el mundo y es consumido como verdura. En este lugar, hay una sintomatología denominada cacarizo del nopal relacionada con la presencia de la chinche roja (Hesperolabops nigriceps) y, a pesar de la importancia del cultivo, no hay estudios de la distribución del cacarizo del nopal. Para determinar la distribución, incidencia y severidad de la sintomatología se realizaron muestreos en huertas de nopal verdura en diferentes puntos geográficos de Milpa Alta, se construyó una escala logarítmica diagramática para evaluar la severidad, se correlacionó el cacarizo del nopal con H. nigriceps y se estimaron los índices de agregación y dispersión de Morisita y Lloyd. El cacarizo del nopal se distribuye en todo Milpa Alta con alguna agregación en el oeste (Morisita=1.768; Lloyd=2.535), igual que H. nigriceps (Morisita=1.003; Lloyd=1.006). Además hubo una relación estrecha entre cacarizo del nopal y H. nigriceps (r=0.765). El daño a las plantas individuales se concentra en el cladodio basal, segundo y tercero. Con la escala logarítmica diagramática, con precisión (r2=0.988) y exactitud (b1=1.012) aceptables, se estableció una incidencia de 56.4±18.5 % y una severidad de 10.5±6.4 % del cacarizo del nopal en Milpa Alta. Esto evidencia la importancia de la sintomatología en plantas de Opuntia, la necesidad de conocer el fenómeno y las condiciones que favorecen su dispersión.
Palabras clave: Hesperolabops nigriceps; nopal verdura; sintomatología
Introduction
Mexico is one of the most important locations as center of origin and domestication of prickly pear or nopal, Opuntia ficus-indica L. (Miller) (Caryophyllales: Cactaceae) (Casas and Barbera, 2002; Griffith, 2004). This plant is exploited in countries of semi-arid and arid zones (Le Houérou et al., 1993; Griffith, 2004); it is used as forage and its fruits are used for human consumption. In Mexico the immature cladodes are also consumed as a vegetable, and are known as nopalitos, or vegetable nopal (Flores, 2001), and approximately 11 000 ha are under cultivation (SIAP, 2013).
In Milpa Alta, in the south of Mexico City, approximately 4,000 ha of vegetable nopal are cultivated (SIAP, 2013). The plants present symptoms known as nopal cacarizo, which is manifested as ochre colored pustules in the mature cladodes. The initial damage appears approximately 5 d after the red chinch bug, Hesperolabops nigriceps Reuter (Hemiptera: Miridae) introduces its stylet in the cladode to feed. In this site a small spot appears with a chlorotic halo which is transformed into a pustule on the epidermis of the cladode. Later, the pustules coalesce and acquire tones that range from yellow to ochre, until they burst, probably due to destruction and dehydration of the tissue sections. Time from onset of the symptoms to final manifestation of damage is approximately 30 d (Palomares-Pérez et al., 2010).
Some producers and agricultural technicians relate nopal cacarizo to the apparent decrease in the photosynthetic rate of the plants and of production, but there is no scientific evidence that confirms this hypothesis. The available information relates these symptoms to the presence and damage from the red chinch bug, Hesperoloabops spp. (Hemiptera: Miridae) (Melgarejo-Moreno, 2000; Méndez-Gallegos et al., 2008; Palomares-Pérez et al., 2010). In some technical manuals it is suggested that nopal cacarizo could result from the interaction of the red chinch bug and some phyto-pathogenic fungus (Mena-Covarrubias and Roas-Gallegos, 2004), but in the revised scientific literature there is no evidence that these symptoms could be considered of pathogenic origin.
Despite the importance of the cultivation of vegetable nopal in the southern region of Mexico City, no studies have been developed to evaluate the damages or distribution of nopal cacarizo (Mann, 1969; Badii and Flores, 2001; Ayala-Escobar et al., 2006; Quezada-Salinas et al., 2006). Therefore, the objective of this research was to determine the incidence, severity and distribution of nopal cacarizo in the region of Milpa Alta, and to confirm the relationship of nopal cacarizo with the presence of H. nigriceps.
Materials and methods
Study area
The Milpa Alta area, with a surface of 28,375 ha (Diario Oficial de la Federación, 1997) is located in the southeast sector of Mexico City between 19° 04’ and 19° 12’ N and 98° 57’ and 99° 08’ W. It has mountainous topography and altitudes of 2,230 to 3,200 m (Rodríguez and López, 2006). The climate is sub-humid temperate with rains in summer [C(w2)(w)], mean annual temperature of 14.4 °C and annual rainfall of 878.9 mm (García, 1988). Rainfall is heterogenic in its distribution, from 580.6 mm in the lower altitudes to 1,200 mm in the higher elevations (IMTA, 1996).
Logarithmic diagrammatic scale
To evaluate the incidence and severity of nopal cacarizo, a logarithmic diagrammatic scale was constructed using the methodology proposed by Mora-Aguilera et al. (2003)[2]. A digital camera of 5 mega pixels was used to randomly photograph 60 mature cladodes of various levels of severity of nopal cacarizo. Then, with the image editor of Adobe Photoshop (Adobe Sytem Incorporated, 2010), the area invaded by the nopal cacarizo was measured using the program ImageTool® (Wilcox et al., 2002) and the upper and lower limits were determined. Once the maximum limit of the scale was defined, this value was put in the program 2LOG ver. I (Osada-Velázquez and Mora-Aguilera, 1997[3] to define the number of classes (Tovar-Soto et al., 2002; Mora-Aguilera et al., 2003[2]; Gomes et al., 2004).
The program 2LOG ver. 1 was used to obtain a logarithmic scale that was converted to a diagrammatic scale through the association of a photographic image whose severity best approximated the mean points of each class. Thus, the diagrammatic scale was defined with five classes (0-4) (Figure 1).
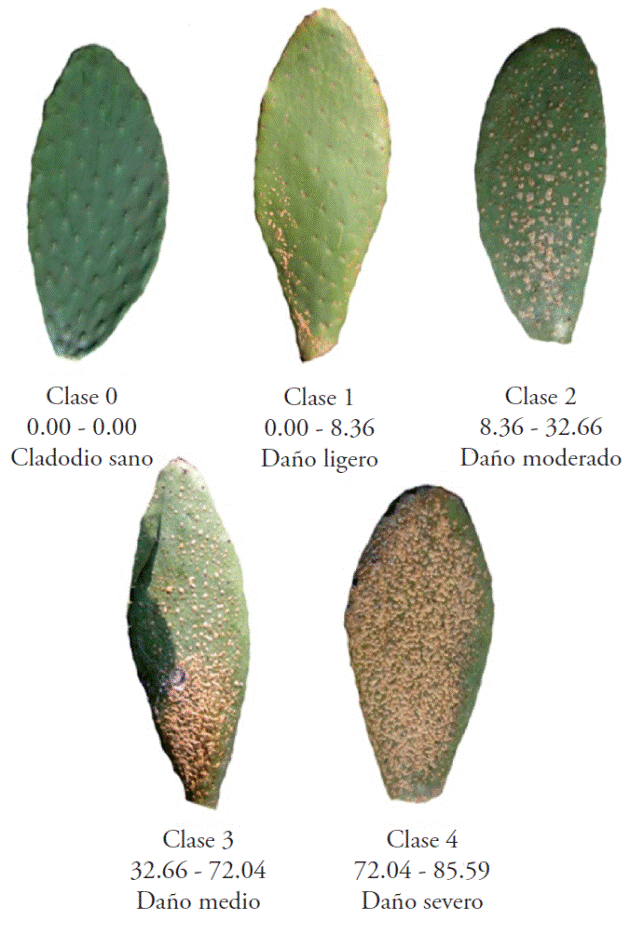
Figure 1 Logarithmic diagrammatic scale of severity of nopal cacarizo. Ranges represent the percentage of the average area invaded in the cladodes.
The diagrammatic scale was validated before using it, for which the real values obtained with the program PhotoShop and Image Tool were used, and those obtained for the evaluator. These data were used to make an analysis of simple linear regression with the program Validar-PER ver. 1.1 (Mora-Aguilera, 2009[4]) and the values of r2 and b1 close to 1.0 indicated maximum precision and exactness, respectively.
Sampling
Simple stratified sampling was carried out based on altitude and cardinal points in Milpa Alta in the spring-summer of 2010. For the distribution of the crop in the study area, five sampling zones were defined by altitude (masl): 1 3070 to 2720, II) 2919 to 2770; III) 2769 to 2620; IV) 2619 to 2470 and V) 2469 to 2320, and in each zone four sampling sites were selected; 20 plots of 5 to 10 years of age and whose plants did not exceed 1.60 m height, where the principal management system of pests and diseases was by fertilization with organic manure and chemical control.
Incidence
Incidence was evaluated as the presence or absence of nopal cacarizo on O. ficus-indica. In each plot 10 randomly selected plants were examined. The response variables were as follows: number of cladodes per plant, and number of cladodes with at least one pustule of nopal cacarizo. Each sampling point was geo-referenced with the global positioning system (GPS 12 XL Garmin Olathe, KS, USA). Determination of incidence was carried out according to the proportion or percentage of plants with symptoms using the following formula (Tovar-Soto et al., 2002):
Severity
To evaluate severity (area of the cladode affected by nopal cacarizo), 10 plants were randomly selected from each plot with the size indicated above. In each plant an estimation was made of the degree of damage in three strata using the logarithmic diagrammatic scale. The basal and second cladodes were considered as low stratum, third cladode as middle stratum and those that grow above the third cladode as upper stratum. Total number of cladodes per stratum was determined along with the number of cladodes per level of damage per stratum. Severity was estimated using the following formula (Tovar-Soto et al., 2002):
The data obtained of incidence and severity were captured on a calculation sheet of the program Excel and were imported to the program SURFER 32 ver. 6.04 (Surface Mapping System, 1997) to determine the distribution of the nopal cacarizo. In addition, the dimension of both was estimated graphically with the data previously recorded on the calculation sheet and later as a graph. Then the options “Classed post and Contour” were used to generate bi and tridimensional maps.
Identification of the insect
To confirm the species of red chinch bug, five specimens were collected from each sampling site and they were taken to the entomology laboratory of the Plant Health department of the Colegio de Postgraduados mounted on entomological needles. The insects were then identified with a dissection microscope and with the keys for the genus Hesperolabops, published by Froeschner (1967). In addition, the species was confirmed by Dr. Harry U. Brailovsky of the Biology Institute of UNAM, specialist in the family Miridae. The specimens were kept in the taxonomy laboratory of the Agricultural Parasitology Department of the Universidad Autónoma Chapingo.
Statistical analysis
For the distribution of nopal cacarizo in the field, the calculations of aggregation and spatial dispersion were used, which were determined through the indices of Morisita and Lloyd (Campbell and Madden, 1990) using the program MorLloyd version 1.0 (Rivera-Valencia and Mora-Aguilera, 2010[5]. The aggregation and spatial dispersion index of H. nigriceps was estimated with the same program.
The experimental design was completely randomized with five treatments; each altitude was considered a treatment and the four plots within each altitude were the replicates. Given that the data did not have normal distribution, even when transformations were attempted (Shapiro-Wilk), they were processed using the non-parametric test of Friedman. Tests were also made of simple correlation between the presence of H. nigriceps and the incidence of nopal cacarizo. The variables were healthy cladode, cladode with nopal cacarizo and the presence of red chinch bug, and cladode with the presence of red chinch bug without nopal cacarizo.
Results and discussion
Incidence and severity of nopal cacarizo
The validation of the logarithmic diagrammatic scale to determine severity of nopal cacarizo, estimated by regression analysis, presented a precision of r2=0.988 and an exactness of b1=1.012, which indicated that the values of severity were adequate for this evaluation. The incidence and severity of the nopal cacarizo in Milpa Alta were 56.4±18.5 % and 10.5±6.4 %, respectively. According to the aggregation indices of Morisita (0.990) and of dispersion of Lloyd (0.980), the incidence of nopal cacarizo presented a uniform type distribution in the region (Figure 2A), whereas the severity was of the aggregated type Morisita (1.767) and Lloyd (2.535) Figure 2B). These data suggest a possible vegetative transmission of the causal agent influenced by agronomic management, as occurs with other phytosanitary problems such as chlorotic spots associated with O. ficus-indica (Alonso-Barrera et al., 2015).
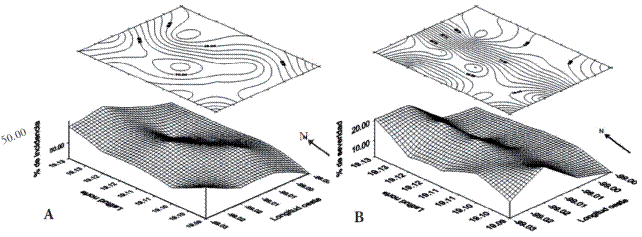
Figure 2 Spatial distribution of incidence (A: uniform type) and severity (B: aggregated type) of nopal cacarizo in Milpa Alta, Mexico City, 2010.
Although the bidimensional maps and the indices of Morisita and Lloyd reveal certain aggregation of the severity of nopal cacarizo in the western zone of Milpa Alta, no statistical differences were found between altitudinal categories (F=0.57; p=0.943), nor between the cardinal points (F=1.0; p=0.464).
Individually, in each plant the highest concentration of nopal cacarizo was measured in the low and middle strata, where the mother, second and third cladodes are located (Table 1) and which correspond to the cladodes of highest age in a plantation and highest concentration of red chinch bug, an observation which coincided with that registered by Palomares-Pérez et al. (2010).
Table 1 Severity of nopal cacarizo by strata in plants of vegetable nopal, O. ficus-indica, in Milpa Alta, Mexico City.
| Grado de severidad (escala diagramática) | Porcentaje de severidad por estrato† | ||
| Bajo (cladodios de 5 a 8 años) | Medio (cladodios de 6 meses a 5 años) | Alto (cladodios menores a 6 meses) | |
†Mean ± standard deviation.
Association of nopal cacarizo-Hesperolabops nigriceps
All of the specimens of chinch bugs collected in the plots corresponded to H. nigriceps, and there was a significant correlation between the presence of H. nigriceps and nopal cacarizo (r=0.765). Furthermore, the indices of Morisita (1.003) and Lloyd (1.006) indicated that the concentration of red chinch bug in Milpa Alta was aggregated and coincided with the areas (west) where the highest severity of nopal cacarizo was concentrated (Figure 3). It is possible that the western topographical zone presents the optimum conditions for the development of this problem or that the phytosanitary management, principally in the cutting and transport of the cladode to be planted, are the factors that favor the development of this damage, as occurs with the dissemination of other problems of nopal such as black spot or chlorotic spots (Quezada-Salinas et al., 2006; Alonso-Barrera et al., 2015).
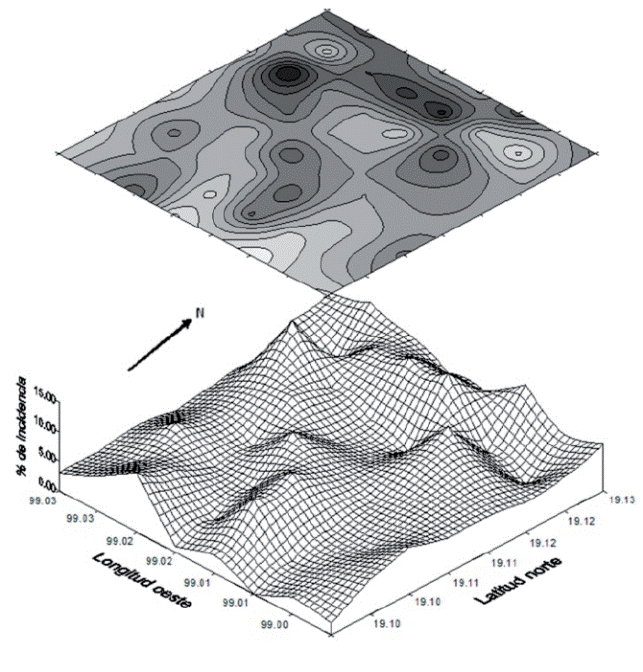
Figure 3 Spatial distribution of the red chinch bug, Hesperolabops nigriceps, in the production zone of vegetable nopal in Milpa Alta, Mexico City.
Nopal cacarizo is a phytosanitary problem of the first order in Milpa Alta according to some technical reports. With this study the symptoms of nopal cacarizo can be considered important and establish that the visual estimations made in the technical studies are probably overestimated, as occurs with other symptoms that are manifested on leaves or stems in the plant of interest (Cadena-Hinajosa et al., 2003; Soltero-Díaz and Williams-Alanís, 2003).
A fundamental factor in the level of damage or severity was the age of the cladodes. The dry pustules are practically limited to the mature cladodes of more than one year of age, while the young cladodes rarely presented this symptom. Palomares-Pérez et al. (2010) mention that the young cladodes are cut by the producers when they present any damage that affects their appearance in the market, and this is probably one of the reasons why the damage is observed only on the mature cladodes and is related to the places where the red chinch bug prefers to feed. Perhaps the concentration of nutrients in mature and immature cladodes, as is reported in studies of nutrition in nopal (Villareal et al., 1963; Saag et al., 1975; Bravo-Hollins, 1978; McGarvie and Parolis, 1979, 1981), is important for defining the feeding locations of this species of insect. In addition to the preference for the feeding sites for H. nigriceps, the symptoms are cumulative, that is, the mature cladodes may have symptoms caused by the feeding of chinch bugs from previous years.
Although no differences were detected between incidence and severity of nopal cacarizo at different altitude and cardinal points, it was evident that using the spatial interpretation and the indices of Morisita and Lloyd, the symptoms presented certain tendency of aggregation in the western zone, where the populations of H. nigriceps were concentrated. This confirmed the relationship nopal cacarizo-H. nigriceps and the aggregation habit of this species, as occurs with other species with gregarious habits of the same family such as Lygus Hesperus Knight and Sahlbergella singularis Haglung (Schotzko and O’Keeffe, 1989; Bisseleus et al., 2011).
The presence of microclimates due to altitudinal changes is a factor which affects the distribution of bees and other pollinating insects (Gonçalves and Stort, 1978; Salamanca-Grosso, 2009). In the case of H. nigriceps the presence of microclimates in the region of Milpa Alta, related to altitude (García, 1988; IMTA, 1996; Rodríguez and López, 2006) does not seem to have an important effect on the distribution of the insect and nopal cacarizo. This could be attributed, at least in part, to the capacity of this species of insect to exploit the nopal resource in the environment of Milpa Alta.
One factor that could influence the distribution of nopal cacarizo is crop management. Although it is the area with greatest surface destined to this crop in Mexico (SIAP, 2013), in Milpa Alta agriculture is a secondary activity and the producers destine relatively less time to management tasks and pest control, including H. nigriceps, compared with producers whose principal activity is agriculture (Vanegas-Rico et al., 2010). Furthermore, apparently not enough attention is given to the origin of cladodes that are used to establish new plantations. The cycle of H. nigriceps is one year and the sites of oviposition in the cladode are not evident (Palomares-Pérez, unpublished data), therefore it is probable that eggs of the insect are present in the material used to establish new plantations (Badii and Flores, 2001), thus favoring the dispersion of the red chinch bug and nopal cacarizo. To date there is no information of the effect of nopal cacarizo on O. ficus-indica. Nevertheless, due to the distribution of these symptoms in Milpa Alta, and the severity that can be reached in some plots, it is convenient to study this phenomenon and its possible consequences on the productive life of the plants.
Conclusion
Nopal cacarizo has uniform distribution in 56% of the area of Milpa Alta, and severity of 10 %. There is positive correlation between nopal cacarizo and the presence of Hesperolabops nigriceps. Besides, management activities of the crop may favor the presence and dispersion of the symptoms.
Literatura citada
Adobe System Incorporated. 2010. Adobe Photoshop CS6 for Windows. [ Links ]
Alonso-Barrera, B., G. Mora-Aguilera, G. Valdovinos-Ponce, D. L. Ochoa-Martínez, E. Rodríguez-Leyva, B. Tlapal-Bolaños, y R. De la Torre-Almaraz. 2015. Asociación de Potexvirus como agente causal de manchas cloróticas en Opuntia ficusindica. Rev. Mex. Fitopatol. 33: 75-86. [ Links ]
Ayala-Escobar, V., M. de J. Yáñez-Morales, U. Braun, J. Z. Groenewald, and P. W. Craus. 2006. Pseudocercospora opuntiae sp. nov., the causal organism of cactus leaf spot in Mexico. Fungal Divers. 21: 1-9. [ Links ]
Badii, H. M., and E. A. Flores. 2001. Prickly pear cacti pest and their control in Mexico. Fla. Entomol. 84: 503-505. [ Links ]
Bisseleua, D. H., B. Yede, and S. Vidal. 2011. Dispersion models and sampling of cacao mirid bug Sahlbergella singularis (Hemiptera: Miridae) on Theobroma cacao in Southern Cameroon. Environ. Entomol. 40: 111-119. [ Links ]
Bravo-Hollins, H. 1978. Las Cactáceas de México. vol. I. UNAM, México. 743 p. [ Links ]
Cadena-Hinojosa, M. A., R. Guzmán-Plazola, M. Díaz-Valasis, T. E. Zavala-Qiontana, O. S. Magaña-Torres, I. H. Almeyda-León, H. López-Delgado, A. Rivera-Péña, y O. Rubio-Covarrubias. 2003. Distribución, incidencia y severidad del pardeamiento y la brotación anormal en los tubérculos de papa (Solanum tuberosum L.) en valles altos y sierras de los estados de México. Rev. Mex. Fitopatol. 21: 248-259. [ Links ]
Campbell, C. L., and L. V. Madden. 1990. Introduction to Plant Disease Epidemiology. John Wiley and Sons. New York, USA. 532 p. [ Links ]
Casas, A., and G. Barbera. 2002. Mesoamerican domestication and diffusion. In: Nobel, P. S. (ed.) Cacti Biology and Uses. University of California, Berkeley. pp: 143-162. [ Links ]
Diario Oficial de la Federación. 1997. Programa delegacional de desarrollo urbano de Milpa Alta. http://www.sideso.df.gob.mx/documentos/progdelegacionales/milpa[1].pdf [ Links ]
Flores, C. 2001. Producción, Industrialización y Comercialización de Nopalitos. Centro de Investigaciones Económicas, Sociales y Tecnológicas de la Agroindustria y la Agricultura Mundial. Universidad Autónoma Chapingo. Chapingo. México. 27 p. [ Links ]
Froeschner, C. R. 1967. Revision of the cactus plant bug genus Hesperolabops spp. Kirkaldy (Hemiptera: Miridae). Proc. United States National Museum, USA. 123: 1-11. [ Links ]
García, E. 1988. Modificaciones al Sistema de Clasificación Climática de Köppen, 5a ed., Instituto de Geografía, UNAM, México 90 p. [ Links ]
Gomesa, M. A., S. J. Michereff, y R. L. M. Mariano. 2004. Elaboração e validação de escala diagramática para Cercosporiose da alface. Summa Phytopathol. 30: 38-42. [ Links ]
Gonçalves, L. S. and A. C. Stort. 1978. Honey bee improvement through behavioral genetics. Annu. Rev. Entomol. 23: 197-213. [ Links ]
Griffith, M. P. 2004. The origins of an important cactus crop, Opuntia ficus-indica (Cactaceae): new molecular evidence. Am. J. Bot. 91: 1915-1921. [ Links ]
IMTA. 1996. Extractor rápido de información climatológica. Instituto Mexicano de Tecnología del Agua. México. In: Rodríguez-Gamiño, M. de L., y J. López-Blanco (eds). Caracterización de unidades biofísicas a partir de indicadores ambientales en Milpa Alta, Centro de México. Investigaciones Geográficas, Boletín del Instituto de Geografía, UNAM, México 60: 46-61. [ Links ]
Le Houérou, H. N., G. F. Popov, and L. See. 1993. Agro-Bioclimatic Classification of Africa. Agrometeorology Series N° 6. Rome, Italy: FAO. 227 pp. [ Links ]
Mann, J. 1969. Cactus-feeding insects and mites. Bull. United States National Museum 256: 1-158. [ Links ]
McGarvie, D., and H. Parolis. 1979. The mucilage of Opuntia ficus-indica. Carbohydr. Res. 69: 171-179. [ Links ]
McGarvie, D., and H. Parolis. 1981. Methylation analysis of the mucilage of Opuntia ficus-indica. Carbohydr. Res. 88: 305-314. [ Links ]
Mena-Covarrubias, J., y S. Rosas-Gallegos. 2004. Guía para el Manejo Integrado de las Plagas del Nopal Tunero. Publicación especial Num. 14. Segunda reimpresión, Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación; Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias, México. 34 p. [ Links ]
Méndez-Gallegos, S. de J., D. Talavera-Magaña, y E. J. García-Herrera. 2008. Identificación y control de las enfermedades más comunes en el nopal. Rev. Salud Pública y Nutrición 14: 105-113. [ Links ]
Melgarejo-Moreno, P. 2000. Tratado de Fruticultura para Zonas Áridas y Semiáridas. Vol. I. Ediciones Mundi-Prensa. Madrid, España, 382 p. [ Links ]
Palomares-Pérez, M., E. Rodríguez-Leyva, H. Brailowsky, and S. Ramírez-Alarcón. 2010. First record of Hesperolabops nigriceps Reuter (Hemiptera: Miridae) on Opuntia ficus-indica in Milpa Alta, México City. Neotrop. Entomol. 39: 829-830. [ Links ]
Quezada-Salinas, A., J. S. Sandoval-Islas, D. Alvarado-Rosales, y E. Cárdenas-Soriano. 2006. Etiología de la mancha negra del nopal (Opuntia ficus-indica Miller) en Tlalnepantla, Morelos, México. Agrociencia 40: 641-643. [ Links ]
Rodríguez, G. M., y B. J. López. 2006. Caracterización de unidades biofísicas a partir de indicadores ambientales en Milpa Alta, centro de México. Investigaciones Geográficas, Boletín del Instituto de Geografía, UNAM, México 60: 46-61. [ Links ]
Saag, K. M. L., G. Sanderson, P. Moyna, and G. Ramos. 1975. Cactaceae mucilage composition. J. Sci. Food Agric. 26: 993-1000. [ Links ]
Salamanca-Grosso, G. 2009. Variabilidad genética del ADN mitocondrial de poblaciones de abejas Apis mellifera (Hymenoptera: Apidae) en Colombia. Zootec. Trop. 27: 373-382. [ Links ]
Schotzko, D. J., and L. E. O’Keeffe. 1989. Geostatistical description of the spatial distribution of Lygus hesperus (Heteroptera: Miridae) in lentils. J. Econ. Entomol. 82: 1277-1288. [ Links ]
SIAP (Servicio de Información Agropecuaria y Pesquera). Cierre de la producción agrícola por cultivo. 2013. Anuario Estadístico de la Producción Agrícola http://www.siap.gob.mx/cierre-de-la-produccion-agricola-por-cultivo/ [ Links ]
Soltero-Díaz, L., y H. Williams-Alanís. 2003. Incidencia y severidad del cornezuelo (Claviceps africana, Frederickson, Mantle and de Millano) en híbridos comerciales de sorgo (Sorghum bicolor (L.) Moench.) en la Ciénaga de Chapala, Jalisco, México. Rev. Mex. Fitopatol. 21: 26-32. [ Links ]
Surface Mapping System. 1997. Surfer (Win32) Version 6.04. Copyright® 1993-97, Golden Software, Inc. [ Links ]
Tovar-Soto, S. A., M. M. Hernández, J. Cristóbal-Alejo, J. Romero-Hijo, y G. Mora-Aguilera . 2002. Escala logarítmica diagramática de severidad de la mancha negra (Colletotrichum gloesporoides Penz.) en chirimoyo (Annona chirimolla Mill.). Rev. Mex. Fitopatol. 20: 103-109. [ Links ]
Vanegas-Rico, J. M., J. R. Lomeli-Flores, E. Rodríguez-Leyva, G. Mora-Aguilera, y J. M. Valdez. 2010. Enemigos naturales de Dactylopius opuntiae (Cockerell) en Opuntia ficus-indica (L.) Miller en el centro de México. Acta Zool. Mex. (n.s.) 26: 415-433. [ Links ]
Villareal, F., P. Rojas-Mendoza, V. Arellano, y J. Moreno. 1963. Estudio químico sobre seis especies de nopales (Opuntia spp.). Ciencia Mx. 22: 59-65. [ Links ]
Wilcox, D., B. Dove, D. McDavid, and D. Greer. 2002. Image Tool for Windows ver.3.00. UTHSCSA. [ Links ]
Received: October 2015; Accepted: April 2016











 texto em
texto em 


