Introduction
The genus Crataegus (Rosaceae: Maloideae; Hummer and Janick, 2009) includes around 280 species that grow in different temperate regions of the Northern Hemisphere in the form of thorny shrubs or small trees with bright green leaves and white flowers (Chang et al., 2002). The fruits are berries that are yellow to red and dark purple when ripe (Chang et al., 2002; Özcan et al., 2005). Hawthorn fruit (Crataegus spp.), or tejocote, as it is called in Mexico (Martínez, 1979), is known as a source of good quality pectin (Wang et al., 2007), with potential applications in the food, pharmaceutical, and a number of other industries, derived from its ability to form gels (Thakur et al., 1997). The field of postharvest preservation of horticultural commodities requires the use of materials such as pectin to formulate biopolymeric films for use as coatings to provide protection against mechanical, chemical or microbiological damage and extend shelf life (Falguera et al., 2011). Nowadays, there is greater awareness of environmental conservation and, based on advantages such as edibility, biodegradability, biocompatibility, and barrier properties (Alparslan et al., 2014), edible films constitute an alternative to plastics for establishment of modified atmospheres. A polymer such as pectin can be suitable for active packaging (Pérez-Espitia et al., 2014). In fact, pectin is applied on processed products (Castro-Freitas et al., 2009), although it was used in combination with other raw materials such as maize starch (Fishman et al., 2000), soy flour (Mariniello et al., 2003), and banana flour (Sothornvit and Pitak, 2007).
Pectin for industrial purposes is obtained from apple pomace and citrus peels (Galus and Lenart, 2013; Pérez-Espitia et al., 2014), but other sources such as mango fruit pulp were explored (Azeredo et al., 2009). In this regard, the hawthorn fruit could be a good source of pectin for use in biopolymeric film formulations, with potential to be included in postharvest conservation strategies of fresh produce, but its characterization for that end has not been carried out. Hawthorn is an underutilized species in Mexico (Nieto-Ángel, 2007), but because of its composition and antioxidant potential, the fruit could be more highly valued (García-Mateos et al., 2012; 2013). Thus, extraction and use of pectin could be an alternative strategy for adding value to this plant genetic resource.
Pectin is highly hydrophilic because it is composed of at least 17 kinds of monosaccharides, of which D-galacturonic acid is the most abundant, followed by D-galactose or L-arabinose, all covalently interconnected to one another (Yapo, 2009). For this reason, the inclusion of a lipid compound may be required during formulation of biopolymeric coatings in order to regulate the hydrophilic-lipophilic balance. For our study, candelilla wax, a natural product obtained from the plant Euphorbia antisyphilitica, was selected to be used in biopolymeric film formulations with hawthorn pectin, because it is recognized as safe (GRAS) (FDA, 2003) and its films have been shown to be good barriers to water vapor (Chick and Hernandez, 2002). Thus, the objectives of this study were to: 1) formulate and evaluate candelilla wax in hawthorn pectin emulsions; 2) obtain edible films by casting the emulsions and evaluating their functional properties; and 3) evaluate the potential application of candelilla wax in hawthorn pectin emulsions in the postharvest conservation of fresh produce. In order to attend the last objective, the oyster mushroom Pleurotus ostreatus was selected as experimental material because it has high economic importance (Gregori et al., 2007), but its shelf life is short due to high respiration and transpiration rates (Villaescusa and Gil, 2003).
Materials and methods
Materials
Hawthorn fruit came from accession 55 of the gene bank of Universidad Autónoma Chapingo, Mexico (19° 29’ 3” N, 98° 53’ 37” W, 2250 masl). Candelilla wax (CW) was provided by Cedrosa S. A. de C. V., Mexico. Other used materials were citrus pectin (CP; methoxyl content 72.9 %; Laitz® S. A. de C. V.; Mexico), carboxymethyl cellulose (CMC; 700 000 g mol-1; Meyer® S. A. de C. V., Mexico), sorbitan monostearate (S60; Laitz® S. A. de C. V., Mexico), glycerol (as a plasticizer), and ethanol (96 %; Meyer® S. A. de C. V., Mexico).
Pectin extraction
Hawthorn pectin (HP) was extracted following the method of Yapo et al. (2007). Fruit pulp was mixed with 0.1 N HCl (1000 mL per 100 g of flesh), heated to 85 °C for 45 min, and filtered through muslin cloth. The filtrate was mixed (1:1) with ethanol (96 % v/v), left to stand at 4 °C for 24 h; after this time the supernatant was decanted. This procedure was repeated three times. The combined precipitates were washed three times with a mixture of ethanol:water (70:30, v/v). Subsequently, the mixture was centrifuged (Sorvall RC-5B centrifuge; Du Pont Instruments, MN, USA) and pectin was purified in distilled water with dialysis membrane (Spectra/ Por 6 Dialysis Membrane, 12 kDa; Spectrum Laboratories Inc., CA, USA). Finally, the polysaccharide was dried in an oven (Memmert, Wisconsin Oven Distributions, LLC, WI, USA) at 30+-2 °C for 24 h. Pectin yield was calculated on a dry basis (Y p , %) with equation (1), where m p and m f are mass (g) of pectin obtained and original amount of hawthorn pulp used, respectively:
The esterification degree (ED, %) was evaluated with the method of Singthong et al. (2004), with 500 mg dry pectin mixed with 2 mL ethanol and 100 mL distilled water. After titration with 0.5 N NaOH (V A , mL), 10 mL of 0.5 N NaOH were added while shaking and subsequently left in repose for 15 min. The mixture was added to 10 mL of 0.5 N HCl and again titrated with 0.5 N NaOH (V B , mL). ED (%) was calculated with equation (2):
Emulsion preparation
Four oil-in-water (O/W) emulsions with different compositions were made. The disperse and continuous phases were prepared separately. The disperse phase comprised CW (0.5 or 1.0 g)+S60 (0.5 or 1.0 g)+glycerol (1.26 g) per 100 g emulsion. The continuous phase had HP or CP (1.0 or 2.0 g)+CMC (0.5 g)+the requisite amount of water to complete 100 g of emulsion. Both disperse and continuous phases were heated to 75+2 °C and mixed separately with an Ultra Turrax T50 homogenizer (IKA Labortechnik, Staufen, Germany). The disperse phase was then added drop by drop to the continuous phase with constant shaking using the same homogenizer at 10 000 rpm for 10 min, maintaining the temperature at 75 °C. The emulsions were coded as E0.5CW,1HP and E0.5CW,1CP , having a disperse phase mass fraction (φ) of 0.0226, and E1CW,2HP and E1CW,2CP , having a φ=0.0326.
Emulsion characterization
The mean volume-surface diameter (d3,2) of the wax droplets in emulsions was measured with a particle size analyzer (Malvern Instruments, Ltd., UK), using samples of 1 mL diluted in distilled water until an obscuration index of 16 % was registered in the equipment. In addition, rheological behavior was evaluated with a Physica MCR 301 rheometer (Anton Paar®, Messtechnik, Germany) to determine the flow properties of emulsions, with cone-plate geometry, in which the rotating cone had a diameter of 50 mm and an angle of 1°. Steady shear measurements were performed at 20 °C by applying shear rates of 0.001 to 1000 s-1 and apparent viscosity was recorded as a function of shear rate.
Film preparation
Aliquots of 6 mL from each emulsion were poured into 9 cm diameter glass Petri dishes and dried in an oven (Memmert, Wisconsin Oven Distributions, LLC, WI, USA) at 30±2 °C for 24 h. Films were obtained and coded as a function of emulsions: F0.5CW,1HP , F0.5CW,1CP , F1CW,2HP, and F1CW,2CP.
Film evaluation
Film thickness (δ, μm) was obtained as the mean of five measurements with a micrometer (Starret Company, USA) at different points of each film. Transparency (T r ) was evaluated with equation (3), using the method of Chana-Thaworn et al. (2011). Rectangular pieces measuring 0.5 cmx4.0 cm were placed in spectrophotometer cells (CS-200PC®, Spectronics Instruments Inc., USA), perpendicular to the light path to evaluate transmittance (T 550) at 550 nm.
Water vapor permeability (WVP) was measured with the method of McHugh et al. (1993) with 125 mL glass containers, 4.5 cm in diameter and 7.5 cm deep. A volume of 35 mL of a saturated KNO3 aqueous solution (Meyer® S. A. de C. V., Mexico) was placed inside each container to generate a constant relative humidity (Rh) of 97 %. Each vessel was covered with the film to be evaluated and the assembly was placed in the headspace of a sealed chamber containing a saturated solution of anhydrous K2CO3 (Meyer® S. A. de C. V., Mexico) at 24±2 °C to develop an internal environment with 54 % Rh. Water vapor flow (r
v
; mol s-1) through the film was calculated by recording the weight loss of the KNO3 solution over a 24-h period. Partial vapor pressures (Pa) were evaluated inside (
In addition, partial vapor pressures were corrected considering the gas diffusion through the static air layer on both sides of the film (Gennadios et al., 1994). The WVP (mol m s-1 m-2 Pa-1) was calculated with equation (5), where A is area of water vapor transmission (m2).
Tensile strength (τ) was evaluated on 8 cmx3 cm rectangular pieces with a texture analyzer (TA-TX2i, Stable Micro Systems, UK). Samples were stretched to rupture point at 2 mm s-1. The resulting force was divided by film thickness and τ was expressed in MPa. In addition, elongation at break (EB) was calculated in percentage with equation (6), where L i and L f were initial and final lengths (mm) of films (Navarro-Tarazaga et al., 2011).
Film application
The mushroom P. ostreatus was used to assess the potential use of edible films of hawthorn pectin and candelilla wax in the postharvest conservation of horticultural products. The mushroom was harvested at Nopaltepec, Mexico (19° 44’ 43” N, 98° 38’ 26” W; 2450 masl). After harvest, it was quickly cooled to 4 °C, cut into 3 cm-5 cm pieces and immersed in a 200 ppm NaClO solution, followed by immersion in a solution of sodium erythorbate (1.0 g 100 mL-1) and citric acid (0.5 g 100 mL-1) for 3 min to delay oxidation (Ventura-Aguilar et al., 2011). Emulsions E0.5CW,1HP and E1CW,2HP were sprayed on the surface of mushroom slices as treatments E1 and E2, respectively. The coated mushroom slices were exposed to a current of air for 30 min, and batches of 150 g of the product from each treatment was put into each of 30 clamshells. Two controls were established: C2 included mushroom slices treated only with NaClO and the antioxidant solution and those of C1 were treated only with the NaClO solution. All batches were placed at 4 °C and 85 % Rh. Every other day, three clamshells from each treatment were assessed in terms of weight loss, firmness, and color. Weight loss was evaluated with a digital scale (Ohaus, USA) relative to weight at the beginning of storage. A texture analyzer (TA-TX2i, Stable Micro Systems, UK) was used to measure firmness with a spherical probe of 5.0 mm in diameter and a routine where the mushroom was deformed up to 4 mm at a velocity of 4 mm s-1. Color was expressed as hue angle, chroma, and lightness and assessed with a Hunter Lab colorimeter (Mini Scan XE Plus 45/0-L, USA).
Data analysis
The study organization during the phase of emulsion and film evaluation was with a completely randomized design and the experimental unit was of one emulsion or one film. We expected that an increment in the concentration of emulsion constituents would affect rheological and mechanical properties and that water vapor barrier results would improve. The phase of film application considered one clamshell with 150 g of product as the experimental unit, and type of treatment (C1, C2, E1, and E2) and storage time were variation factors. It was expected that at least one of the emulsions would have a beneficial effect in prolonging shelf life. All measurements were carried out in triplicate and data was used for ANOVA and means were compared with Tukey test (p≤0.05).
Results and discussion
Hawthorn pectin yield and esterification degree
Purified hawthorn pectin yield (Y p ) was 4.3±0.3 %. Values vary between 7 and 15 % in cases without a purification treatment (Methacanon et al., 2014; Wang et al., 2007). This confirms the report of Yapo (2009), who stated that pectin yield depends on the desired purity. Esterification degree was 76.9±0.1 %, which corresponded to high methoxyl.
Emulsion flow properties
Apparent viscosity (η), as a function of emulsion shear rate (
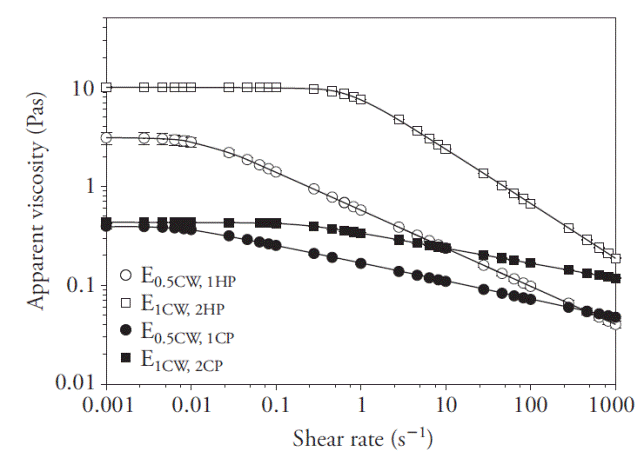
Figure 1 Change of apparent viscosity (η) of emulsions (E) as a function of shear rate (
Table 1 Parameters of the modified Carreau model during rheological characterization of emulsions.
| Emulsion code† | Model parameter¶, § | R2 | ||
| η 0 (Pa s) | λ (s) | n (dimensionless) | ||
| E0.5CW,1HP | 3.09 (±0.7) b | 77.52 (±8.9) b | 0.61 (±0.01) b | 0.90 (±0.004) |
| E1CW,2HP | 9.99 (±0.3) a | 1.36 (±0.1) d | 0.45 (±0.01) c | 0.99 (±0.003) |
| E0.5CW,1CP | 0.39 (±0.1) c | 116.71 (±3.7) a | 0.82 (±0.01) a | 0.91 (±0.015) |
| E1CW,2CP | 0.43 (±0.1) c | 5.35 (±1.5) c | 0.85 (±0.02) a | 0.93 (±0.002) |
†CW: candelilla wax; HP: hawthorn pectin, CP: citric pectin. Subscript numbers indicate concentration of HP, CP, or CW in percentage. ¶Model parameters; η 0: low shear rate limiting viscosity; l: characteristic time constant related to the relaxation times of biopolymer in the emulsion; n: shear thinning exponent that adjusts the slope of the shear thinning region; R2: determination coefficient. §Values in parenthesis are standard errors. Means in a column followed by different letters are significantly different (Tukey, p≤0.05)
The values of parameters in equation (7) are in Table 1. Emulsions based on HP showed higher η 0 values (p≤0.05) than emulsions based on CP, regardless of their pectin concentration and φ values, which coincided with the report of Wang et al. (2007), who found that HP tended to exhibit four to six times higher viscosity than citrus or apple pomace pectins. Moreover, Pal (2011) indicated that emulsion rheology is sensitive to biopolymer concentration since an increase in solids in the continuous phase may cause viscosity to increase. On the other hand, McClements (2004) stated that relatively higher φ values produce an increase in particle-particle interactions, causing greater slippage between them in a field of flow, resulting also in higher viscosity. In our study, E1CW,2HP exhibited higher η 0 than E0.5CW,1HP (p≤0.05), which was expected; however, E1CW,2CP displayed a η 0 value similar to E0.5CW,1HP (p≤0.05), which was unexpected.
The behavior of the CP emulsions can be explained by taking into account two offsetting effects. On one hand, a higher concentration of CP tended to increase η 0, but on the other, when φ increased, droplet sizes increased (see Emulsions droplet size sub-section), effectively decreasing the number of droplets per unit volume, resulting in a decrease in η 0. Thus, the two effects compensated each other.
Parameter λ (Table 1) is related to the relaxation time of an emulsion, and its inverse (1/λ) is the critical shear rate (
Emulsion droplet size
The emulsions had the following d3,2 values: 1.79 (±0.01) μm (E0.5CW,1HP)=1.75 (±0.01) μm (E1CW,2HP)=1.71 (±0.02) μm (E0.5CW,1CP)<1.99 (±0.02) μm (E1CW,2CP) (honest significant difference [HSD]=0.19 μm). According to McClements (2004), during emulsification, even after shearing has ceased, the droplets are in continual motion and frequently collide with one another because of their Brownian motion and gravity. It is expected that movement and collision frequency are modulated by the apparent viscosity of the continuous phase and by the mass fraction of the disperse phase. Therefore, it is possible that droplet size in E0.5CW,1HP and E1CW,2HP was non-significantly different because higher φ was balanced with higher apparent viscosity in the latter emulsion. On the other hand, droplet size in E1CW,2CP was significantly higher than in E0.5CW,1CP, probably because the former had higher φ than the latter, but their apparent viscosity was similar throughout the entire shear rate range studied (see Emulsion flow properties sub-section).
Film thickness ( δ )
Higher pectin concentrations and φ values produced significantly thicker films, but the source of the pectin (hawthorn or citrus) had a non-significant effect on this variable. Film thickness varied from 47.3 to 94.3 μm (Table 2). Thickness depends on the method of preparation, and on an industrial scale, values between 150 and 1040 um can be obtained through casting or extrusion procedures (Pérez-Espitia et al., 2014). In our study, a casting method was used and constant volumes were applied to reduce variability. Galus and Lenart (2013) showed that an increase in pectin concentration can increase thickness because of the colloidal properties of this compound. However, the increment in thickness was also associated with an increment of lipids in films (Ghasemlou et al., 2011; Ayala-Zavala et al., 2012).
Table 2 Thickness, transparency, tensile strength, elongation at break, and water vapor permeability of edible films.
| Parameter† | Film code¶, § | HSD | |||
| F0.5CW,1HP | F1CW,2HP | F0.5CW,1CP | F1CW,2CP | ||
| δ | 63.0 (±3.0) b | 94.3 (±5.1) a | 47.3 (±10.2) b | 102.0 (±8.5) a | 19.10 |
| T r | 1.98 (±0.04) a | 1.43 (±0.05) b | 2.03 (±0.06) a | 1.56 (±0.03) b | 0.26 |
| τ | 0.16 (±0.04) b | 0.22 (±0.01) a | 0.14 (±0.01) b | 0.06 (±0.03) c | 0.06 |
| EB | 57.2 (±2.1) a | 4.4 (±1.8) d | 23.9 (±2.8) b | 15.4 (±2.5) c | 5.46 |
| WVPx1014 | 9.3 (±0.7) a | 1.3 (±0.3 b | 9.2 (±2.0) a | 1.2 (±0.3) b | 6.01 |
†Films parameters; δ: thickness (μm); T r : transparency (%); τ: tensile strength (MPa); EB: elongation at break (%); WVP: water vapor permeability (mol m s-1 m-2 Pa-1). ¶CW: candelilla wax, HP: hawthorn pectin, CP: citric pectin. Subscript numbers indicate concentration of HP, CP, or CW in percentage. §Values in parenthesis are standard errors. Means in a row followed by different letters are significantly different. HSD: honest significant difference (Tukey, p≤0.05)
Film transparency
All films were opaque even though CMC was incorporated into the formulations; according to Aulin et al. (2013), CMC has the ability to make films transparent. It is possible that CW droplets scatter in emulsions and reflect light in the same way as a beam of light passing through the medium (Tyndall effect). This effect is more pronounced with higher disperse phase concentrations. In our study, the increase in φ caused a reduction in transparency, regardless of the source of pectin (Table 2). Transparency varied as follows: F0.5CW,1CP (2.03)=F0.5CW,1HP (1.98)>F1CW,2CP (1.56)=F1CW,2HP (1.43). Besides, it is reported that the opaque appearance may increase in edible films if the lipid component is present in higher concentrations (Fabra et al., 2009; Rodrigues et al., 2014).
Mechanical properties of films
Mechanical properties of polysaccharidebased films are often reported in terms of tensile strength (τ) and elongation at break (EB). The τ accounts for the film’s mechanical resistance due to cohesion forces between chains, while EB measures its plasticity, which is the capacity to stretch before breaking (Galus and Lenart, 2013). A high τ is generally necessary for edible films to withstand the normal stress that occurs during their application, subsequent shipping, and handling. However, due to their structure, films with high τ show low EB (Galus and Lenart, 2013). In our study, τ increased from 0.155 to 0.219 MPa as the HP concentration increased, but decreased from 0.136 to 0.063 MPa as CP concentration increased in films. According to Silva-Weiss et al. (2013), τ can increase or decrease as a function of the interaction between ingredients. Thus, based on rheological properties, CP films came from emulsions with a weaker structure than those of HP films, reflected in lower τ of the former. However, the values we found were low. Maftoonazad et al. (2007) reported τ of 2.0-7.5 MPa in pectin-beeswax films, for polysaccharide concentrations ranging from 2.0 to 9.0 %. Besides, Farris et al. (2011) found 11.09 MPa in gelatin-pectin films and indicated that ionic interactions between the positively charged gelatin and the negatively charged pectin produced hydrogels with homogeneous molecular arrangement that improved tensile strength. The inclusion of a lipid disperse phase can reduce τ because of a relaxation of the polymeric matrix (Fabra et al., 2009; Navarro-Tarazaga et al., 2011). In the particular case of CP films, the reduction of τ in the measure that φ increased can possibly be attributed to interrupted aggregation of the CP pectin chains in the network by relatively large disperse phase droplets, which could favor the chains’ sliding during film stretching, showing plastic (non-elastic) behavior (Bonilla et al., 2012). In contrast, in the case of HP films, as pectin concentration increased, a more coherent and intermingled polysaccharide matrix arose, diminishing the effect of CW in structure relaxation, as was evidenced by an increased τ. The increase in pectin and CW concentrations, however, caused a significant reduction in the films’ ability to stretch before breaking (EB ), with values ranging from 57.2 to 4.4 % in HP films and from 23.9 to 15.4 % in the CP films (Table 2), suggesting an increment in structure rigidity. Our results indicate that HP forms self-supported films that have good mechanical properties, but are highly brittle. In order to increase the elasticity of films, plasticizers, such as sorbitol and glycerol, should be added. Plasticizers act chemically between polymeric molecular chains, reducing cohesion forces and allowing the structure to extend. Maftoonazad et al. (2007) reported that EB increased when sorbitol was added to pectin-based films; they attributed such behavior to the replacement of pectin-pectin hydrogen bonds with pectin-sorbitol hydrogen bonds, reducing direct interaction between polymer chains and increasing chain segmental mobility, which enhanced film elongation at break. In our study, plasticization was achieved with a constant amount of glycerol in films, regardless of pectin concentration. However, it is possible that the replacement of pectin-pectin hydrogen bonds with pectin-glycerol hydrogen bonds was not enough to produce an increase in EB.
Water vapor permeability
The increment in pectin and CW concentrations produced a significant reduction in water vapor permeability (WVP), from 9.26x10-14 to 1.25x10-14 mol m s-1 m-2 Pa-1 (average values), regardless of the pectin source used (Table 2). This behavior was expected since the incorporation of a lipid compound into a polymeric film causes reduction in WVP (Bahram et al., 2014; Baldwin and Hagenmaier, 2012). Bosquez-Molina et al. (2003) reported that mesquite gum-based films decreased WVP by 29.1 % when candelilla wax (1.75 % w/w) was added to the polymeric matrix, while Ruíz-Ramos et al. (2006) reported a decrease of 34.6 % in WVP when 2 % w/w CW was added to mesquite gum-chitosan based films. Likewise, Chick and Hernandez (2002) reported a reduction of 27.3 % in WVP of casein-based films when 5 % CW was added. The WVP values of films of our study were lower than typical values reported in the literature for other pectin-based films, which is a positive characteristic. Particularly, the values found were lower than those of carrageenan-pectin films (9.5x10-11 mol m s-1 m-2 Pa-1; Alves et al., 2010) or films with pectin as the sole ingredient (9.6x10-12 mol m s-1 m-2 Pa-1; Galus and Lenart, 2013), with no lipid addition. WVP is an important variable when a biopolymeric film is to be used in the postharvest conservation of horticultural products, which can undergo high transpiration rates and loss of quality when they are exposed to environments with low relative humidity (Morillon et al., 2002). Therefore, it is very important to use films with low WVP. Thus, we underline this effect of CW in our study; its incorporation may improve the potential of pectin films to protect products from dehydration.
Film application
The application of edible coatings on horticultural commodities has several purposes: to reduce water losses and solutes, to limit gaseous exchange with the environment, and to act as a vehicle for incorporating additives (Martín-Belloso et al., 2005). Since pectin is a polymer with high hydrophilicity, it is necessary to verify that the coatings based on pectin-candelilla wax are useful in reducing transpiration when they are applied on horticultural products. The edible mushroom P. ostreatus has a short shelf life and one of the main deterioration factors of this product is a high rate of transpiration, which causes high weight losses. In addition, the fungus undergoes rapid deterioration in postharvest, which is accompanied by high respiration rates (Villaescusa and Gil, 2003) and suggests high metabolic activity. Emulsions based on HP-CW were applied on slices of P. ostreatus in order to verify, first, that their water barrier properties are high enough to reduce transpiration rate and, second, that changes in quality attributes are reduced sufficiently to lengthen shelf life. Good performance of coatings on this type of material would be evidence of its potential for application on other horticultural products.
The material of the treatment without antioxidant and without coating (C1) deteriorated rapidly; deterioration was characterized by the presence of dark spots and evidence of decay by day 10, indicating the end of its shelf life. This situation was accompanied by high weight loss rate, although no significant difference was found between the fungus treated with an antioxidant solution (C2) and the material coated with the emulsion E0.5CW,1HP (E1). In contrast, application of the emulsion E1CW,2HP (E2) significantly reduced weight loss of the fungus relative to the control treatments (Figure 2A). Since the practice of adding a lipid compound has proved to reduce weight loss (Bibi and Baloch, 2014), the beneficial effect of E2 was attributed to the higher presence of candelilla wax, which was coherent with the lower water vapor permeability (WVP) found in the corresponding films.
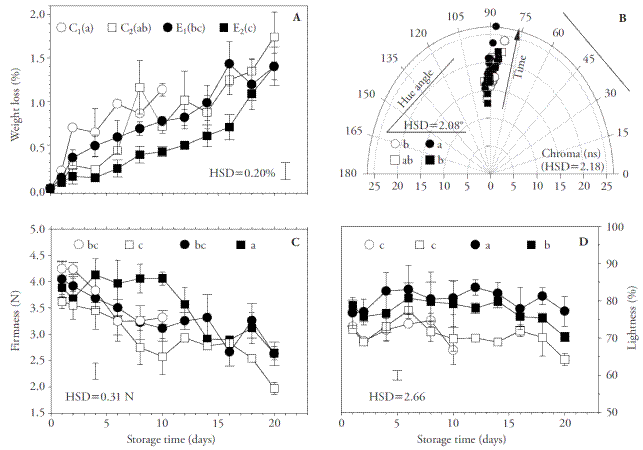
Figure 2 Physiological behavior of Pleurotus ostreatus during 20 days of storage expressed as cumulative weight loss (A), hue angle and chroma (B), firmness (C), and lightness (D). C1: control treatment without antioxidant and without coating; C2: control treatment with antioxidant solution but without no additional coating; E1: material treated with antioxidant solution and coated with 0.5 % CW and 1 % HP emulsion (E0.5CW,1HP); E2: material treated with antioxidant solution and coated with 1 % CW and 2 % HP (E1CW,2HP) emulsion, where CW is candelilla wax and HP is hawthorn pectin. Different letters indicate significant difference. HSD: honest significant difference (Tukey, p≤0.05). Error bars correspond to standard error.
Firmness of the material in both control treatments and that of treatment E1 suffered continuous reduction during storage, while the mushrooms in treatment E2 remained significantly firmer than the rest during the first 10 days of storage, although afterwards there was a gradual decrease in that mechanical property (Figure 2C). The material of all treatments exhibited a yellowish tonality and, although hue had the lowest value in treatment C1 and the highest in treatment E2, the differences had no practical importance. In the case of chroma, there were no significant differences (Figure 2B), but lightness was significantly higher in material coated with a HP-CW emulsion than in either control treatment (Figure 2D), underlining that the coating improved the appearance of the mushroom. Falguera et al. (2011) reported that application of biopolymeric coatings on the surface of fresh produce may induce a modified atmosphere effect inside tissue, affecting color, firmness, sensory quality, microbial growth, and ethylene production, and results in longer shelf life. In our study, the beneficial effects obtained with emulsions of 2 % HP and 1 % CW suggest that the corresponding coatings exhibited properties as barriers to O2 and CO2 that were high enough to reduce metabolic activity. However, permeability of coatings to these gases should be evaluated in future studies to contribute more information that could explain the benefits found. Nevertheless, HP and CW coatings with 2 and 1 %, respectively, could be used as part of a postharvest conservation strategy for horticultural products, since they exhibited good potential to reduce deterioration factors in the oyster mushroom P. ostreatus. Moreover, the use of pectin extracted from hawthorn fruit in coating formulations may constitute a feasible strategy for adding value to this species.
Conclusions
Emulsions based on hawthorn pectin and candelilla wax exhibited rheological behavior that corresponded to a pseudoplastic fluid, which was coherent with the modified Carreau model. The increment in pectin concentration allowed a broader Newtonian phase, with a stable particle size in the disperse phase.
The polymeric films showed tensile strength that increased with increments in pectin concentration, causing, at the same time, a reduction in elongation at break and increased opacity. Water vapor permeability was low and, thus, these films can be good barriers to water vapor exchange. Films based on hawthorn pectin and candelilla wax exhibited good potential for use in postharvest conservation of the oyster mushroom Pleurotus ostreatus.











 texto em
texto em 


